Research Progress on the Regulating Factors of Muscle Fiber Heterogeneity in Livestock: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Skeletal Muscle Fibers
1.2. Classification Methods of Skeletal Muscle Fiber
2. Effect of the Composition of Types of Muscle Fibers on Meat Quality
3. Potential Factors Influencing Myofibers’ Heterogeneity
3.1. Individual Characteristics
3.1.1. Breed
3.1.2. Age
3.1.3. Sex
3.1.4. Muscle Area
3.2. Feeding Patterns
3.2.1. Nutritional Regulation
3.2.2. Environmental Temperatures
3.2.3. Hormone Treatments
3.3. Genetic Regulation
3.3.1. Non-Coding RNAs
miRNA
LncRNA
CircRNA
3.3.2. Marker Genes of Myofiber Transformation
The MYHs Family
PGC-1α
Other Genes
Signaling Pathways
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sans, P.; Combris, P. World meat consumption patterns: An overview of the last fifty years (1961–2011). Meat Sci. 2015, 109, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Milford, A.B.; Le Mouel, C.; Bodirsky, B.L.; Rolinski, S. Drivers of meat consumption. Appetite 2019, 141, 104313. [Google Scholar] [CrossRef] [PubMed]
- Devatkal, S.K.; Naveena, B.M.; Kotaiah, T. Quality, composition, and consumer evaluation of meat from slow-growing broilers relative to commercial broilers. Poult. Sci. 2019, 98, 6177–6186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Thakali, K.; Morse, P.; Shelby, S.; Chen, J.; Apple, J.; Huang, Y. Comparison of Growth Performance and Meat Quality Traits of Commercial Cross-Bred Pigs versus the Large Black Pig Breed. Animals 2021, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.T.; Kim, G.D.; Hwang, Y.H.; Ryu, Y.C. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013, 95, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Cretoiu, D.; Pavelescu, L.; Duica, F.; Radu, M.; Suciu, N.; Cretoiu, S.M. Myofibers. Adv. Exp. Med. Biol. 2018, 1088, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.; Maves, L. Skeletal muscle fiber type: Using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.; Joo, S.T. Poultry Meat Quality in Relation to Muscle Growth and Muscle Fiber Characteristics. Korean J. Food Sci. Anim. Resour. 2017, 37, 873–883. [Google Scholar] [CrossRef]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How Muscle Structure and Composition Influence Meat and Flesh Quality. Sci. World J. 2016, 2016, 3182746. [Google Scholar] [CrossRef]
- Wang, H.H. The perspective of meat and meat-alternative consumption in China. Meat Sci. 2022, 194, 108982. [Google Scholar] [CrossRef]
- Machovina, B.; Feeley, K.J.; Ripple, W.J. Biodiversity conservation: The key is reducing meat consumption. Sci. Total Environ. 2015, 536, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Picard, B.; Berri, C.; Lefaucheur, L.; Molette, C.; Sayd, T.; Terlouw, C. Skeletal muscle proteomics in livestock production. Brief. Funct. Genom. 2010, 9, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jin, J.; Xu, Z.; Zuo, B. Functions and Regulatory Mechanisms of lncRNAs in Skeletal Myogenesis, Muscle Disease and Meat Production. Cells 2019, 8, 1107. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Joo, S.T.; Ryu, Y.C. Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality. Meat Sci. 2010, 86, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Thaete, F.L.; Kelley, D.E. Composition of skeletal muscle evaluated with computed tomography. Ann. N. Y. Acad. Sci. 2000, 904, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef]
- Dong, H.; Tsai, S.Y. Mitochondrial Properties in Skeletal Muscle Fiber. Cells 2023, 12, 2183. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, B.; Schiaffino, S.; Reggiani, C. Mechanisms modulating skeletal muscle phenotype. Compr. Physiol. 2013, 3, 1645–1687. [Google Scholar] [CrossRef]
- Pette, D.; Staron, R.S. Mammalian skeletal muscle fiber type transitions. Int. Rev. Cytol. 1997, 170, 143–223. [Google Scholar] [CrossRef]
- Rehfeldt, C.; Te Pas, M.F.; Wimmers, K.; Brameld, J.M.; Nissen, P.M.; Berri, C.; Valente, L.M.; Power, D.M.; Picard, B.; Stickland, N.C.; et al. Advances in research on the prenatal development of skeletal muscle in animals in relation to the quality of muscle-based food. I. Regulation of myogenesis and environmental impact. Animal 2011, 5, 703–717. [Google Scholar] [CrossRef]
- Zhang, M.; McLennan, I.S. Primary myotubes preferentially mature into either the fastest or slowest muscle fibers. Dev. Dyn. 1998, 213, 147–157. [Google Scholar] [CrossRef]
- Buller, A.J.; Eccles, J.C.; Eccles, R.M. Differentiation of fast and slow muscles in the cat hind limb. J. Physiol. 1960, 150, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Hoh, J.F. Myogenic regulation of mammalian skeletal muscle fibres. News Physiol. Sci. 1991, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sawano, S.; Mizunoya, W. History and development of staining methods for skeletal muscle fiber types. Histol. Histopathol. 2022, 37, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Westerblad, H.; Bruton, J.D.; Katz, A. Skeletal muscle: Energy metabolism, fiber types, fatigue and adaptability. Exp. Cell Res. 2010, 316, 3093–3099. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Preller, M.; Manstein, D.J. Myosin structure, allostery, and mechano-chemistry. Structure 2013, 21, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, H.L.; Houdusse, A.; Robert-Paganin, J. Myosin Structures. Adv. Exp. Med. Biol. 2020, 1239, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, H.L.; Houdusse, A. Structural and functional insights into the Myosin motor mechanism. Annu. Rev. Biophys. 2010, 39, 539–557. [Google Scholar] [CrossRef]
- Abdelmoez, A.M.; Sardon Puig, L.; Smith, J.A.B.; Gabriel, B.M.; Savikj, M.; Dollet, L.; Chibalin, A.V.; Krook, A.; Zierath, J.R.; Pillon, N.J. Comparative profiling of skeletal muscle models reveals heterogeneity of transcriptome and metabolism. Am. J. Physiol. Cell Physiol. 2020, 318, C615–C626. [Google Scholar] [CrossRef]
- Jensen, T.E.; Leutert, R.; Rasmussen, S.T.; Mouatt, J.R.; Christiansen, M.L.; Jensen, B.R.; Richter, E.A. EMG-normalised kinase activation during exercise is higher in human gastrocnemius compared to soleus muscle. PLoS ONE 2012, 7, e31054. [Google Scholar] [CrossRef] [PubMed]
- Bottinelli, R.; Reggiani, C. Human skeletal muscle fibres: Molecular and functional diversity. Prog. Biophys. Mol. Biol. 2000, 73, 195–262. [Google Scholar] [CrossRef] [PubMed]
- Gaver, K.D. A guide through the accreditation maze. Hosp. Community Psychiatry 1982, 33, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S. Muscle fiber type diversity revealed by anti-myosin heavy chain antibodies. FEBS J. 2018, 285, 3688–3694. [Google Scholar] [CrossRef] [PubMed]
- Bandman, E.; Rosser, B.W. Evolutionary significance of myosin heavy chain heterogeneity in birds. Microsc. Res. Tech. 2000, 50, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Barnard, E.A.; Lyles, J.M.; Pizzey, J.A. Fibre types in chicken skeletal muscles and their changes in muscular dystrophy. J. Physiol. 1982, 331, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Fry, C.S.; Mula, J.; Jackson, J.R.; Lee, J.D.; Peterson, C.A.; Yang, L. Automated fiber-type-specific cross-sectional area assessment and myonuclei counting in skeletal muscle. J. Appl. Physiol. 2013, 115, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
- Hamalainen, N.; Pette, D. Patterns of myosin isoforms in mammalian skeletal muscle fibres. Microsc. Res. Tech. 1995, 30, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Bhaskaran, S.; Van Remmen, H. Muscle fiber type diversification during exercise and regeneration. Free Radic. Biol. Med. 2016, 98, 56–67. [Google Scholar] [CrossRef]
- Jin, T.E.; Witzemann, V.; Brecht, M. Fiber types of the intrinsic whisker muscle and whisking behavior. J. Neurosci. 2004, 24, 3386–3393. [Google Scholar] [CrossRef]
- Ketelings, L.; Havermans, R.C.; Kremers, S.P.J.; de Boer, A. How Different Dimensions Shape the Definition of Meat Alternative Products: A Scoping Review of Evidence between 2000 and 2021. Curr. Dev. Nutr. 2023, 7, 101960. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.M.; Vicente, A.F. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef]
- Rogowski, B. Meat in human nutrition. World Rev. Nutr. Diet. 1980, 34, 46–101. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef]
- Gonzalez, N.; Marques, M.; Nadal, M.; Domingo, J.L. Meat consumption: Which are the current global risks? A review of recent (2010–2020) evidences. Food Res. Int. 2020, 137, 109341. [Google Scholar] [CrossRef] [PubMed]
- Geletu, U.S.; Usmael, M.A.; Mummed, Y.Y.; Ibrahim, A.M. Quality of Cattle Meat and Its Compositional Constituents. Vet. Med. Int. 2021, 2021, 7340495. [Google Scholar] [CrossRef]
- Matarneh, S.K.; Silva, S.L.; Gerrard, D.E. New Insights in Muscle Biology that Alter Meat Quality. Annu. Rev. Anim. Biosci. 2021, 9, 355–377. [Google Scholar] [CrossRef]
- Taheri-Garavand, A.; Fatahi, S.; Omid, M.; Makino, Y. Meat quality evaluation based on computer vision technique: A review. Meat Sci. 2019, 156, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; He, Y.; He, Y.; Yan, Z.; Chen, J.; Zhao, R.; Sui, X.; Zhang, L.; Du, X.; Irwin, D.M.; et al. Comparative Proteomic Analysis of Glycolytic and Oxidative Muscle in Pigs. Genes 2023, 14, 361. [Google Scholar] [CrossRef]
- Mo, M.; Zhang, Z.; Wang, X.; Shen, W.; Zhang, L.; Lin, S. Molecular mechanisms underlying the impact of muscle fiber types on meat quality in livestock and poultry. Front. Vet. Sci. 2023, 10, 1284551. [Google Scholar] [CrossRef]
- Komiya, Y.; Mizunoya, W.; Kajiwara, K.; Yokoyama, I.; Ogasawara, H.; Arihara, K. Correlation between skeletal muscle fiber type and responses of a taste sensing system in various beef samples. Anim. Sci. J. 2020, 91, e13425. [Google Scholar] [CrossRef]
- Ryu, Y.C.; Kim, B.C. Comparison of histochemical characteristics in various pork groups categorized by postmortem metabolic rate and pork quality. J. Anim. Sci. 2006, 84, 894–901. [Google Scholar] [CrossRef] [PubMed]
- LeMaster, M.N.; Ha, M.; Dunshea, F.R.; Chauhan, S.; D’Souza, D.; Warner, R.D. Impact of cooking temperature on pork longissimus, and muscle fibre type, on quality traits and protein denaturation of four pork muscles. Meat Sci. 2024, 209, 109395. [Google Scholar] [CrossRef]
- Lee, S.H.; Choe, J.H.; Choi, Y.M.; Jung, K.C.; Rhee, M.S.; Hong, K.C.; Lee, S.K.; Ryu, Y.C.; Kim, B.C. The influence of pork quality traits and muscle fiber characteristics on the eating quality of pork from various breeds. Meat Sci. 2012, 90, 284–291. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, S.; Kim, J.M. Genetic correlation between biopsied and post-mortem muscle fibre characteristics and meat quality traits in swine. Meat Sci. 2022, 186, 108735. [Google Scholar] [CrossRef]
- Huo, W.; Weng, K.; Gu, T.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Effect of muscle fiber characteristics on meat quality in fast- and slow-growing ducks. Poult. Sci. 2021, 100, 101264. [Google Scholar] [CrossRef]
- Weng, K.; Huo, W.; Li, Y.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Fiber characteristics and meat quality of different muscular tissues from slow- and fast-growing broilers. Poult. Sci. 2022, 101, 101537. [Google Scholar] [CrossRef]
- Weng, K.; Huo, W.; Gu, T.; Bao, Q.; Hou, L.E.; Zhang, Y.; Zhang, Y.; Xu, Q.; Chen, G. Effects of marketable ages on meat quality through fiber characteristics in the goose. Poult. Sci. 2021, 100, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Kim, G.D.; Jeong, J.Y.; Hur, S.J.; Joo, S.T. The relationship between muscle fiber characteristics and meat quality traits of highly marbled Hanwoo (Korean native cattle) steers. Meat Sci. 2010, 86, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Song, S.; Jung, E.Y.; Jeong, J.Y.; Joo, S.T.; Kim, G.D. Comparison of beef quality influenced by freeze-thawing among different beef cuts having different muscle fiber characteristics. Meat Sci. 2020, 169, 108206. [Google Scholar] [CrossRef]
- Bai, X.; Yin, F.; Ru, A.; Tian, W.; Li, J.; Zhang, G.; Chen, Q.; Chai, R.; Xiao, K.; Zhu, C.; et al. Muscle fiber composition affects the postmortem redox characteristics of yak beef. Food Chem. 2022, 397, 133797. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; He, J.; Luo, Y.; Yan, H.; Chen, H.; Zheng, P.; et al. Dietary lycopene supplementation improves meat quality, antioxidant capacity and skeletal muscle fiber type transformation in finishing pigs. Anim. Nutr. 2022, 8, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jiang, G.; Wang, Y.; Yan, E.; He, L.; Guo, J.; Yin, J.; Zhang, X. Maternal consumption of l-malic acid enriched diets improves antioxidant capacity and glucose metabolism in offspring by regulating the gut microbiota. Redox Biol. 2023, 67, 102889. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.C.; Kim, B.C. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 2005, 71, 351–357. [Google Scholar] [CrossRef]
- Park, J.; Moon, S.S.; Song, S.; Cheng, H.; Im, C.; Du, L.; Kim, G.D. Comparative review of muscle fiber characteristics between porcine skeletal muscles. J. Anim. Sci. Technol. 2024, 66, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.D.; Yang, H.S.; Jeong, J.Y. Comparison of Characteristics of Myosin Heavy Chain-based Fiber and Meat Quality among Four Bovine Skeletal Muscles. Korean J. Food Sci. Anim. Resour. 2016, 36, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.Y.; Luo, J.; Lei, H.G.; Jiang, Y.Z.; Bai, L.; Li, M.Z.; Tang, G.Q.; Li, X.W.; Zhang, S.H.; Zhu, L. Effects of muscle fiber type on glycolytic potential and meat quality traits in different Tibetan pig muscles and their association with glycolysis-related gene expression. Genet. Mol. Res. 2015, 14, 14366–14378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, Y.; Wu, W.; Hou, L.; Chen, H.; Zuo, B.; Xiong, Y.; Yang, J. Skeletal Muscle-Specific Overexpression of PGC-1alpha Induces Fiber-Type Conversion through Enhanced Mitochondrial Respiration and Fatty Acid Oxidation in Mice and Pigs. Int. J. Biol. Sci. 2017, 13, 1152–1162. [Google Scholar] [CrossRef]
- Mashima, D.; Oka, Y.; Gotoh, T.; Tomonaga, S.; Sawano, S.; Nakamura, M.; Tatsumi, R.; Mizunoya, W. Correlation between skeletal muscle fiber type and free amino acid levels in Japanese Black steers. Anim. Sci. J. 2019, 90, 604–609. [Google Scholar] [CrossRef]
- Verdiglione, R.; Cassandro, M. Characterization of muscle fiber type in the pectoralis major muscle of slow-growing local and commercial chicken strains. Poult. Sci. 2013, 92, 2433–2437. [Google Scholar] [CrossRef]
- Hocquette, J.F. Endocrine and metabolic regulation of muscle growth and body composition in cattle. Animal 2010, 4, 1797–1809. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Shan, T.; Wu, T.; Zhu, L.N.; Ren, Y.; An, S.; Wang, Y. Comparisons of different muscle metabolic enzymes and muscle fiber types in Jinhua and Landrace pigs. J. Anim. Sci. 2011, 89, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, L.; Tong, X.; Li, R.; Jin, E.; Ren, M.; Gao, Y.; Gu, Y.; Li, S. Transcriptomic Profiling of Meat Quality Traits of Skeletal Muscles of the Chinese Indigenous Huai Pig and Duroc Pig. Genes 2023, 14, 1548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Ma, C.; Wang, W.; Wang, H.; Jiang, Y. Comparative Transcriptomic Analysis of mRNAs, miRNAs and lncRNAs in the Longissimus dorsi Muscles between Fat-Type and Lean-Type Pigs. Biomolecules 2022, 12, 1294. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Lin, Z.; Xie, F.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y.; Sun, J. Identification of circRNA-associated ceRNA networks using longissimus thoracis of pigs of different breeds and growth stages. BMC Genom. 2022, 23, 294. [Google Scholar] [CrossRef] [PubMed]
- Buzala, M.; Janicki, B. Review: Effects of different growth rates in broiler breeder and layer hens on some productive traits. Poult. Sci. 2016, 95, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Aberle, E.D.; Stewart, T.S. Growth of fiber types and apparent fiber number in skeletal muscle of broiler- and layer-type chickens. Growth 1983, 47, 135–144. [Google Scholar] [PubMed]
- Zhao, J.P.; Zhao, G.P.; Jiang, R.R.; Zheng, M.Q.; Chen, J.L.; Liu, R.R.; Wen, J. Effects of diet-induced differences in growth rate on metabolic, histological, and meat-quality properties of 2 muscles in male chickens of 2 distinct broiler breeds. Poult. Sci. 2012, 91, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Lim, A.J.; Muir, W.I.; Groves, P.J. Comparison of performance and carcass composition of a novel slow-growing crossbred broiler with fast-growing broiler for chicken meat in Australia. Poult. Sci. 2021, 100, 100966. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Ma, J.; Zhang, Y.; Zhang, H. Integrating genome and transcriptome profiling for elucidating the mechanism of muscle growth and lipid deposition in Pekin ducks. Sci. Rep. 2017, 7, 3837. [Google Scholar] [CrossRef]
- Wu, N.; Gu, T.; Lu, L.; Cao, Z.; Song, Q.; Wang, Z.; Zhang, Y.; Chang, G.; Xu, Q.; Chen, G. Roles of miRNA-1 and miRNA-133 in the proliferation and differentiation of myoblasts in duck skeletal muscle. J. Cell Physiol. 2019, 234, 3490–3499. [Google Scholar] [CrossRef]
- Wegner, J.; Albrecht, E.; Fiedler, I.; Teuscher, F.; Papstein, H.J.; Ender, K. Growth- and breed-related changes of muscle fiber characteristics in cattle. J. Anim. Sci. 2000, 78, 1485–1496. [Google Scholar] [CrossRef]
- Albrecht, E.; Lembcke, C.; Wegner, J.; Maak, S. Prenatal muscle fiber development and bundle structure in beef and dairy cattle. J. Anim. Sci. 2013, 91, 3666–3673. [Google Scholar] [CrossRef] [PubMed]
- Stavaux, D.; Art, T.; McEntee, K.; Reznick, M.; Lekeux, P. Muscle fibre type and size, and muscle capillary density in young double-muscled blue Belgian cattle. Zentralbl Vet. A 1994, 41, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, N.M.; Garcia, F.; Jurie, C.; Agabriel, J.; Micol, D.; Bauchart, D.; Listrat, A.; Picard, B. Meta-analysis of the effect of animal maturity on muscle characteristics in different muscles, breeds, and sexes of cattle. J. Anim. Sci. 2008, 86, 2872–2887. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, S.; Yan, P. The meat quality, muscle fiber characteristics and fatty acid profile in Jinjiang and F1 SimmentalxJinjiang yellow cattle. Asian-Australas. J. Anim. Sci. 2018, 31, 301–308. [Google Scholar] [CrossRef]
- Bittante, G.; Cecchinato, A.; Tagliapietra, F.; Verdiglione, R.; Simonetto, A.; Schiavon, S. Crossbred young bulls and heifers sired by double-muscled Piemontese or Belgian Blue bulls exhibit different effects of sexual dimorphism on fattening performance and muscularity but not on meat quality traits. Meat Sci. 2018, 137, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Bunger, L.; Navajas, E.A.; Stevenson, L.; Lambe, N.R.; Maltin, C.A.; Simm, G.; Fisher, A.V.; Chang, K.C. Muscle fibre characteristics of two contrasting sheep breeds: Scottish Blackface and Texel. Meat Sci. 2009, 81, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.B.; Moody, W.G.; Kemp, J.D.; Ely, D.G. Effect of breed, slaughter weight and sex on histological properties of ovine muscle. J. Anim. Sci. 1981, 52, 1019–1025. [Google Scholar] [CrossRef]
- Thompson, L.V. Effects of age and training on skeletal muscle physiology and performance. Phys. Ther. 1994, 74, 71–81. [Google Scholar] [CrossRef]
- Ying, F.; Zhang, L.; Bu, G.; Xiong, Y.; Zuo, B. Muscle fiber-type conversion in the transgenic pigs with overexpression of PGC1alpha gene in muscle. Biochem. Biophys. Res. Commun. 2016, 480, 669–674. [Google Scholar] [CrossRef]
- Wang, B.; Nie, W.; Fu, X.; de Avila, J.M.; Ma, Y.; Zhu, M.J.; Maquivar, M.; Parish, S.M.; Busboom, J.R.; Nelson, M.L.; et al. Neonatal vitamin A injection promotes cattle muscle growth and increases oxidative muscle fibers. J. Anim. Sci. Biotechnol. 2018, 9, 82. [Google Scholar] [CrossRef]
- Wank, V.; Fischer, M.S.; Walter, B.; Bauer, R. Muscle growth and fiber type composition in hind limb muscles during postnatal development in pigs. Cells Tissues Organs 2006, 182, 171–181. [Google Scholar] [CrossRef]
- Ortiz, A.; Tejerina, D.; Garcia-Torres, S.; Gonzalez, E.; Morcillo, J.F.; Mayoral, A.I. Effect of Animal Age at Slaughter on the Muscle Fibres of Longissimus thoracis and Meat Quality of Fresh Loin from Iberian x Duroc Crossbred Pig under Two Production Systems. Animals 2021, 11, 2143. [Google Scholar] [CrossRef]
- Katsumata, M.; Yamaguchi, T.; Ishida, A.; Ashihara, A. Changes in muscle fiber type and expression of mRNA of myosin heavy chain isoforms in porcine muscle during pre- and postnatal development. Anim. Sci. J. 2017, 88, 364–371. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, X.; Ai, N.; Wang, K.; Zhang, P.; Li, X.; LiuFu, S.; Liu, X.; Jiang, J.; Gu, J.; et al. Integrated analysis of circRNA, lncRNA, miRNA and mRNA to reveal the ceRNA regulatory network of postnatal skeletal muscle development in Ningxiang pig. Front. Cell Dev. Biol. 2023, 11, 1185823. [Google Scholar] [CrossRef]
- Smith, D.P.; Fletcher, D.L. Chicken breast muscle fiber type and diameter as influenced by age and intramuscular location. Poult. Sci. 1988, 67, 908–913. [Google Scholar] [CrossRef]
- Ono, Y.; Iwamoto, H.; Takahara, H. The relationship between muscle growth and the growth of different fiber types in the chicken. Poult. Sci. 1993, 72, 568–576. [Google Scholar] [CrossRef]
- Shu, J.; Ji, G.; Zhang, M.; Tu, Y.; Shan, Y.; Liu, Y.; Ju, X.; Zhang, D. Molecular Cloning, Characterization, and Temporal Expression Profile of Troponin I Type 1 (TNNI1) Gene in Skeletal Muscle During Early Development of Gaoyou Duck (Anas Platyrhynchos Domestica). Anim. Biotechnol. 2019, 30, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Baeza, E.; Salichon, M.R.; Marche, G.; Wacrenier, N.; Dominguez, B.; Culioli, J. Effects of age and sex on the structural, chemical and technological characteristics of mule duck meat. Br. Poult. Sci. 2000, 41, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Picard, B.; Gagniere, H.; Geay, Y.; Hocquette, J.F.; Robelin, J. Study of the influence of age and weaning on the contractile and metabolic characteristics of bovine muscle. Reprod. Nutr. Dev. 1995, 35, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Costagliola, A.; Wojcik, S.; Pagano, T.B.; De Biase, D.; Russo, V.; Iovane, V.; Grieco, E.; Papparella, S.; Paciello, O. Age-Related Changes in Skeletal Muscle of Cattle. Vet. Pathol. 2016, 53, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Jurie, C.; Picard, B.; Geay, Y. Changes in the metabolic and contractile characteristics of muscle in male cattle between 10 and 16 months of age. Histochem. J. 1999, 31, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Bakhsh, A.; Lee, J.G.; Joo, S.T. Differences in Muscle Fiber Characteristics and Meat Quality by Muscle Type and Age of Korean Native Black Goat. Food Sci. Anim. Resour. 2019, 39, 988–999. [Google Scholar] [CrossRef] [PubMed]
- White, N.A.; McGavin, M.D.; Smith, J.E. Age-related changes in percentage of fiber types and mean fiber diameters of the ovine quadriceps muscles. Am. J. Vet. Res. 1978, 39, 1297–1302. [Google Scholar] [PubMed]
- Roneus, M.; Lindholm, A.; Asheim, A. Muscle characteristics in Thoroughbreds of different ages and sexes. Equine Vet. J. 1991, 23, 207–210. [Google Scholar] [CrossRef]
- Esbjornsson, M.E.; Dahlstrom, M.S.; Gierup, J.W.; Jansson, E.C. Muscle fiber size in healthy children and adults in relation to sex and fiber types. Muscle Nerve 2021, 63, 586–592. [Google Scholar] [CrossRef] [PubMed]
- English, A.W.; Widmer, C.G. Sex differences in rabbit masseter muscle function. Cells Tissues Organs 2003, 174, 87–96. [Google Scholar] [CrossRef]
- English, A.W.; Eason, J.; Schwartz, G.; Shirley, A.; Carrasco, D.I. Sexual dimorphism in the rabbit masseter muscle: Myosin heavy chain composition of neuromuscular compartments. Cells Tissues Organs 1999, 164, 179–191. [Google Scholar] [CrossRef]
- English, A.W.; Schwartz, G. Development of sex differences in the rabbit masseter muscle is not restricted to a critical period. J. Appl. Physiol. 2002, 92, 1214–1222. [Google Scholar] [CrossRef]
- Baeza, E.; Marche, G.; Wacrenier, N. Effect of sex on muscular development of Muscovy ducks. Reprod. Nutr. Dev. 1999, 39, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Palencia, J.Y.P.; Garbossa, C.A.P.; Betarelli, R.P.; Fonseca, L.S.; Lanferdini, E.; Guimaraes, G.C.; Zangeronimo, M.G.; Schinckel, A.P.; Abreu, M.L.T. Swine foetal myogenesis in different gestation periods. J. Anim. Physiol. Anim. Nutr. 2018, 102, e99–e105. [Google Scholar] [CrossRef] [PubMed]
- Larzul, C.; Lefaucheur, L.; Ecolan, P.; Gogue, J.; Talmant, A.; Sellier, P.; Le Roy, P.; Monin, G. Phenotypic and genetic parameters for longissimus muscle fiber characteristics in relation to growth, carcass, and meat quality traits in large white pigs. J. Anim. Sci. 1997, 75, 3126–3137. [Google Scholar] [CrossRef] [PubMed]
- Sawano, S.; Oza, K.; Murakami, T.; Nakamura, M.; Tatsumi, R.; Mizunoya, W. Effect of Gender, Rearing, and Cooking on the Metabolomic Profile of Porcine Muscles. Metabolites 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, K.; Tabata, S.; Shiba, N.; Gotoh, T.; Nishimura, S.; Iwanoto, H. Myofibre composition and total collagen content in M. iliotibialis lateralis and M. pectoralis of Silkie and White Leghorn chickens. Br. Poult. Sci. 2000, 41, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.M.; Meyer, H.H. Influences of gender, time of castration, genotype, birthtype and feeding regimen on lamb longissimus fiber type proportions. J. Anim. Sci. 1988, 66, 2476–2483. [Google Scholar] [CrossRef]
- Pattanakuhar, S.; Pongchaidecha, A.; Chattipakorn, N.; Chattipakorn, S.C. The effect of exercise on skeletal muscle fibre type distribution in obesity: From cellular levels to clinical application. Obes. Res. Clin. Pract. 2017, 11, 112–132. [Google Scholar] [CrossRef]
- Kernell, D. Muscle regionalization. Can. J. Appl. Physiol. 1998, 23, 1–22. [Google Scholar] [CrossRef]
- Picard, B.; Lefaucheur, L.; Berri, C.; Duclos, M.J. Muscle fibre ontogenesis in farm animal species. Reprod. Nutr. Dev. 2002, 42, 415–431. [Google Scholar] [CrossRef]
- Kim, G.D.; Yang, H.S.; Jeong, J.Y. Intramuscular variations of proteome and muscle fiber type distribution in semimembranosus and semitendinosus muscles associated with pork quality. Food Chem. 2018, 244, 143–152. [Google Scholar] [CrossRef]
- Ju, X.; Liu, Y.; Shan, Y.; Ji, G.; Zhang, M.; Tu, Y.; Zou, J.; Chen, X.; Geng, Z.; Shu, J. Analysis of potential regulatory LncRNAs and CircRNAs in the oxidative myofiber and glycolytic myofiber of chickens. Sci. Rep. 2021, 11, 20861. [Google Scholar] [CrossRef] [PubMed]
- Nagasao, J.; Fukasawa, H.; Yoshioka, K.; Miyamoto, M.; Iwaki, Y.; Kajiwara, K.; Sato, K.; Arihara, K. Skeletal Muscle Fibre Type Changes in an Avian Model of Hepatic Fibrosis. J. Comp. Pathol. 2021, 183, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hosotani, M.; Kametani, K.; Ohno, N.; Hiramatsu, K.; Kawasaki, T.; Hasegawa, Y.; Iwasaki, T.; Watanabe, T. The unique physiological features of the broiler pectoralis major muscle as suggested by the three-dimensional ultrastructural study of mitochondria in type IIb muscle fibers. J. Vet. Med. Sci. 2021, 83, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Chriki, S.; Picard, B.; Jurie, C.; Reichstadt, M.; Micol, D.; Brun, J.P.; Journaux, L.; Hocquette, J.F. Meta-analysis of the comparison of the metabolic and contractile characteristics of two bovine muscles: Longissimus thoracis and semitendinosus. Meat Sci. 2012, 91, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Oury, M.P.; Dumont, R.; Jurie, C.; Hocquette, J.F.; Picard, B. Specific fibre composition and metabolism of the rectus abdominis muscle of bovine Charolais cattle. BMC Biochem. 2010, 11, 12. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, X.; Chu, M.; Guo, X.; Pei, J.; Xiong, L.; Ma, X.; Bao, P.; Liang, C.; Yan, P. Whole transcriptome analyses and comparison reveal the metabolic differences between oxidative and glycolytic skeletal muscles of yak. Meat Sci. 2022, 194, 108948. [Google Scholar] [CrossRef]
- Konno, T.; Watanabe, K. Morpho-functional relationship between muscular architecture and proportion of myofiber types in ovine antebrachial musculature. Okajimas Folia Anat. Jpn. 2012, 89, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Konno, T.; Watanabe, K. Distribution of myofiber types in the crural musculature of sheep. Okajimas Folia Anat. Jpn. 2012, 89, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Bou, T.; Han, H.; Mongke, T.; Zhao, R.; La, X.; Ding, W.; Jia, Z.; Liu, H.; Tiemuqier, A.; An, T.; et al. Fast and slow myofiber-specific expression profiles are affected by noncoding RNAs in Mongolian horses. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2022, 41, 100942. [Google Scholar] [CrossRef]
- Lexell, J.; Jarvis, J.C.; Currie, J.; Downham, D.Y.; Salmons, S. Fibre type composition of rabbit tibialis anterior and extensor digitorum longus muscles. J. Anat. 1994, 185 Pt 1, 95–101. [Google Scholar]
- Wu, Y.; Zhao, J.; Xu, C.; Ma, N.; He, T.; Zhao, J.; Ma, X.; Thacker, P.A. Progress towards pig nutrition in the last 27 years. J. Sci. Food Agric. 2020, 100, 5102–5110. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, X.; Schilling, M.W.; Li, X.; Tabler, G.T.; Peebles, E.D.; Zhai, W. Effects of broiler genetic strain and dietary amino acid reduction on meat yield and quality (part II). Poult. Sci. 2021, 100, 101033. [Google Scholar] [CrossRef]
- Deane, C.S.; Bass, J.J.; Crossland, H.; Phillips, B.E.; Atherton, P.J. Animal, Plant, Collagen and Blended Dietary Proteins: Effects on Musculoskeletal Outcomes. Nutrients 2020, 12, 2670. [Google Scholar] [CrossRef]
- Turkozu, D.; Sanlier, N. L-theanine, unique amino acid of tea, and its metabolism, health effects, and safety. Crit. Rev. Food Sci. Nutr. 2017, 57, 1681–1687. [Google Scholar] [CrossRef]
- Chen, X.; Chen, L.; Qin, Y.; Mao, Z.; Jia, G.; Zhao, H.; Liu, G.; Huang, Z. Effect of dietary L-theanine supplementation on skeletal muscle fiber type transformation in weaning piglets. Anim. Biotechnol. 2022, 33, 1389–1397. [Google Scholar] [CrossRef]
- Dai, H.; Chen, X.; Chen, D.; Yu, B.; He, J.; Chen, H.; Yan, H.; Zheng, P.; Luo, Y.; Huang, Z. Effects of dietary l-theanine supplementation on pork quality and muscle fiber type transformation in finishing pigs. J. Sci. Food Agric. 2023, 103, 2106–2115. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Zhao, X.; Chen, K.; Geng, Z. Effect of L-theanine on meat quality, muscle amino acid profiles, and antioxidant status of broilers. Anim. Sci. J. 2020, 91, e13351. [Google Scholar] [CrossRef]
- Qui, N.H. Immune-boosting role of L-theanine in broiler poultry production under stress conditions. Open Vet. J. 2022, 12, 250–255. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Chen, K.; Zhao, X.; Geng, Z. Effect of l-theanine on growth performance, intestinal development and health, and peptide and amino acid transporters expression of broilers. J. Sci. Food Agric. 2020, 100, 1718–1725. [Google Scholar] [CrossRef]
- Saeed, M.; Khan, M.S.; Kamboh, A.A.; Alagawany, M.; Khafaga, A.F.; Noreldin, A.E.; Qumar, M.; Safdar, M.; Hussain, M.; Abd El-Hack, M.E.; et al. L-theanine: An astounding sui generis amino acid in poultry nutrition. Poult. Sci. 2020, 99, 5625–5636. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Li, Z.; Yu, Y.; Yang, L.; Zhang, P.; Shen, W.; Wan, F.; He, J.; Xiao, W.; et al. Alterations of endotoxin distribution across different biofluids and relevant inflammatory responses by supplementing L-theanine in dairy cows during heat stress. Anim. Nutr. 2021, 7, 1253–1257. [Google Scholar] [CrossRef]
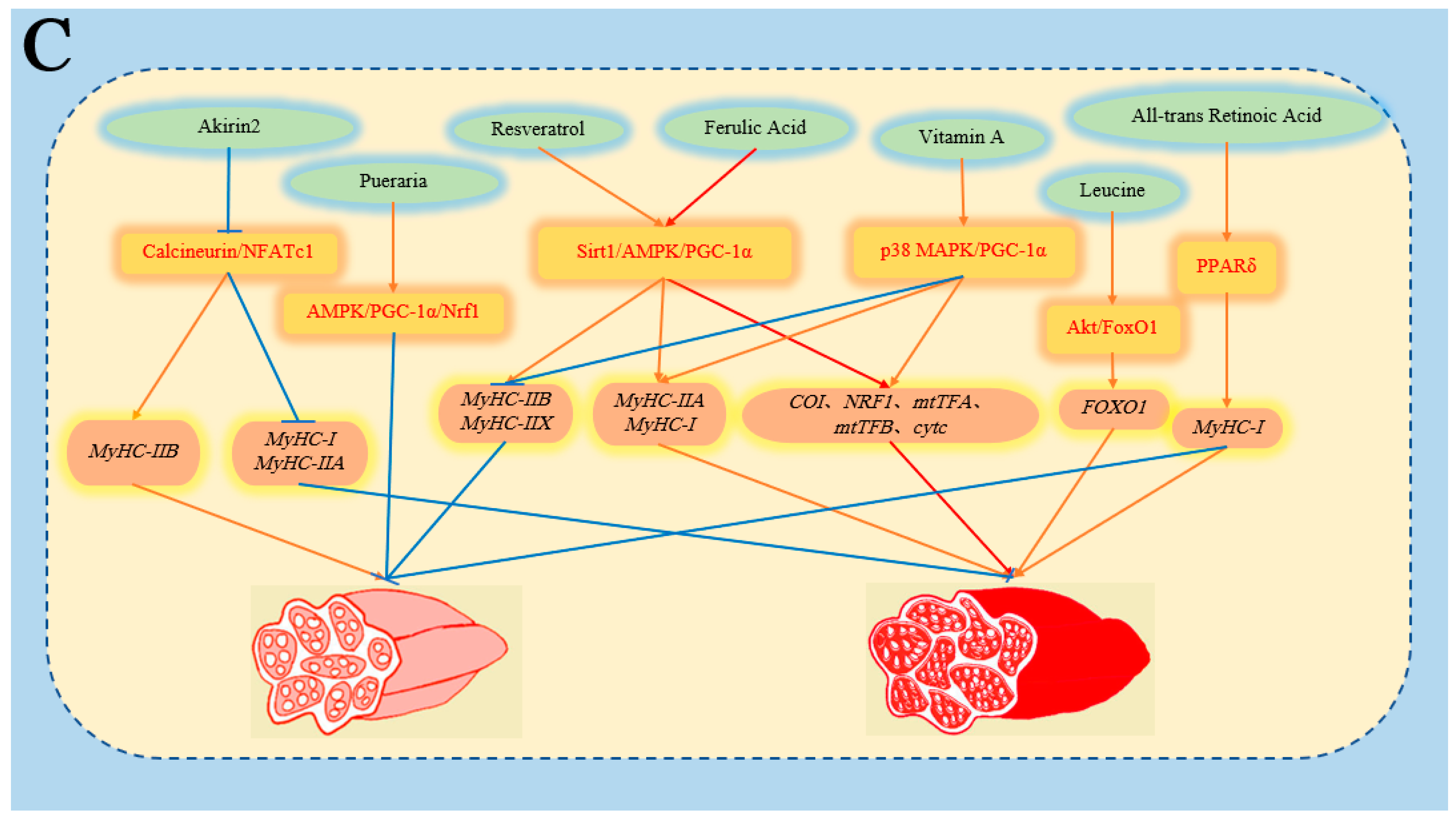
- Toniolo, L.; Concato, M.; Giacomello, E. Resveratrol, a Multitasking Molecule That Improves Skeletal Muscle Health. Nutrients 2023, 15, 3413. [Google Scholar] [CrossRef]
- Niu, W.; Wang, H.; Wang, B.; Mao, X.; Du, M. Resveratrol improves muscle regeneration in obese mice through enhancing mitochondrial biogenesis. J. Nutr. Biochem. 2021, 98, 108804. [Google Scholar] [CrossRef]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Chen, H.; Luo, Y.; He, J.; Zheng, P.; Yu, J.; Yu, B. Resveratrol regulates muscle fiber type conversion via miR-22-3p and AMPK/SIRT1/PGC-1alpha pathway. J. Nutr. Biochem. 2020, 77, 108297. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, J.; Yu, B.; Zheng, P.; Huang, Z.; Mao, X.; He, J.; Yu, J.; Chen, J.; Chen, D. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 2015, 102, 15–21. [Google Scholar] [CrossRef]
- Meng, Q.; Sun, S.; Bai, Y.; Luo, Z.; Li, Z.; Shi, B.; Shan, A. Effects of dietary resveratrol supplementation in sows on antioxidative status, myofiber characteristic and meat quality of offspring. Meat Sci. 2020, 167, 108176. [Google Scholar] [CrossRef]
- Li, J.; Liang, R.; Mao, Y.; Yang, X.; Luo, X.; Qian, Z.; Zhang, Y.; Zhu, L. Effect of dietary resveratrol supplementation on muscle fiber types and meat quality in beef cattle. Meat Sci. 2022, 194, 108986. [Google Scholar] [CrossRef]
- Cui, Y.; Qi, J.; Li, J.; Zhang, Y.; Yang, X.; Xin, L.; Niu, L.; Xu, B.; Qian, Z.; Zhu, L.; et al. Effects of dietary resveratrol supplementation in cattle on the anti-oxidative capacity and meat quality of beef steaks under high-oxygen packaging. Meat Sci. 2023, 204, 109238. [Google Scholar] [CrossRef]
- Yu, Q.; Fang, C.; Ma, Y.; He, S.; Ajuwon, K.M.; He, J. Dietary resveratrol supplement improves carcass traits and meat quality of Pekin ducks. Poult. Sci. 2021, 100, 100802. [Google Scholar] [CrossRef]
- Gunawan, A.M.; Richert, B.T.; Schinckel, A.P.; Grant, A.L.; Gerrard, D.E. Ractopamine induces differential gene expression in porcine skeletal muscles. J. Anim. Sci. 2007, 85, 2115–2124. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, L.; Huang, Z.; Jia, G.; Liu, G.; Zhao, H. Effect of dietary leucine supplementation on skeletal muscle fiber type transformation in weaning piglets. Anim. Biotechnol. 2022, 33, 546–554. [Google Scholar] [CrossRef]
- Chen, X.; Jia, G.; Liu, G.; Zhao, H.; Huang, Z. Effects of apple polyphenols on myofiber-type transformation in longissimus dorsi muscle of finishing pigs. Anim. Biotechnol. 2021, 32, 246–253. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, X.; Huang, Z.; Chen, D.; Li, M.; Yu, B.; He, J.; Luo, Y.; Yan, H.; Zheng, P. Dihydromyricetin improves meat quality and promotes skeletal muscle fiber type transformations via AMPK signaling in growing-finishing pigs. Food Funct. 2022, 13, 3649–3659. [Google Scholar] [CrossRef]
- Bee, G.; Calderini, M.; Biolley, C.; Guex, G.; Herzog, W.; Lindemann, M.D. Changes in the histochemical properties and meat quality traits of porcine muscles during the growing-finishing period as affected by feed restriction, slaughter age, or slaughter weight. J. Anim. Sci. 2007, 85, 1030–1045. [Google Scholar] [CrossRef]
- Hergenreder, J.E.; Legako, J.F.; Dinh, T.T.N.; Spivey, K.S.; Baggerman, J.O.; Broadway, P.R.; Beckett, J.L.; Branine, M.E.; Johnson, B.J. Zinc Methionine Supplementation Impacts Gene and Protein Expression in Calf-Fed Holstein Steers with Minimal Impact on Feedlot Performance. Biol. Trace Element. Res. 2016, 171, 315–327. [Google Scholar] [CrossRef]
- Luo, Y.; Ju, N.; Chang, J.; Ge, R.; Zhao, Y.; Zhang, G. Dietary alpha-lipoic acid supplementation improves postmortem color stability of the lamb muscles through changing muscle fiber types and antioxidative status. Meat Sci. 2022, 193, 108945. [Google Scholar] [CrossRef]
- Spooner, H.C.; Derrick, S.A.; Maj, M.; Manjarin, R.; Hernandez, G.V.; Tailor, D.S.; Bastani, P.S.; Fanter, R.K.; Fiorotto, M.L.; Burrin, D.G.; et al. High-Fructose, High-Fat Diet Alters Muscle Composition and Fuel Utilization in a Juvenile Iberian Pig Model of Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 4195. [Google Scholar] [CrossRef]
- Ren, Q.; Li, H.; Xu, F.; Zhu, Y.; Zhang, X.; Fan, T.; Wei, Z.; Yuan, F.; Han, F.; Cong, R. Effect of high-concentrate diets on mRNA expression of genes related to muscle fiber type and metabolism of psoas major muscle in goats. Anim. Sci. J. 2022, 93, e13725. [Google Scholar] [CrossRef]
- Meale, S.J.; Ruiz-Sanchez, A.L.; Dervishi, E.; Roy, B.C.; Paradis, F.; Juarez, M.; Aalhus, J.; Lopez-Campos, O.; Das, C.; Li, C.; et al. Impact of genetic potential for residual feed intake and diet fed during early- to mid-gestation in beef heifers on carcass characteristics and meat quality attributes of their castrated male offspring. Meat Sci. 2021, 182, 108637. [Google Scholar] [CrossRef]
- Brown, A.D.; Fogarty, M.J.; Sieck, G.C. Mitochondrial morphology and function varies across diaphragm muscle fiber types. Respir. Physiol. Neurobiol. 2022, 295, 103780. [Google Scholar] [CrossRef]
- Makida, S.; Kametani, K.; Hosotani, M.; Takahashi, N.; Iwasaki, T.; Hasegawa, Y.; Takaya, T.; Ueda, H.; Watanabe, T. Three-dimensional structural analysis of mitochondria composing each subtype of fast-twitch muscle fibers in chicken. J. Vet. Med. Sci. 2022, 84, 809–816. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Wu, R.; Guo, G.; Liu, Y.; Zeng, B.; Liao, X.; Wang, Y.; Wang, X. DHA alleviates diet-induced skeletal muscle fiber remodeling via FTO/m(6)A/DDIT4/PGC1alpha signaling. BMC Biol. 2022, 20, 39. [Google Scholar] [CrossRef]
- He, X.; Duan, Y.; Yao, K.; Li, F.; Hou, Y.; Wu, G.; Yin, Y. beta-Hydroxy-beta-methylbutyrate, mitochondrial biogenesis, and skeletal muscle health. Amino Acids 2016, 48, 653–664. [Google Scholar] [CrossRef]
- Hines, E.A.; Coffey, J.D.; Starkey, C.W.; Chung, T.K.; Starkey, J.D. Improvement of maternal vitamin D status with 25-hydroxycholecalciferol positively impacts porcine fetal skeletal muscle development and myoblast activity. J. Anim. Sci. 2013, 91, 4116–4122. [Google Scholar] [CrossRef]
- Fainberg, H.P.; Almond, K.L.; Li, D.; Rauch, C.; Bikker, P.; Symonds, M.E.; Mostyn, A. Impact of maternal dietary fat supplementation during gestation upon skeletal muscle in neonatal pigs. BMC Physiol. 2014, 14, 6. [Google Scholar] [CrossRef]
- Ehrenborg, E.; Krook, A. Regulation of skeletal muscle physiology and metabolism by peroxisome proliferator-activated receptor delta. Pharmacol. Rev. 2009, 61, 373–393. [Google Scholar] [CrossRef]
- Marquez, D.C.; Paulino, M.F.; Renno, L.N.; Villadiego, F.C.; Ortega, R.M.; Moreno, D.S.; Martins, L.S.; de Almeida, D.M.; Gionbelli, M.P.; Manso, M.R.; et al. Supplementation of grazing beef cows during gestation as a strategy to improve skeletal muscle development of the offspring. Animal 2017, 11, 2184–2192. [Google Scholar] [CrossRef]
- Ithurralde, J.; Perez-Clariget, R.; Saadoun, A.; Genovese, P.; Cabrera, C.; Lopez, Y.; Feed, O.; Bielli, A. Gestational nutrient restriction under extensive grazing conditions: Effects on muscle characteristics and meat quality in heavy lambs. Meat Sci. 2021, 179, 108532. [Google Scholar] [CrossRef]
- Zhu, M.J.; Ford, S.P.; Means, W.J.; Hess, B.W.; Nathanielsz, P.W.; Du, M. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J. Physiol. 2006, 575, 241–250. [Google Scholar] [CrossRef]
- Stickland, N.; Bayol, S.; Ashton, C.; Rehfeldt, C. Manipulation of muscle fibre number during prenatal development. In Muscle Development of Livestock Animals: Physiology, Genetics and Meat Quality; CABI Publishing: Wallingford, UK, 2004. [Google Scholar]
- Hu, C.; Yang, Y.; Chen, M.; Hao, X.; Wang, S.; Yang, L.; Yin, Y.; Tan, C. A maternal high-fat/low-fiber diet impairs glucose tolerance and induces the formation of glycolytic muscle fibers in neonatal offspring. Eur. J. Nutr. 2021, 60, 2709–2718. [Google Scholar] [CrossRef]
- Lu, Z.Q.; Ren, Y.; Zhou, X.H.; Yu, X.F.; Huang, J.; Yu, D.Y.; Wang, X.X.; Wang, Y.Z. Maternal dietary linoleic acid supplementation promotes muscle fibre type transformation in suckling piglets. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1130–1136. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Yang, X.; Sun, Q.; Huang, R.; Xing, J.; Zhao, R. Maternal dietary protein induces opposite myofiber type transition in Meishan pigs at weaning and finishing stages. Meat Sci. 2011, 89, 221–227. [Google Scholar] [CrossRef]
- Cerisuelo, A.; Baucells, M.D.; Gasa, J.; Coma, J.; Carrion, D.; Chapinal, N.; Sala, R. Increased sow nutrition during midgestation affects muscle fiber development and meat quality, with no consequences on growth performance. J. Anim. Sci. 2009, 87, 729–739. [Google Scholar] [CrossRef]
- Wang, L.; Zuo, X.; Zhao, W.; Zhou, G.; Luo, L.; Yang, K.; Shu, G.; Wang, S.; Gao, P.; Zhu, X.; et al. Effect of maternal dietary supplementation with phytosterol esters on muscle development of broiler offspring. Acta Biochim. Pol. 2020, 67, 135–141. [Google Scholar] [CrossRef]
- Huang, Z.; Dai, H.; Li, S.; Wang, Z.; Wei, Q.; Ning, Z.; Guo, Y.; Shi, F.; Lv, Z. Maternal supplementation with mulberry-leaf flavonoids improves the development of skeletal muscle in the offspring of chickens. Anim. Nutr. 2024, 18, 72–83. [Google Scholar] [CrossRef]
- Gao, J.; Lv, Z.; Li, C.; Yue, Y.; Zhao, X.; Wang, F.; Guo, Y. Maternal zinc supplementation enhanced skeletal muscle development through increasing protein synthesis and inhibiting protein degradation of their offspring. Biol. Trace Element. Res. 2014, 162, 309–316. [Google Scholar] [CrossRef]
- Maresca, S.; Valiente, S.L.; Rodriguez, A.M.; Testa, L.M.; Long, N.M.; Quintans, G.I.; Pavan, E. The influence of protein restriction during mid- to late gestation on beef offspring growth, carcass characteristic and meat quality. Meat Sci. 2019, 153, 103–108. [Google Scholar] [CrossRef]
- Nascimento, K.B.; Galvao, M.C.; Meneses, J.A.M.; Ramirez-Zamudio, G.D.; Pereira, D.G.; Paulino, P.V.R.; Casagrande, D.R.; Gionbelli, T.R.S.; Ladeira, M.M.; Duarte, M.S.; et al. Maternal protein supplementation during mid-gestation improves offspring performance and metabolism in beef cows. J. Anim. Sci. 2024, 102, skae058. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Camacho, L.E.; Ebarb, S.M.; Swanson, K.C.; Vonnahme, K.A.; Stelzleni, A.M.; Johnson, S.E. Realimentation of nutrient restricted pregnant beef cows supports compensatory fetal muscle growth. J. Anim. Sci. 2013, 91, 4797–4806. [Google Scholar] [CrossRef]
- Fahey, A.J.; Brameld, J.M.; Parr, T.; Buttery, P.J. The effect of maternal undernutrition before muscle differentiation on the muscle fiber development of the newborn lamb. J. Anim. Sci. 2005, 83, 2564–2571. [Google Scholar] [CrossRef]
- Nordby, D.J.; Field, R.A.; Riley, M.L.; Kercher, C.J. Effects of maternal undernutrition during early pregnancy on growth, muscle cellularity, fiber type and carcass composition in lambs. J. Anim. Sci. 1987, 64, 1419–1427. [Google Scholar] [CrossRef]
- Lefaucheur, L.; Le Dividich, J.; Mourot, J.; Monin, G.; Ecolan, P.; Krauss, D. Influence of environmental temperature on growth, muscle and adipose tissue metabolism, and meat quality in swine. J. Anim. Sci. 1991, 69, 2844–2854. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, S.; Zeng, Z.; Xing, S.; Chen, D.; Yu, B.; He, J.; Huang, Z.; Luo, Y.; Zheng, P.; et al. Effects of Cold Exposure on Performance and Skeletal Muscle Fiber in Weaned Piglets. Animals 2021, 11, 2148. [Google Scholar] [CrossRef]
- Kim, S.; Nakayama, C.; Kondoh, D.; Okazaki, T.; Yoneda, E.; Tomita, K.; Sasaki, M.; Muranishi, Y. Seasonal adaptation of Mangalica pigs in terms of muscle morphology and metabolism. Anat. Histol. Embryol. 2024, 53, e12982. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, L.; Ecolan, P.; Lossec, G.; Gabillard, J.C.; Butler-Browne, G.S.; Herpin, P. Influence of early postnatal cold exposure on myofiber maturation in pig skeletal muscle. J. Muscle Res. Cell Motil. 2001, 22, 439–452. [Google Scholar] [CrossRef]
- Hirabayashi, M.; Ijiri, D.; Kamei, Y.; Tajima, A.; Kanai, Y. Transformation of Skeletal Muscle from Fast- to Slow-Twitch during Acquisition of Cold Tolerance in the Chick. Endocrinology 2005, 146, 399–405. [Google Scholar] [CrossRef]
- Ijiri, D.; Miura, M.; Kanai, Y.; Hirabayashi, M. Increased mass of slow-type skeletal muscles and depressed myostatin gene expression in cold-tolerant chicks. Zool. Sci. 2009, 26, 277–283. [Google Scholar] [CrossRef]
- Ferreira, I.B.; Matos Junior, J.B.; Sgavioli, S.; Vicentini, T.I.; Morita, V.S.; Boleli, I.C. Vitamin C prevents the effects of high rearing temperatures on the quality of broiler thigh meat1. Poult. Sci. 2015, 94, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Duchamp, C.; Cohen-Adad, F.; Rouanet, J.L.; Barre, H. Histochemical arguments for muscular non-shivering thermogenesis in muscovy ducklings. J. Physiol. 1992, 457, 27–45. [Google Scholar] [CrossRef]
- Chikani, V.; Ho, K.K. Action of GH on skeletal muscle function: Molecular and metabolic mechanisms. J. Mol. Endocrinol. 2014, 52, R107–R123. [Google Scholar] [CrossRef]
- Young, J.A.; Zhu, S.; List, E.O.; Duran-Ortiz, S.; Slama, Y.; Berryman, D.E. Musculoskeletal Effects of Altered GH Action. Front. Physiol. 2022, 13, 867921. [Google Scholar] [CrossRef]
- Mjaaland, M.; Unneberg, K.; Jenssen, T.G.; Bjoro, T.; Lindal, S.; Revhaug, A. Metabolic effects of two regimens of growth hormone given before operation in piglets. Eur. J. Surg. 1995, 161, 639–646. [Google Scholar] [PubMed]
- Ge, X.; Yu, J.; Jiang, H. Growth hormone stimulates protein synthesis in bovine skeletal muscle cells without altering insulin-like growth factor-I mRNA expression. J. Anim. Sci. 2012, 90, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.L.; Tornell, J.; Schulze, W.; Hoebeke, J.; Isaksson, O.G.; Sandstedt, J.; Hjalmarson, A. Myocardial hypertrophy in transgenic mice overexpressing the bovine growth hormone (bGH) gene. J. Intern. Med. 2000, 247, 546–552. [Google Scholar] [CrossRef]
- Hemmings, K.M.; Daniel, Z.C.; Buttery, P.J.; Parr, T.; Brameld, J.M. Differential effects of short-term beta agonist and growth hormone treatments on expression of myosin heavy chain IIB and associated metabolic genes in sheep muscle. Animal 2015, 9, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Milioto, C.; Malena, A.; Maino, E.; Polanco, M.J.; Marchioretti, C.; Borgia, D.; Pereira, M.G.; Blaauw, B.; Lieberman, A.P.; Venturini, R.; et al. Beta-agonist stimulation ameliorates the phenotype of spinal and bulbar muscular atrophy mice and patient-derived myotubes. Sci. Rep. 2017, 7, 41046. [Google Scholar] [CrossRef]
- Shackelford, S.D.; Wheeler, T.L.; Koohmaraie, M. The effects of in utero exposure of lambs to a beta-adrenergic agonist on prenatal and postnatal muscle growth, carcass cutability, and meat tenderness. J. Anim. Sci. 1995, 73, 2986–2993. [Google Scholar] [CrossRef]
- Barnes, T.L.; Cadaret, C.N.; Beede, K.A.; Schmidt, T.B.; Petersen, J.L.; Yates, D.T. Hypertrophic muscle growth and metabolic efficiency were impaired by chronic heat stress, improved by zilpaterol supplementation, and not affected by ractopamine supplementation in feedlot lambs1. J. Anim. Sci. 2019, 97, 4101–4113. [Google Scholar] [CrossRef]
- Gunawan, A.M.; Yen, C.N.; Richert, B.T.; Schinckel, A.P.; Grant, A.L.; Gerrard, D.E. Ractopamine-induced fiber type-specific gene expression in porcine skeletal muscles is independent of growth. J. Anim. Sci. 2020, 98, skaa341. [Google Scholar] [CrossRef]
- Li, H.; Gariepy, C.; Jin, Y.; Font, I.F.M.; Fortin, J.; Rocha, L.M.; Faucitano, L. Effects of ractopamine administration and castration method on muscle fiber characteristics and sensory quality of the longissimus muscle in two Pietrain pig genotypes. Meat Sci. 2015, 102, 27–34. [Google Scholar] [CrossRef]
- Paulk, C.B.; Tokach, M.D.; Nelssen, J.L.; Burnett, D.D.; Vaughn, M.A.; Phelps, K.J.; Dritz, S.S.; Derouchey, J.M.; Goodband, R.D.; Woodworth, J.C.; et al. Effect of dietary zinc and ractopamine hydrochloride on pork chop muscle fiber type distribution, tenderness, and color characteristics. J. Anim. Sci. 2014, 92, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Depreux, F.F.; Grant, A.L.; Anderson, D.B.; Gerrard, D.E. Paylean alters myosin heavy chain isoform content in pig muscle. J. Anim. Sci. 2002, 80, 1888–1894. [Google Scholar] [CrossRef] [PubMed]
- Rehfeldt, C.; Schadereit, R.; Weikard, R.; Reichel, K. Effect of clenbuterol on growth, carcase and skeletal muscle characteristics in broiler chickens. Br. Poult. Sci. 1997, 38, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Ijiri, D.; Ishitani, K.; Shimamoto, S.; Ishimaru, Y.; Ohtsuka, A. The effects of intraperitoneal clenbuterol injection on protein degradation and myostatin expression differ between the sartorius and pectoral muscles of neonatal chicks. Gen. Comp. Endocrinol. 2014, 206, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Davis, S.K.; Wilson, J.J.; Stone, R.T.; Wu, F.Y.; Garcia, D.K.; Lunt, D.K.; Schiavetta, A.M. Bovine fast-twitch myosin light chain 1: Cloning and mRNA amount in muscle of cattle treated with clenbuterol. Am. J. Physiol. 1995, 268, E858–E865. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Henckel, P.; Oksbjerg, N.; Sejrsen, K. The effect of cimaterol on muscle fiber characteristics, capillary supply, and metabolic potentials of longissimus and semitendinosus muscles from young Friesian bulls. J. Anim. Sci. 1994, 72, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Ren, H.; Gao, S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: Roles in skeletal muscle growth and differentiation. Gen. Comp. Endocrinol. 2010, 167, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Oksbjerg, N.; Gondret, F.; Vestergaard, M. Basic principles of muscle development and growth in meat-producing mammals as affected by the insulin-like growth factor (IGF) system. Domest. Anim. Endocrinol. 2004, 27, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Gerrard, D.E.; Okamura, C.S.; Ranalletta, M.A.; Grant, A.L. Developmental expression and location of IGF-I and IGF-II mRNA and protein in skeletal muscle. J. Anim. Sci. 1998, 76, 1004–1011. [Google Scholar] [CrossRef]
- Biagetti, B.; Simo, R. GH/IGF-1 Abnormalities and Muscle Impairment: From Basic Research to Clinical Practice. Int. J. Mol. Sci. 2021, 22, 415. [Google Scholar] [CrossRef]
- Nagasao, J.; Fukasawa, H.; Yoshioka, K.; Fujimura, N.; Kobayashi, M.; Tsunemi, Y.; Nomoto, A.; Mitsui, S.; Murata, H.; Yokoyama, I.; et al. Research Note: Expression of IGF-1 and IGF-1 receptor proteins in skeletal muscle fiber types in chickens with hepatic fibrosis. Poult. Sci. 2022, 101, 102045. [Google Scholar] [CrossRef]
- Liu, H.H.; Wang, J.W.; Zhang, R.P.; Chen, X.; Yu, H.Y.; Jin, H.B.; Li, L.; Han, C.C.; Xu, F.; Kang, B.; et al. In ovo feeding of IGF-1 to ducks influences neonatal skeletal muscle hypertrophy and muscle mass growth upon satellite cell activation. J. Cell Physiol. 2012, 227, 1465–1475. [Google Scholar] [CrossRef]
- Yue, M.; Tian, Y.G.; Wang, Y.J.; Gu, Y.; Bayaer, N.; Hu, Q.; Gu, W.W. Associated analysis of single nucleotide polymorphisms found on exon 3 of the IGF-1 gene with Tibetan miniature pig growth traits. Genet. Mol. Res. 2014, 13, 1263–1269. [Google Scholar] [CrossRef]
- Wei, H.K.; Zhou, Y.; Jiang, S.; Tao, Y.X.; Sun, H.; Peng, J.; Jiang, S. Feeding a DHA-enriched diet increases skeletal muscle protein synthesis in growing pigs: Association with increased skeletal muscle insulin action and local mRNA expression of insulin-like growth factor 1. Br. J. Nutr. 2013, 110, 671–680. [Google Scholar] [CrossRef]
- Shaikh, S.; Bloomfield, F.H.; Bauer, M.K.; Phua, H.H.; Gilmour, R.S.; Harding, J.E. Amniotic IGF-I supplementation of growth-restricted fetal sheep alters IGF-I and IGF receptor type 1 mRNA and protein levels in placental and fetal tissues. J. Endocrinol. 2005, 186, 145–155. [Google Scholar] [CrossRef]
- Bloomfield, F.H.; van Zijl, P.L.; Bauer, M.K.; Phua, H.H.; Harding, J.E. Effect of pulsatile growth hormone administration to the growth-restricted fetal sheep on somatotrophic axis gene expression in fetal and placental tissues. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E333–E339. [Google Scholar] [CrossRef]
- Quinn, L.S.; Haugk, K.L. Overexpression of the type-1 insulin-like growth factor receptor increases ligand-dependent proliferation and differentiation in bovine skeletal myogenic cultures. J. Cell Physiol. 1996, 168, 34–41. [Google Scholar] [CrossRef]
- Zeigerer, A.; Sekar, R.; Kleinert, M.; Nason, S.; Habegger, K.M.; Muller, T.D. Glucagon’s Metabolic Action in Health and Disease. Compr. Physiol. 2021, 11, 1759–1783. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Fernandez-Fernandez, C.; Lopez-Pereiro, Y.; Castro-Calvo, I.; Carneiro-Freire, N. The effects of glucagon and the target of rapamycin (TOR) on skeletal muscle protein synthesis and age-dependent sarcopenia in humans. Clin. Nutr. ESPEN 2021, 44, 15–25. [Google Scholar] [CrossRef]
- Luo, J.; Shen, Y.L.; Lei, G.H.; Zhu, P.K.; Jiang, Z.Y.; Bai, L.; Li, Z.M.; Tang, Q.G.; Li, W.X.; Zhang, H.S.; et al. Correlation between three glycometabolic-related hormones and muscle glycolysis, as well as meat quality, in three pig breeds. J. Sci. Food Agric. 2017, 97, 2706–2713. [Google Scholar] [CrossRef]
- Shen, L.; Lei, H.; Zhang, S.; Li, X.; Li, M.; Jiang, X.; Zhu, K.; Zhu, L. Comparison of energy metabolism and meat quality among three pig breeds. Anim. Sci. J. 2014, 85, 770–779. [Google Scholar] [CrossRef]
- Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-specific microRNAs in skeletal muscle development. Dev. Biol. 2016, 410, 1–13. [Google Scholar] [CrossRef]
- Cannataro, R.; Carbone, L.; Petro, J.L.; Cione, E.; Vargas, S.; Angulo, H.; Forero, D.A.; Odriozola-Martinez, A.; Kreider, R.B.; Bonilla, D.A. Sarcopenia: Etiology, Nutritional Approaches, and miRNAs. Int. J. Mol. Sci. 2021, 22, 9724. [Google Scholar] [CrossRef]
- McCarthy, J.J. The MyomiR network in skeletal muscle plasticity. Exerc. Sport. Sci. Rev. 2011, 39, 150–154. [Google Scholar] [CrossRef]
- Hong, J.S.; Noh, S.H.; Lee, J.S.; Kim, J.M.; Hong, K.C.; Lee, Y.S. Effects of polymorphisms in the porcine microRNA miR-1 locus on muscle fiber type composition and miR-1 expression. Gene 2012, 506, 211–216. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, H.; Zhou, P.; Zhang, Z.; Liu, J.; Zhang, H. MicroRNA-152 Promotes Slow-Twitch Myofiber Formation via Targeting Uncoupling Protein-3 Gene. Animals 2019, 9, 669. [Google Scholar] [CrossRef]
- Wang, X.Y.; Chen, X.L.; Huang, Z.Q.; Chen, D.W.; Yu, B.; He, J.; Luo, J.Q.; Luo, Y.H.; Chen, H.; Zheng, P.; et al. MicroRNA-499-5p regulates porcine myofiber specification by controlling Sox6 expression. Animal 2017, 11, 2268–2274. [Google Scholar] [CrossRef]
- Li, B.; Yin, D.; Li, P.; Zhang, Z.; Zhang, X.; Li, H.; Li, R.; Hou, L.; Liu, H.; Wu, W. Profiling and Functional Analysis of Circular RNAs in Porcine Fast and Slow Muscles. Front. Cell Dev. Biol. 2020, 8, 322. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; Yu, J.; Zheng, P.; Huang, Z.; Luo, Y.; Luo, J.; Mao, X.; Yan, H.; He, J.; et al. Butyrate promotes slow-twitch myofiber formation and mitochondrial biogenesis in finishing pigs via inducing specific microRNAs and PGC-1alpha expression1. J. Anim. Sci. 2019, 97, 3180–3192. [Google Scholar] [CrossRef]
- Liu, X.; Trakooljul, N.; Hadlich, F.; Murani, E.; Wimmers, K.; Ponsuksili, S. MicroRNA-mRNA regulatory networking fine-tunes the porcine muscle fiber type, muscular mitochondrial respiratory and metabolic enzyme activities. BMC Genom. 2016, 17, 531. [Google Scholar] [CrossRef]
- Fan, D.; Yao, Y.; Liu, Y.; Yan, C.; Li, F.; Wang, S.; Yu, M.; Xie, B.; Tang, Z. Regulation of myo-miR-24-3p on the Myogenesis and Fiber Type Transformation of Skeletal Muscle. Genes 2024, 15, 269. [Google Scholar] [CrossRef]
- Wang, W.; Yu, Q.; Cao, Q.; Ye, H.; Zhang, C.; Dong, Z.; Feng, D.; Zuo, J. MicroRNA-27a inhibits porcine type I muscle fibre gene expression by directly targeting peroxisome proliferator-activated receptor-gamma coactivator-1alpha. J. Anim. Physiol. Anim. Nutr. 2023, 107, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Chen, H.; He, J.; Luo, J.; Zheng, P.; Yu, J.; et al. Leucine promotes porcine myofibre type transformation from fast-twitch to slow-twitch through the protein kinase B (Akt)/forkhead box 1 signalling pathway and microRNA-27a. Br. J. Nutr. 2019, 121, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Wu, F.; Liu, Y.; Xiao, J.; Xu, M.; Yu, Q.; Xia, M.; He, X.; Zou, S.; Tan, H.; et al. MicroRNA Transcriptome Profile Analysis in Porcine Muscle and the Effect of miR-143 on the MYH7 Gene and Protein. PLoS ONE 2015, 10, e0124873. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, H.; Liu, R.; Jin, L.; Tang, Q.; Wang, X.; Jiang, A.; Hu, Y.; Li, Z.; Zhu, L.; et al. The miRNA Transcriptome Directly Reflects the Physiological and Biochemical Differences between Red, White, and Intermediate Muscle Fiber Types. Int. J. Mol. Sci. 2015, 16, 9635–9653. [Google Scholar] [CrossRef]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; He, J.; Yan, H.; Luo, Y.; Chen, H.; Zheng, P.; et al. Resveratrol regulates muscle fiber type gene expression through AMPK signaling pathway and miR-22-3p in porcine myotubes. Anim. Biotechnol. 2022, 33, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, M.; Shan, Y.; Ji, G.; Ju, X.; Tu, Y.; Sheng, Z.; Xie, J.; Zou, J.; Shu, J. miRNA-mRNA network regulation in the skeletal muscle fiber phenotype of chickens revealed by integrated analysis of miRNAome and transcriptome. Sci. Rep. 2020, 10, 10619. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zhang, M.; Shan, Y.J.; Pang, L.C.; Ji, G.G.; Ju, X.J.; Tu, Y.J.; Shi, S.Y.; Bai, H.; Zou, J.M.; et al. Transcriptome sequencing analysis of the role of miR-499-5p and SOX6 in chicken skeletal myofiber specification. Front. Genet. 2022, 13, 1008649. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Cai, B.; Jiang, L.; Abdalla, B.A.; Li, Z.; Nie, Q.; Zhang, X. lncRNA-Six1 Is a Target of miR-1611 that Functions as a ceRNA to Regulate Six1 Protein Expression and Fiber Type Switching in Chicken Myogenesis. Cells 2018, 7, 243. [Google Scholar] [CrossRef]
- Horikawa, A.; Ogasawara, H.; Okada, K.; Kobayashi, M.; Muroya, S.; Hojito, M. Grazing-induced changes in muscle microRNA-206 and -208b expression in association with myogenic gene expression in cattle. Anim. Sci. J. 2015, 86, 952–960. [Google Scholar] [CrossRef]
- Muroya, S.; Taniguchi, M.; Shibata, M.; Oe, M.; Ojima, K.; Nakajima, I.; Chikuni, K. Profiling of differentially expressed microRNA and the bioinformatic target gene analyses in bovine fast- and slow-type muscles by massively parallel sequencing. J. Anim. Sci. 2013, 91, 90–103. [Google Scholar] [CrossRef]
- Greene, M.A.; Powell, R.; Bruce, T.; Bridges, W.C.; Duckett, S.K. miRNA transcriptome and myofiber characteristics of lamb skeletal muscle during hypertrophic growth(1). Front. Genet. 2022, 13, 988756. [Google Scholar] [CrossRef] [PubMed]
- Martone, J.; Mariani, D.; Desideri, F.; Ballarino, M. Non-coding RNAs Shaping Muscle. Front. Cell Dev. Biol. 2019, 7, 394. [Google Scholar] [CrossRef]
- Wang, S.; Tan, B.; Xiao, L.; Zhao, X.; Zeng, J.; Hong, L.; Yang, J.; Cai, G.; Zheng, E.; Wu, Z.; et al. Comprehensive Analysis of Long Noncoding RNA Modified by m(6)A Methylation in Oxidative and Glycolytic Skeletal Muscles. Int. J. Mol. Sci. 2022, 23, 4600. [Google Scholar] [CrossRef]
- Dou, M.; Yao, Y.; Ma, L.; Wang, X.; Shi, X.; Yang, G.; Li, X. The long noncoding RNA MyHC IIA/X-AS contributes to skeletal muscle myogenesis and maintains the fast fiber phenotype. J. Biol. Chem. 2020, 295, 4937–4949. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Gan, M.; Tang, Q.; Tang, G.; Jiang, Y.; Li, M.; Chen, L.; Bai, L.; Shuai, S.; Wang, J.; et al. Comprehensive Analysis of lncRNAs and circRNAs Reveals the Metabolic Specialization in Oxidative and Glycolytic Skeletal Muscles. Int. J. Mol. Sci. 2019, 20, 2855. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Q.; Hua, L.; Chen, J.; Zhang, J.; Bai, H.; Li, H.; Xu, B.; Shi, Z.; Cao, H.; et al. Comprehensive Analysis of Differentially Expressed mRNA, lncRNA and circRNA and Their ceRNA Networks in the Longissimus Dorsi Muscle of Two Different Pig Breeds. Int. J. Mol. Sci. 2019, 20, 1107. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Li, X.; Du, Y.; Zhao, J.; Ge, C. Regulation of Non-Coding RNA in the Growth and Development of Skeletal Muscle in Domestic Chickens. Genes 2022, 13, 1033. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.A.; Wang, Z.; Yang, X.; Ma, M.; Li, Z.; Nie, Q. LncRNA-FKBP1C regulates muscle fiber type switching by affecting the stability of MYH1B. Cell Death Discov. 2021, 7, 73. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, X.; Odame, E.; Xu, X.; Chen, Y.; Ye, J.; Zhou, H.; Dai, D.; Kyei, B.; Zhan, S.; et al. CircRNA-Protein Interactions in Muscle Development and Diseases. Int. J. Mol. Sci. 2021, 22, 3262. [Google Scholar] [CrossRef]
- Cao, H.; Liu, J.; Du, T.; Liu, Y.; Zhang, X.; Guo, Y.; Wang, J.; Zhou, X.; Li, X.; Yang, G.; et al. Circular RNA screening identifies circMYLK4 as a regulator of fast/slow myofibers in porcine skeletal muscles. Mol. Genet. Genom. 2022, 297, 87–99. [Google Scholar] [CrossRef]
- Yang, C.; Wu, L.; Guo, Y.; Li, Y.; Deng, M.; Liu, D.; Liu, G.; Sun, B. Expression profile and bioinformatics analysis of circRNA and its associated ceRNA networks in longissimus dorsi from Lufeng cattle and Leiqiong cattle. BMC Genom. 2023, 24, 499. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, Z.; Deng, K.; Li, J.; Liu, Z.; Huang, X.; Wang, F.; Fan, Y. circUSP13 facilitates the fast-to-slow myofiber shift via the MAPK/ERK signaling pathway in goat skeletal muscles. J. Cell Physiol. 2024, 239, e31226. [Google Scholar] [CrossRef]
- Weiss, A.; Leinwand, L.A. The mammalian myosin heavy chain gene family. Annu. Rev. Cell Dev. Biol. 1996, 12, 417–439. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, M.; Akolkar, D.B.; Kinoshita, S.; Watabe, S. The expression of multiple myosin heavy chain genes during skeletal muscle development of torafugu Takifugu rubripes embryos and larvae. Gene 2013, 515, 144–154. [Google Scholar] [CrossRef]
- He, Y.M.; Gu, M.M. Research progress of myosin heavy chain genes in human genetic diseases. Yi Chuan 2017, 39, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.C.; Park, H.B.; Ahn, J.S.; Han, S.H.; Lee, J.B.; Lim, H.T.; Yoo, C.K.; Jung, E.J.; Kim, D.H.; Sun, W.S.; et al. A functional regulatory variant of MYH3 influences muscle fiber-type composition and intramuscular fat content in pigs. PLoS Genet. 2019, 15, e1008279. [Google Scholar] [CrossRef]
- Brown, D.M.; Brameld, J.M.; Parr, T. Expression of the myosin heavy chain IIB gene in porcine skeletal muscle: The role of the CArG-Box promoter response element. PLoS ONE 2014, 9, e114365. [Google Scholar] [CrossRef]
- Cho, E.S.; Lee, K.T.; Kim, J.M.; Lee, S.W.; Jeon, H.J.; Lee, S.H.; Hong, K.C.; Kim, T.H. Association of a single nucleotide polymorphism in the 5′ upstream region of the porcine myosin heavy chain 4 gene with meat quality traits in pigs. Anim. Sci. J. 2016, 87, 330–335. [Google Scholar] [CrossRef]
- Rossi, A.C.; Mammucari, C.; Argentini, C.; Reggiani, C.; Schiaffino, S. Two novel/ancient myosins in mammalian skeletal muscles: MYH14/7b and MYH15 are expressed in extraocular muscles and muscle spindles. J. Physiol. 2010, 588, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Diao, Z.; Shimokawa, F.; Yoshioka, H.; Itoyama, E.; Matsumura, M.; Murakami, M.; Kitamura, S.; Nagase, H.; Matsui, T.; Funaba, M. Possibility of uncoupling protein 1 expression in bovine fast-twitch muscle fibers. J. Vet. Med. Sci. 2023, 85, 587–591. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Kinoshita, S.; Bhuiyan, S.S.; Asakawa, S.; Watabe, S. Stimulatory and inhibitory mechanisms of slow muscle-specific myosin heavy chain gene expression in fish: Transient and transgenic analysis of torafugu MYH(M86-2) promoter in zebrafish embryos. Exp. Cell Res. 2013, 319, 820–837. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pan, Y.; Cheng, J.; Zhu, X.; Chu, W.; Meng, Y.Y.; Bin, S.; Zhang, J. Characterization of myosin heavy chain (MYH) genes and their differential expression in white and red muscles of Chinese perch, Siniperca chuatsi. Int. J. Biol. Macromol. 2023, 250, 125907. [Google Scholar] [CrossRef] [PubMed]
- Zechner, C.; Lai, L.; Zechner, J.F.; Geng, T.; Yan, Z.; Rumsey, J.W.; Collia, D.; Chen, Z.; Wozniak, D.F.; Leone, T.C.; et al. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab. 2010, 12, 633–642. [Google Scholar] [CrossRef]
- Liang, H.; Ward, W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Li, J.; Ying, F.; Zuo, B.; Xu, Z. Analysis of differential gene expression of the transgenic pig with overexpression of PGC1alpha in muscle. Mol. Biol. Rep. 2019, 46, 3427–3435. [Google Scholar] [CrossRef]
- Yang, K.; Wang, L.; Zhou, G.; Lin, X.; Peng, J.; Wang, L.; Luo, L.; Wang, J.; Shu, G.; Wang, S.; et al. Phytol Promotes the Formation of Slow-Twitch Muscle Fibers through PGC-1alpha/miRNA but Not Mitochondria Oxidation. J. Agric. Food Chem. 2017, 65, 5916–5925. [Google Scholar] [CrossRef]
- Kong, S.; Cai, B.; Nie, Q. PGC-1alpha affects skeletal muscle and adipose tissue development by regulating mitochondrial biogenesis. Mol. Genet. Genom. 2022, 297, 621–633. [Google Scholar] [CrossRef]
- Ijiri, D.; Kanai, Y.; Hirabayashi, M. Possible roles of myostatin and PGC-1alpha in the increase of skeletal muscle and transformation of fiber type in cold-exposed chicks: Expression of myostatin and PGC-1alpha in chicks exposed to cold. Domest. Anim. Endocrinol. 2009, 37, 12–22. [Google Scholar] [CrossRef]
- Shu, J.T.; Xu, W.J.; Zhang, M.; Song, W.T.; Shan, Y.J.; Song, C.; Zhu, W.Q.; Zhang, X.Y.; Li, H.F. Transcriptional co-activator PGC-1alpha gene is associated with chicken skeletal muscle fiber types. Genet. Mol. Res. 2014, 13, 895–905. [Google Scholar] [CrossRef]
- Khan, M.; Couturier, A.; Kubens, J.F.; Most, E.; Mooren, F.C.; Kruger, K.; Ringseis, R.; Eder, K. Niacin supplementation induces type II to type I muscle fiber transition in skeletal muscle of sheep. Acta Vet. Scand. 2013, 55, 85. [Google Scholar] [CrossRef]
- Valle, I.; Alvarez-Barrientos, A.; Arza, E.; Lamas, S.; Monsalve, M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 2005, 66, 562–573. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Liu, Y.; Liang, R.; Mao, Y.; Yang, X.; Zhang, Y.; Zhu, L. Effect of resveratrol on skeletal slow-twitch muscle fiber expression via AMPK/PGC-1alpha signaling pathway in bovine myotubes. Meat Sci. 2023, 204, 109287. [Google Scholar] [CrossRef]
- Zhang, Y.; Otomaru, K.; Oshima, K.; Goto, Y.; Oshima, I.; Muroya, S.; Sano, M.; Roh, S.; Gotoh, T. Maternal Nutrition During Gestation Alters Histochemical Properties, and mRNA and microRNA Expression in Adipose Tissue of Wagyu Fetuses. Front. Endocrinol. 2021, 12, 797680. [Google Scholar] [CrossRef]
- Jin, Z.; Choe, H.M.; Lv, S.; Chang, S.; Yin, X. Esophageal striated muscle hypertrophy and muscle fiber type transformation in MSTN knockout pigs. Transgenic Res. 2022, 31, 341–349. [Google Scholar] [CrossRef]
- Xuan, M.F.; Luo, Z.B.; Wang, J.X.; Guo, Q.; Han, S.Z.; Jin, S.S.; Kang, J.D.; Yin, X.J. Shift from slow- to fast-twitch muscle fibres in skeletal muscle of newborn heterozygous and homozygous myostatin-knockout piglets. Reprod. Fertil. Dev. 2019, 31, 1628–1636. [Google Scholar] [CrossRef]
- Lv, S.T.; Gao, K.; Choe, H.M.; Jin, Z.Y.; Chang, S.Y.; Quan, B.H.; Yin, X.J. Effects of myostatin gene knockout on porcine extraocular muscles. Anim. Biotechnol. 2023, 34, 2150–2158. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zeng, W.; Ma, M.; Wei, Z.; Liu, H.; Liu, X.; Wang, M.; Shi, X.; Zeng, J.; Yang, L.; et al. Precise editing of myostatin signal peptide by CRISPR/Cas9 increases the muscle mass of Liang Guang Small Spotted pigs. Transgenic Res. 2020, 29, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Zhou, Y.; Yang, J.; Li, J.; Peng, Y.; Zhang, X.; Miao, Y.; Jiang, W.; Bu, G.; Hou, L.; et al. Targeted overexpression of PPARgamma in skeletal muscle by random insertion and CRISPR/Cas9 transgenic pig cloning enhances oxidative fiber formation and intramuscular fat deposition. FASEB J. 2021, 35, e21308. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Zhang, Y.; Ma, J.; Du, M.; Xu, H.; Wang, J.; Zhang, L.; Li, W.; Hou, Y.; Liu, X.; et al. FHL3 promotes the formation of fast glycolytic muscle fibers by interacting with YY1 and muscle glycolytic metabolism. Cell Mol. Life Sci. 2023, 80, 27. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, X.; Liu, K.; Li, B.; Chao, Z.; Jiang, A.; Li, R.; Li, P.; Liu, H.; Wu, W. Comprehensive Analysis of Porcine Prox1 Gene and Its Relationship with Meat Quality Traits. Animals 2019, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zha, C.; Jiang, A.; Chao, Z.; Hou, L.; Liu, H.; Huang, R.; Wu, W. A Combined Differential Proteome and Transcriptome Profiling of Fast- and Slow-Twitch Skeletal Muscle in Pigs. Foods 2022, 11, 2842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, S.; Luo, W.; Ren, T.; Huang, X.; Li, W.; Zhang, X. Sox6 Differentially Regulates Inherited Myogenic Abilities and Muscle Fiber Types of Satellite Cells Derived from Fast- and Slow-Type Muscles. Int. J. Mol. Sci. 2022, 23, 11327. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, N.; Jasnic, J.; Novkovic, M.; Milosevic, E.; Boskovic, S.; Kojic, A.; Popic, K.; Stankovic, M.; Wang, Y.; Milenkovic, S.; et al. Cloning and expression profiling of muscle regulator ANKRD2 in domestic chicken Gallus gallus. Histochem. Cell Biol. 2020, 154, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shen, Z.; Zhou, X.; Yang, S.; Yan, F.; He, K.; Zhao, A. Identification of Differentially Expressed Genes in Different Types of Broiler Skeletal Muscle Fibers Using the RNA-seq Technique. Biomed. Res. Int. 2020, 2020, 9478949. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Ye, F.; He, L.; Liu, Y.; Zhao, X.; Yin, H.; Li, D.; Xu, H.; Zhu, Q.; Wang, Y. Molecular Cloning, Expression Profiling, and Marker Validation of the Chicken Myoz3 Gene. Biomed. Res. Int. 2017, 2017, 5930918. [Google Scholar] [CrossRef]
- Ye, M.; Ye, F.; He, L.; Luo, B.; Yang, F.; Cui, C.; Zhao, X.; Yin, H.; Li, D.; Xu, H.; et al. Transcriptomic analysis of chicken Myozenin 3 regulation reveals its potential role in cell proliferation. PLoS ONE 2017, 12, e0189476. [Google Scholar] [CrossRef] [PubMed]
- Weimer, K.; DiMario, J.X. Muscle fiber type specific activation of the slow myosin heavy chain 2 promoter by a non-canonical E-box. Biochem. Biophys. Res. Commun. 2016, 469, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.R.; Jiang, X.S.; Du, H.R.; Zhu, Q.; Li, X.C.; Yang, C.W.; Liu, Y.P. Characterization of the expression profiles of calpastatin (CAST) gene in chicken. Mol. Biol. Rep. 2012, 39, 1839–1843. [Google Scholar] [CrossRef]
- Mitchell, D.L.; DiMario, J.X. Bimodal, reciprocal regulation of fibroblast growth factor receptor 1 promoter activity by BTEB1/KLF9 during myogenesis. Mol. Biol. Cell 2010, 21, 2780–2787. [Google Scholar] [CrossRef]
- Hennebry, A.; Berry, C.; Siriett, V.; O’Callaghan, P.; Chau, L.; Watson, T.; Sharma, M.; Kambadur, R. Myostatin regulates fiber-type composition of skeletal muscle by regulating MEF2 and MyoD gene expression. Am. J. Physiol. Cell Physiol. 2009, 296, C525–C534. [Google Scholar] [CrossRef] [PubMed]
- Wellmann, K.B.; Kim, J.; Urso, P.M.; Smith, Z.K.; Johnson, B.J. Evaluation of vitamin A status on myogenic gene expression and muscle fiber characteristics. J. Anim. Sci. 2021, 99, skab075. [Google Scholar] [CrossRef] [PubMed]
- Coles, C.A.; Wadeson, J.; Knight, M.I.; Cafe, L.M.; Johns, W.H.; White, J.D.; Greenwood, P.L.; McDonagh, M.B. A disintegrin and metalloprotease-12 is type I myofiber specific in Bos taurus and Bos indicus cattle. J. Anim. Sci. 2014, 92, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, R.N.; Kochan, K.J.; Torres, A.K.; Du, M.; Riley, D.G.; Gill, C.A.; Herring, A.D.; Sanders, J.O.; Riggs, P.K. Skeletal Muscle Expression of Actinin-3 (ACTN3) in Relation to Feed Efficiency Phenotype of F(2) Bos indicus—Bos taurus Steers. Front. Genet. 2022, 13, 796038. [Google Scholar] [CrossRef] [PubMed]
- Soria, L.A.; Corva, P.M.; Branda Sica, A.; Villarreal, E.L.; Melucci, L.M.; Mezzadra, C.A.; Papaleo Mazzucco, J.; Fernandez Macedo, G.; Silvestro, C.; Schor, A.; et al. Association of a novel polymorphism in the bovine PPARGC1A gene with growth, slaughter and meat quality traits in Brangus steers. Mol. Cell Probes 2009, 23, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Waddell, J.N.; Kuang, S.; Tellam, R.L.; Cockett, N.E.; Bidwell, C.A. Identification of genes directly responding to DLK1 signaling in Callipyge sheep. BMC Genom. 2018, 19, 283. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, Y.; Jia, G.; Zhao, H.; Liu, G.; Huang, Z. Effects of Active Immunization Against Akirin2 on Muscle Fiber-type Composition in Pigs. Anim. Biotechnol. 2019, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Chen, H.; Yu, J.; Luo, Y.; Zheng, P.; He, J. Effects of dietary ferulic acid supplementation on growth performance and skeletal muscle fiber type conversion in weaned piglets. J. Sci. Food Agric. 2021, 101, 5116–5123. [Google Scholar] [CrossRef] [PubMed]
- Men, X.M.; Xu, Z.W.; Tao, X.; Deng, B.; Qi, K.K. FNDC5 expression closely correlates with muscle fiber types in porcine longissimus dorsi muscle and regulates myosin heavy chains (MyHCs) mRNA expression in C2C12 cells. PeerJ 2021, 9, e11065. [Google Scholar] [CrossRef]
- Shi, X.E.; Song, Z.Y.; Yang, Q.M.; Liu, Y.G.; Yang, G.S. Correlation of forkhead box transcription factor O1 and myosin heavy chain isoforms in porcine skeletal muscle. Genet. Mol. Res. 2014, 13, 10231–10240. [Google Scholar] [CrossRef]
- Saneyasu, T.; Tsuchihashi, T.; Kitashiro, A.; Tsuchii, N.; Kimura, S.; Honda, K.; Kamisoyama, H. The IGF-1/Akt/S6 pathway and expressions of glycolytic myosin heavy chain isoforms are upregulated in chicken skeletal muscle during the first week after hatching. Anim. Sci. J. 2017, 88, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Saneyasu, T.; Nakano, Y.; Tsuchii, N.; Kitashiro, A.; Tsuchihashi, T.; Kimura, S.; Honda, K.; Kamisoyama, H. Differential regulation of protein synthesis by skeletal muscle type in chickens. Gen. Comp. Endocrinol. 2019, 284, 113246. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.F.; Ding, Q.L.; Li, Y.M.; Fang, W.R. Identification of Differentially Expressed Genes and Pathways for Myofiber Characteristics in Soleus Muscles between Chicken Breeds Differing in Meat Quality. Anim. Biotechnol. 2017, 28, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shang, H.; Zhao, X.; Qu, M.; Peng, T.; Guo, B.; Hu, Y.; Song, X. Radix Puerarin Extract (Puerarin) Could Improve Meat Quality of Heat-Stressed Beef Cattle Through Changing Muscle Antioxidant Ability and Fiber Characteristics. Front. Vet. Sci. 2020, 7, 615086. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Wellmann, K.B.; Smith, Z.K.; Johnson, B.J. All-trans retinoic acid increases the expression of oxidative myosin heavy chain through the PPARdelta pathway in bovine muscle cells derived from satellite cells. J. Anim. Sci. 2018, 96, 2763–2776. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhao, J.; Li, F.; Zhao, X.; Feng, J.; Su, Y.; Wang, B.; Zhao, J. Vitamin A regulates mitochondrial biogenesis and function through p38 MAPK-PGC-1alpha signaling pathway and alters the muscle fiber composition of sheep. J. Anim. Sci. Biotechnol. 2024, 15, 18. [Google Scholar] [CrossRef]
- Hou, Y.; Su, L.; Su, R.; Luo, Y.; Wang, B.; Yao, D.; Zhao, L.; Jin, Y. Effect of feeding regimen on meat quality, MyHC isoforms, AMPK, and PGC-1alpha genes expression in the biceps femoris muscle of Mongolia sheep. Food Sci. Nutr. 2020, 8, 2262–2270. [Google Scholar] [CrossRef]
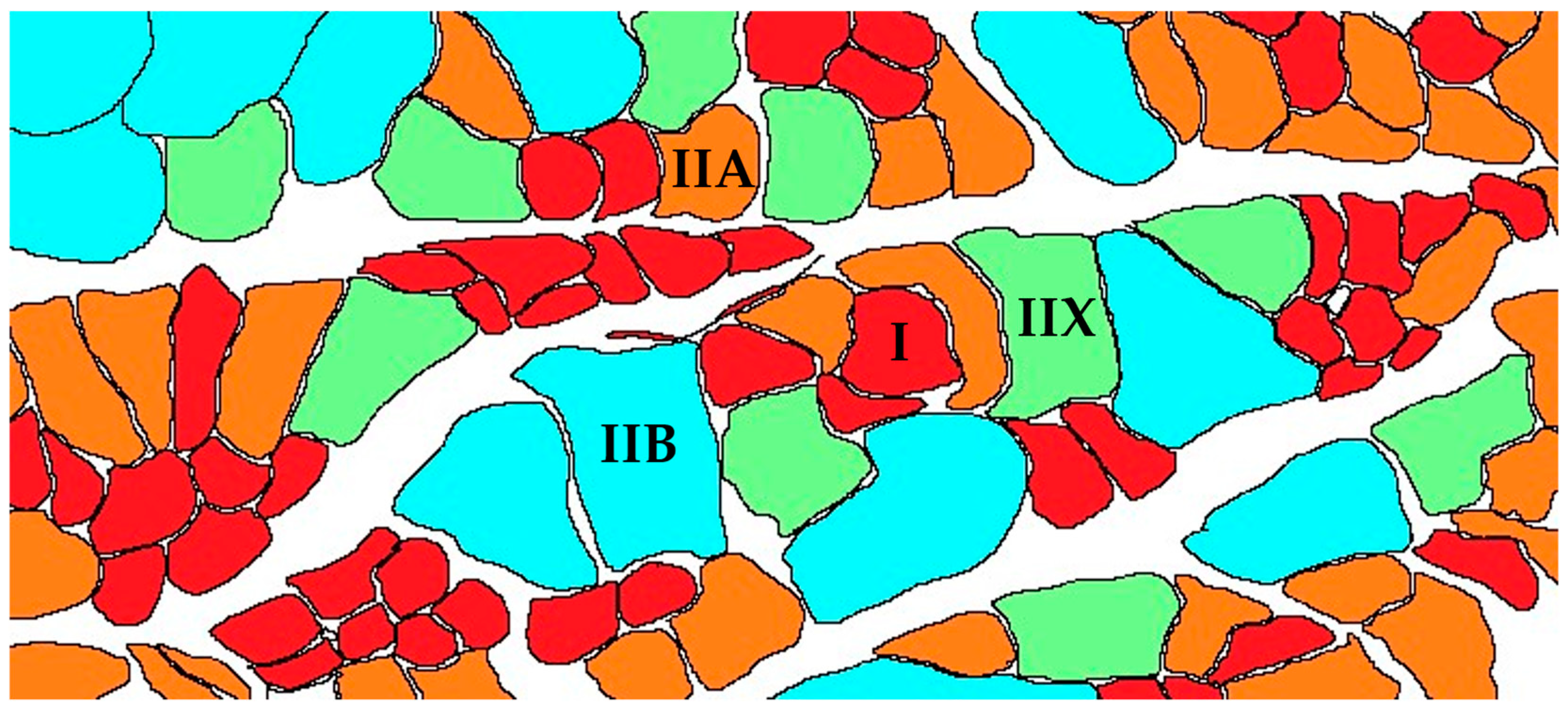

| Type of Muscle Fiber and Traits | Type I | Type IIA | Type IIX and Type IIB |
|---|---|---|---|
| Color | Bright red | Red | White |
| Twitching speed | + | ++ | +++ |
| Characteristics of energy metabolism | Oxidative metabolism | Oxidative metabolism and glycolytic metabolism | Glycolytic metabolism |
| Fatigue resistance | +++ | ++ | + |
| Cross-sectional area (CSA) | + | ++ | +++ |
| Glycogen | + | ++ | +++ |
| Mitochondrial content | +++ | ++ | + |
| ATPase activity | + | ++ | +++ |
| Exercise | Low-intensity endurance | Moderate-intensity aerobic | Short bursts of high intensity |
| Species | miRNAs | Target Genes | Regulation of the Types of Muscle Fiber | References | ||
|---|---|---|---|---|---|---|
| Slow Muscle | Fast Muscle | Transformation of Muscle Fibers | ||||
| Sus scrofa | miR-1 | / | + | / | / | [226] |
| miR-152 | / | + | / | / | [227] | |
| miR-499-5p | SOX6 | + | / | / | [228,229] | |
| miR-30 | Prdm1 | / | + | / | [231] | |
| miR-24-3p | Nek4, Pim1, Nlk Pskh1, Mapk14 | − | + | Slow muscle → fast muscle | [232] | |
| miR-27a | PGC-1α | − | + | / | [233] | |
| FOXO1 | + | − | Fast muscle → slow muscle | [234] | ||
| miR-133a-3p | TEAD1 | + | − | / | [230] | |
| miR-208b miR-499-5p | Sp3 Sox6 | + | − | / | ||
| ssc-miR-143-3p | MYH7 | + | / | / | [235] | |
| miR-125b miR-126 miR-128 miR-486 miR-99a/b | IGF-1 | + | − | / | [236] | |
| miR-22-3p | / | − | + | / | [237] | |
| Gallus gallus | gga-miR-143-5p gga-miR-499-5p gga-miR-129-3p | CaN/NFAT signalling pathway | + | / | / | [238] |
| gga-miR-196-5p | CALM1 | + | / | Fast muscle → slow muscle | ||
| miR-499-5p | SOX6 | + | − | Fast muscle → slow muscle | [239] | |
| miR-1611 | Six1 | + | / | Fast muscle → slow muscle | [240] | |
| Bos taurus | miR-208 miR-206 miR-10b | / | / | / | +* | [241] |
| miR-196a miR-885 | HMGAI, SEMA3A RICTOR, IGFI IGF2BP1, IGF2BP3 | − | + | / | [242] | |
| miR-208b | / | + | − | / | ||
| Species | lncRNAs/circRNAs | Possible Mechanism of Action | Regulation of the Types of Muscle Fibers | Reference | ||
|---|---|---|---|---|---|---|
| Slow Muscle | Fast Muscle | Transformation of Muscle Fibers | ||||
| Sus scrofa | MSTRG.14200.1 | After knockdown, the expression of MyHC-I protein is upregulated and the expression of MyHC-IIB protein is downregulated | + | − | Fast muscle → slow muscle | [213] |
| Lnc MyHC--IIA/X | Sponging miR-130b | − | + | Slow muscle → fast muscle | [246] | |
| circRNA290 | Sponging miR-27b, targeting Foxj3 | / | / | + | [247] | |
| circRNA9210 | Sponging miR-23a, targeting MEF2C | / | / | + | [247] | |
| circMYLK4 | Regulates mitochondria-related factors | + | / | / | [252] | |
| circKANSL1L | After overexpression, the expression of MyHC-I and MyHC-IIB is upregulated | + | + | +* | [75] | |
| Gallus gallus | LncRNA-Six1 LncRNA-FKBP1C Lnc-EDCH1 Lnc-IRS1 | Cis-regulator or trans-regulator Sponging miRNAs Coding short molecular micropeptides | / | / | +* (Oxidation type) | [249] |
| LncRNA-FKBP1C | Specifically interacts with MYH1B and enhances the stability of MYH1B protein | + | − | Fast muscle → slow muscle | ||
| lncXR_001466942.2 | After overexpression, PPP1R36 was downregulated and HSPA2 was upregulated | + | / | / | [121] | |
| lncXR_003077811.1 | After knockdown, TBX3 was targeted and upregulated | + | / | / | ||
| lncXR_001465741.2 | After knockdown, CALM2 was targeted and downregulated | + | / | / | ||
| lncXR_003074785.1 | Sponging miR-193-3p, targeting PPARGC1A | + | / | / | ||
| Bos taurus | ceRNA | Competitive linear splicing of MYH6 and MYH7 | − | / | / | [253] |
| Capra hircus | circUSP13 | After overexpression, mitochondrial homeostasis and autophagy are regulated through the IGF1/MAPK/ERK signaling pathway | + | − | Fast muscle → slow muscle | [222] |
| Bos mutus | Lnc-005603 | Targeting PPARGC1A | / | / | +* | [126] |
| novel_circ_0006099 | Sponging miR-129, targeting MYH7 | + | / | +* | ||
| Species | MYHs Family/PGC-1α | Possible Mechanism of Action | Regulation of the Type of Muscle Fibers | Reference | ||
|---|---|---|---|---|---|---|
| Slow Muscle | Fast Muscle | Transformation of Muscle Fibers | ||||
| Sus scrofa | MYH3 | Through the MYH3Q variant | + | / | / | [258] |
| MYH4 | A 3 bp difference in the CArG/E-box area | − | + | Slow muscle → fast muscle | [259] | |
| The SNP g.-1398G>T site regulates the number and cross-sectional area of Type IIA muscle fibers | + | / | / | [260] | ||
| PGC-1α | Negatively regulates the expression of mitochondrial genes and inhibits oxidative metabolism | − | + | Slow muscle → fast muscle | [233] | |
| Positively regulates the expression of mitochondrial genes and promotes oxidative metabolism | + | − | Fast muscle → slow muscle | [230] | ||
| Upregulates MyOG, and downregulates MSTN and FOXO1 | + | − | Fast muscle → slow muscle | [91] | ||
| Enhances the function of mitochondrial oxidative respiration and fatty acid oxidative metabolism | + | − | Fast muscle → slow muscle | [68] | ||
| Through the fat metabolic pathway | / | / | +* | [267] | ||
| Activates the PPARδ signaling pathway, and upregulates the expression levels of miR-208 and miR-499 | + | / | / | [268] | ||
| Gallus gallus | MYH14 | Codesslow tonic myosin | + | / | / | [261] |
| PGC-1α | Upregulated expression of PGC-1α | + | − | Fast muscle → slow muscle | [270] | |
| G646A gene and H1 haplotype | / | / | +* | [271] | ||
| Bos taurus | MYHs family | Interacts with UCP1 | / | + | / | [262] |
| Capra hircus | PGC-1α | After administration of niacin, levels of PPARGC1A mRNA, which encodes PGC-1α, increased | + | − | Fast muscle → slow muscle | [272] |
| Danio rerio | MYHM86-2 | Sox6 acts as a suppressor of transcription | + | − | / | [263] |
| Species | Genes | Possible Mechanism of Action | Regulation of the Types of Muscle Fiber | Reference | ||
|---|---|---|---|---|---|---|
| Slow Muscle | Fast Muscle | Transformation of Muscle Fiber | ||||
| Sus scrofa | MSTN | After knockout, the expression of MRF, MEF2C, MyOD and MyOG was regulated | − | + | Slow muscle → fast muscle | [276,277,278] |
| PPARγ | Phosphorylation of CAMKII and p38 MAPK promotes the expression of MEF2 and PGC-1αZ | + | / | / | [280] | |
| FHL3 | Inhibition of YY1 combined with MyHC-IIB | − | + | / | [281] | |
| Prox1 | The trend of expression in porcine skeletal muscle was the same as that of MyHC-I and opposite to that of MyHC-IIB | + | − | +* | [282] | |
| Gallus gallus | SOX6 | Activation of MEF2C and inhibition of Nfix | + | / | / | [284] |
| ANKRD2 | Preferentially expressed in red slow muscle fibers of chickens | + | / | +* | [285] | |
| Myoz3 | The PPAR signaling pathway regulates the conversion of muscle fibers and promotes the expression of the fast muscle marker gene MYH1F | − | + | +* | [287,288] | |
| MyHC-IIA | The cis-element interacts with E47 protein | + | − | +* | [289] | |
| Bos taurus | MSTN | After knockout, MyoD was upregulated and MEF2C was downregulated | − | + | Slow muscle → fast muscle | [292] |
| F94L | / | − | + | / | [293] | |
| ADAM12 | Through phosphorylation and glycosylation | + | − | Fast muscle → slow muscle | [294] | |
| ACTN3 | / | − | + | / | [295] | |
| Ovis aries | RIP140 PGC-1β | β-agonists upregulate RIP140 and downregulate PGC-1β through the PPARδ signaling pathway | − | + | Slow muscle → fast muscle | [196] |
| RTL1 DLK1 | Directly targeting the upregulation of DNTTIP1 and PDE4, promoting the upregulation of MYH4 and the downregulation of MYH7 | − | + | / | [297] | |
| Species | Signaling Pathways | Regulation of the Types of Muscle Fibers | Reference | ||
|---|---|---|---|---|---|
| Slow Muscle | Fast Muscle | Transformation of Muscle Fibers | |||
| Sus scrofa | Akirin2 inhibits the expression of NFATc1, PGC-1α, MEF2C and MCIP1 through the Calcineurin/NFATc1 signaling pathway | − | + | Slow muscle → fast muscle | [298] |
| Ferulic acid positively regulates the expression of mitochondrial transcription genes through the Sirt1/AMPK/PGC-1α signaling pathway | + | − | Fast muscle → slow muscle | [299] | |
| FNDC5 directly positively regulates the expression of MyHC-IIA mRNA through the PGC-1α/FNDC5 signaling axis | / | + | / | [300] | |
| FOXO1 regulates the type of muscle fiber through the PI3K/PKB and MAPK/ERK signaling pathways and the calcineurin/NFAT signaling cascade | − | + | Slow muscle → fast muscle | [301] | |
| Leucine reduces protein levels of its downstream transcription factor FoxO1 by activating the Akt/FoxO1 signaling pathway | + | − | Fast muscle → slow muscle | [234] | |
| Fatty acid metabolism, degradation, and elongation pathways may be related to the formation of types of porcine skeletal muscle fiber | / | / | +* | [283] | |
| Gallus gallus | IGF-1 affects protein synthesis in the pectoral muscle of poultry through the AKT/S6 signaling pathway, and IGF-1 is coordinated with the mRNA of the MSTN gene | − | + | +* | [302] |
| The Hedgehog signaling pathway and calcium signaling pathway are key pathways that promote the formation of slow muscle fibers | + | / | / | [304] | |
| Bos taurus | Resveratrol increases the expression of MyHC-IIA and MyHC-I proteins and decreases the expression of MyHC-IIB and MyHC-IIX proteins through the AMPK/SIRT1/PGC-1α signaling pathway | + | − | Fast muscle → slow muscle | [274] |
| Pueraria may regulate the type of muscle fibers through the AMPK/PGC-1α/Nrf1 signaling pathway | + | − | Fast muscle → slow muscle | [305] | |
| ATRA upregulates the expression of MyHC-I-related genes through the PPARδ signaling pathway | + | − | Fast muscle → slow muscle | [306] | |
| Ovis aries | Vitamin A stimulates mitochondrial biogenesis by activating the p38 MAPK/PGC-1α signaling pathway, inducing slow muscle fibers and inhibiting fast muscle fibers | + | − | / | [307] |
| The AMPK/PGC-1α signaling pathway is involved in the conversion of Type II muscle fibers to Type I muscle fibers in grazing sheep | + | − | Fast muscle → slow muscle | [308] | |
| PARK7 and PDE4D are potential targets of DLK1 by activating the cAMP signaling pathway and the PI3K/AKT pathway, respectively | − | + | Slow muscle → fast muscle | [297] | |
| Bos mutus | MYH7, TNNT1, TPM3, and MYL3 regulate the distribution of muscle fibers mainly through the PPAR signaling pathway and the PI3K/Akt signaling pathway | / | / | +* | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, D.; Liu, Y. Research Progress on the Regulating Factors of Muscle Fiber Heterogeneity in Livestock: A Review. Animals 2024, 14, 2225. https://doi.org/10.3390/ani14152225
Wang Y, Zhang D, Liu Y. Research Progress on the Regulating Factors of Muscle Fiber Heterogeneity in Livestock: A Review. Animals. 2024; 14(15):2225. https://doi.org/10.3390/ani14152225
Chicago/Turabian StyleWang, Yufei, Donghao Zhang, and Yiping Liu. 2024. "Research Progress on the Regulating Factors of Muscle Fiber Heterogeneity in Livestock: A Review" Animals 14, no. 15: 2225. https://doi.org/10.3390/ani14152225





