Effects of Herd Establishment Time and Structure on Group-on-Individual Aggression Intensity in Farm Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
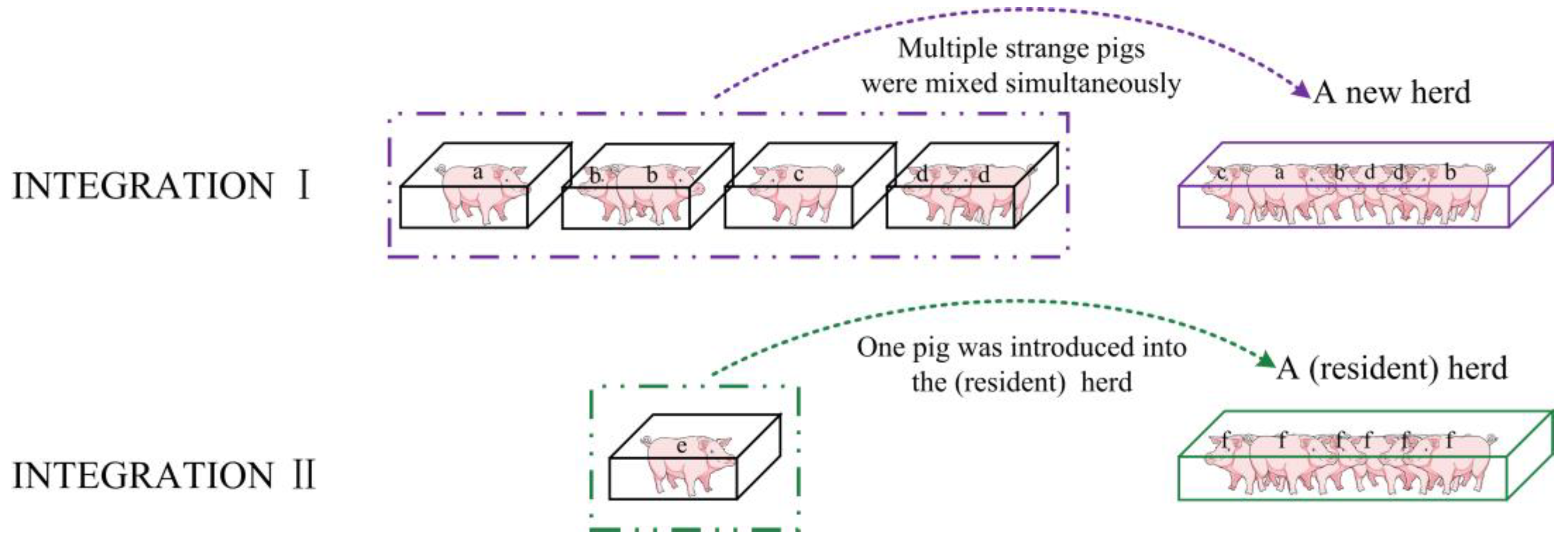
2.1. Pig Herd and Rearing Conditions
2.2. Methods for Identifying Aggressive Behavior and Quantifying Its Intensity
2.3. Experimental Hypothesis and Protocol
2.4. Behavior Data Collection
2.5. Classification and Assessment Method of Skin Lesions
2.6. Assessment of Aggression Intensity and Score Allocation
2.7. Aggression Intensity Analysis Method and Evaluation Index
2.7.1. Levenberg–Marquardt Algorithm
2.7.2. Parabolic Model Based on the Levenberg–Marquardt Algorithm
2.7.3. Conjoint Analysis Model
2.8. Statistical Analysis
3. Results
3.1. Differences in Aggression Intensity across Herds
3.2. Relationship between Herd Establishment Time and Aggression Intensity
3.3. Interaction Effect between Herd’s Establishment Time and Structure Composition
3.4. Contribution Rate and Utility Value of Factors Affecting Aggression Intensity
4. Discussion
4.1. Effect of the Structural Composition of Herd on Its Aggression Intensity
4.2. Effect of the Establishment Time of Herd on Its Aggression Intensity
4.3. Experimental Design and Its Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dawkins, M.S. Farm Animal Welfare: Beyond “Natural” Behavior. Science 2023, 379, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Lymbery, P. An Urgent Call for Global Action to Shift to Regenerative Farming. Nat. Food 2021, 2, 846–847. [Google Scholar] [CrossRef] [PubMed]
- Publications Office of the European Union. Council Directive 2008/120/EC of 18 December 2008 Laying down Minimum Standards for the Protection of Pigs (Codified Version), CELEX1. Available online: https://op.europa.eu/en/publication-detail/-/publication/d6d1224d-0de3-4913-8ea0-8f6825b84275/language-en/format-PDF (accessed on 19 July 2024).
- Spoolder, H.A.M.; Geudeke, M.J.; Van der Peet-Schwering, C.M.C.; Soede, N.M. Group Housing of Sows in Early Pregnancy: A Review of Success and Risk Factors. Livest. Sci. 2009, 125, 1–14. [Google Scholar] [CrossRef]
- Razdan, P.; Tummaruk, P.; Kindahl, H.; Rodriguez-Martinez, H.; Hultén, F.; Einarsson, S. Hormonal Profiles and Embryo Survival of Sows Subjected to Induced Stress during Days 13 and 14 of Pregnancy. Anim. Reprod. Sci. 2004, 81, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Elmore, M.R.P.; Garner, J.P.; Johnson, A.K.; Kirkden, R.D.; Richert, B.T.; Pajor, E.A. Getting around Social Status: Motivation and Enrichment Use of Dominant and Subordinate Sows in a Group Setting. Appl. Anim. Behav. Sci. 2011, 133, 154–163. [Google Scholar] [CrossRef]
- Razdan, P. Stress and Early Pregnancy in Sow. Doctoral Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2003. [Google Scholar]
- Kongsted, A.G. Stress and Fear as Possible Mediators of Reproduction Problems in Group Housed Sows: A Review. Acta Agric. Scand. Sect. Anim. Sci. 2004, 54, 58–66. [Google Scholar] [CrossRef]
- Arey, D.S. Time Course for the Formation and Disruption of Social Organisation in Group-Housed Sows. Appl. Anim. Behav. Sci. 1999, 62, 199–207. [Google Scholar] [CrossRef]
- Camp Montoro, J.; Pessoa, J.; Solà-Oriol, D.; Muns, R.; Gasa, J.; Manzanilla, E.G. Effect of Phase Feeding, Space Allowance and Mixing on Productive Performance of Grower-Finisher Pigs. Animals 2022, 12, 390. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca de Oliveira, A.C.; Costa, L.B.; Weber, S.H.; Ramayo-Caldas, Y.; Dalmau, A. Mixed Management in Growing and Finishing Pigs: Differences between Gender and Their Impacts on Behavior, Growth Performance, and Physiological Parameters. PLoS ONE 2023, 18, e0284481. [Google Scholar] [CrossRef] [PubMed]
- Hyun, Y.; Ellis, M.; Riskowski, G.; Johnson, R.W. Growth Performance of Pigs Subjected to Multiple Concurrent Environmental Stressors. J. Anim. Sci. 1998, 76, 721–727. [Google Scholar] [CrossRef]
- Holden, P.J.; Ensminger, M.E. Swine Science; Wang, A.G., Translator; China Agricultural University Press: Beijing, China, 2007. [Google Scholar]
- Coutellier, L.; Arnould, C.; Boissy, A.; Orgeur, P.; Prunier, A.; Veissier, I.; Meunier-Salaün, M.-C. Pig’s Responses to Repeated Social Regrouping and Relocation during the Growing-Finishing Period. Appl. Anim. Behav. Sci. 2007, 105, 102–114. [Google Scholar] [CrossRef]
- Kranz, V.A.; Horback, K.M.; Parsons, T.D.; Pierdon, M.K. Sow Behavior during Introduction to a Large Dynamic Group Is Influenced by Familiarity and Method. Appl. Anim. Behav. Sci. 2022, 250, 105624. [Google Scholar] [CrossRef]
- Bokma, S.; Kersjes, G.J.K.; Bokma, S.; Kersjes, G.J.K. The Introduction of Pregnant Sows in an Established Group. Proc. Int. Congr. Appl. Ethol. Farm Anim. Skara 1988, 1988, 166–169. [Google Scholar]
- Turner, S.P.; Horgan, G.W.; Edwards, S.A. Effect of Social Group Size on Aggressive Behaviour between Unacquainted Domestic Pigs. Appl. Anim. Behav. Sci. 2001, 74, 203–215. [Google Scholar] [CrossRef]
- Ayo, J.O.; Oladele, S.B.; Fayomi, A. Stress and Its Adverse on Modern Swine Production. Pig News Inf. 1998, 19, 51N–56N. [Google Scholar]
- Stevens, B.; Karlen, G.M.; Morrison, R.; Gonyou, H.W.; Butler, K.L.; Kerswell, K.J.; Hemsworth, P.H. Effects of Stage of Gestation at Mixing on Aggression, Injuries and Stress in Sows. Appl. Anim. Behav. Sci. 2015, 165, 40–46. [Google Scholar] [CrossRef]
- Arey, D.S.; Edwards, S.A. Factors Influencing Aggression between Sows after Mixing and the Consequences for Welfare and Production. Livest. Prod. Sci. 1998, 56, 61–70. [Google Scholar] [CrossRef]
- Greenwood, E.C.; Plush, K.J.; Van Wettere, W.H.E.J.; Hughes, P.E. Hierarchy Formation in Newly Mixed, Group Housed Sows and Management Strategies Aimed at Reducing Its Impact. Appl. Anim. Behav. Sci. 2014, 160, 1–11. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th ed.; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Tinbergen, N. Some Aspects of Ethology, the Biological Study of Animal Behaviour. Adv. Sci. 1955, 12, 17–19. [Google Scholar]
- Tinbergen, N. The Work of the Animal Behaviour Research Group in the Department of Zoology, University of Oxford. Anim. Behav. 1963, 11, 206–209. [Google Scholar] [CrossRef]
- Lorenz, K. Rat. In On Aggression; Routledge Press: London, UK, 1967; pp. 152–160. [Google Scholar]
- Mukoda, N.; Tweheyo, M. Understanding the Integration Process of Captive Chimpanzees Pan Troglodytes in the Uganda Wildlife Education Centre. Acta Zool. Sin. 2007, 53, 257–267. [Google Scholar] [CrossRef]
- Bao, J. Animal Welfare; China Agriculture Press: Beijing, China, 2021; pp. 78–79. [Google Scholar]
- Li, Y.Z.; Wang, L.H.; Johnston, L.J. Sorting by Parity to Reduce Aggression toward First-Parity Sows in Group-Gestation Housing Systems1. J. Anim. Sci. 2012, 90, 4514–4522. [Google Scholar] [CrossRef] [PubMed]
- Drews, C. The Concept and Definition of Dominance in Animal Behaviour. Behaviour 1993, 125, 283–313. [Google Scholar] [CrossRef]
- Driessen, B.; Van Beirendonck, S.; Buyse, J. The Impact of Grouping on Skin Lesions and Meat Quality of Pig Carcasses. Animals 2020, 10, 544. [Google Scholar] [CrossRef] [PubMed]
- Desire, S.; Turner, S.P.; D’Eath, R.B.; Doeschl-Wilson, A.B.; Lewis, C.R.G.; Roehe, R. Genetic Associations of Short- and Long-Term Aggressiveness Identified by Skin Lesion with Growth, Feed Efficiency, and Carcass Characteristics in Growing Pigs. J. Anim. Sci. 2015, 93, 3303–3312. [Google Scholar] [CrossRef] [PubMed]
- Angarita, B.K.; Cantet, R.J.C.; Wurtz, K.E.; O’Malley, C.I.; Siegford, J.M.; Ernst, C.W.; Turner, S.P.; Steibel, J.P. Estimation of Indirect Social Genetic Effects for Skin Lesion Count in Group-Housed Pigs by Quantifying Behavioral Interactions1. J. Anim. Sci. 2019, 97, 3658–3668. [Google Scholar] [CrossRef] [PubMed]
- Levenberg, K. A Method for the Solution of Certain Non-Linear Problems in Least Squares. Q. Appl. Math. 1944, 2, 164–168. [Google Scholar] [CrossRef]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Clementz, B.A.; Iacono, W.G.; Grove, W.M. The Construct Validity of Root-Mean-Square Error for Quantifying Smooth-Pursuit Eye Tracking Abnormalities in Schizophrenia. Biol. Psychiatry 1996, 39, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Henseler, J.; Sarstedt, M. Goodness-of-Fit Indices for Partial Least Squares Path Modeling. Comput. Stat. 2013, 28, 565–580. [Google Scholar] [CrossRef]
- Hauser, J.R.; Eggers, F.; Selove, M. The Strategic Implications of Scale in Choice-Based Conjoint Analysis. Mark. Sci. 2019, 38, 1059–1081. [Google Scholar] [CrossRef]
- Etzkorn, J.R.; Tuttle, S.D.; Lim, I.; Feit, E.M.; Sobanko, J.F.; Shin, T.M.; Neal, D.E.; Miller, C.J. Patients Prioritize Local Recurrence Risk over Other Attributes for Surgical Treatment of Facial Melanomas—Results of a Stated Preference Survey and Choice-Based Conjoint Analysis. J. Am. Acad. Dermatol. 2018, 79, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Hemsworth, P.H.; Rice, M.; Nash, J.; Giri, K.; Butler, K.L.; Tilbrook, A.J.; Morrison, R.S. Effects of Group Size and Floor Space Allowance on Grouped Sows: Aggression, Stress, Skin Injuries, and Reproductive Performance1. J. Anim. Sci. 2013, 91, 4953–4964. [Google Scholar] [CrossRef] [PubMed]
- Andersen, I.L.; Nævdal, E.; Bakken, M.; Bøe, K.E. Aggression and Group Size in Domesticated Pigs, Sus Scrofa: ‘When the Winner Takes It All and the Loser Is Standing Small. Anim. Behav. 2004, 68, 965–975. [Google Scholar] [CrossRef]
- Yang, T.; Yang, C.F.; Chizari, M.D.; Maheswaranathan, N.; Burke, K.J.; Borius, M.; Inoue, S.; Chiang, M.C.; Bender, K.J.; Ganguli, S.; et al. Social Control of Hypothalamus-Mediated Male Aggression. Neuron 2017, 95, 955–970. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.P.; Briffa, M. Fight Tactics in Wood Ants: Individuals in Smaller Groups Fight Harder but Die Faster. Proc. R. Soc. B Biol. Sci. 2011, 278, 3243–3250. [Google Scholar] [CrossRef]
- Tinbergen, N. Ethology and Stress Diseases. Science 1974, 185, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Schuppli, C.; Nellissen, L.; Carvajal, L.; Ashbury, A.M.; Oliver-Caldwell, N.; Rahmaeti, T.; Laumer, I.; Haun, D. Ecological, Social, and Intrinsic Factors Affecting Wild Orangutans’ Curiosity, Assessed Using a Field Experiment. Sci. Rep. 2023, 13, 13184. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, A.L.; Wettere, W.H.V.; Hughes, P.E. Space Requirements to Optimize Welfare and Performance in Group Housed Pigs-A Review. Am. J. Anim. Vet. Sci. 2012, 7, 48–54. [Google Scholar] [CrossRef]
- Morris-Drake, A.; Christensen, C.; Kern, J.M.; Radford, A.N. Experimental field evidence that out-group threats influence within-group behavior. Behav. Ecol. 2019, 30, 1425–1435. [Google Scholar] [CrossRef]
- Schaffner, C.M.; French, J.A. Group Size and Aggression: ‘Recruitment Incentives’ in a Cooperatively Breeding Primate. Anim. Behav. 1997, 54, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Mount, N.C.; Seabrook, M.F. A Study of Aggression When Group Housed Sows Are Mixed. Appl. Anim. Behav. Sci. 1993, 36, 377–383. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carre, G.; Garcia Marquez, J.R.; Gruber, B.; Lafourcade, B.; Leitao, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Vander Weele, T.J. Confounding and Effect Modification: Distribution and Measure. Epidemiol. Methods 2012, 1, 55–82. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis; Pearson Education Limited: London, UK, 2013; ISBN 978-1-292-02190-4. [Google Scholar]
- Muir, W.W. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. R. Stat. Soc. J. Ser. Gen. 1981, 144, 367–368. [Google Scholar] [CrossRef]



| Description for Skin Lesions | Resident Herd | ||
|---|---|---|---|
| Aggression Intensity | Scoring | ||
| Integrated pigs | Without skin lesions | / | 0 |
| Slight skin lesions on individual parts | Slight | 1 | |
| Minor skin lesions on individual parts | Minor | 2 | |
| Multiple minor skin lesions on individual parts | Mild | 3 | |
| Moderate skin lesions on individual parts | Moderate | 4 | |
| Severe skin lesions across the whole body | Severe | 5 | |
| Serious skin lesions across the whole body | Serious | 6 | |
| Resident pigs | Slight skin lesions on individual parts | / | −1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Shi, Z.; Li, H.; Liu, H.; Xiao, Z.; Wang, H.; Pu, S. Effects of Herd Establishment Time and Structure on Group-on-Individual Aggression Intensity in Farm Pigs. Animals 2024, 14, 2229. https://doi.org/10.3390/ani14152229
Wang Z, Shi Z, Li H, Liu H, Xiao Z, Wang H, Pu S. Effects of Herd Establishment Time and Structure on Group-on-Individual Aggression Intensity in Farm Pigs. Animals. 2024; 14(15):2229. https://doi.org/10.3390/ani14152229
Chicago/Turabian StyleWang, Zhen, Zhengxiang Shi, Hao Li, Hui Liu, Zhaowei Xiao, Hao Wang, and Shihua Pu. 2024. "Effects of Herd Establishment Time and Structure on Group-on-Individual Aggression Intensity in Farm Pigs" Animals 14, no. 15: 2229. https://doi.org/10.3390/ani14152229





