MiR-20a-5p Targeting the TGFBR2 Gene Regulates Inflammatory Response of Chicken Macrophages Infected with Avian Pathogenic E. coli
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Tissues Collection
2.3. Cell Culture and Passage
2.4. RT-qPCR
2.5. Bioinformatics Analysis of TGFBR2
2.6. Prediction of Target Genes
2.7. Construction of Expression Vectors and Transient Transfection
2.8. Dual-Luciferase Assay
2.9. Western Blotting
2.10. Cell Viability Assay and Apoptosis Assay
2.11. Nitric Oxide (NO) Production Assay
2.12. Statistical Analysis
3. Results
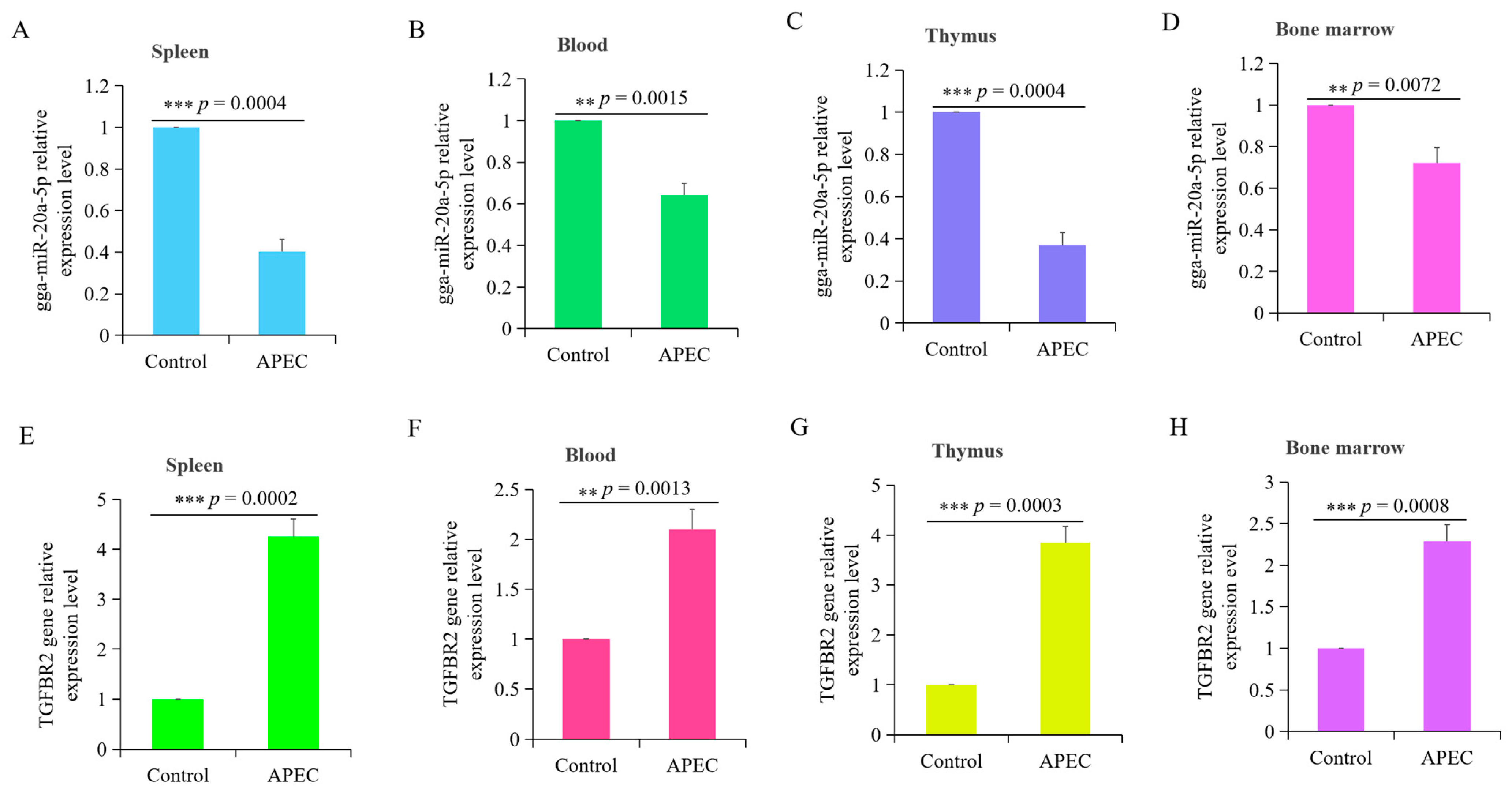
3.1. APEC Infection Significantly Influenced the Expression of gga-miR-20a-5p and TGFBR2
3.2. Bioinformatics Analysis of gga-miR-20a-5p and TGFBR2
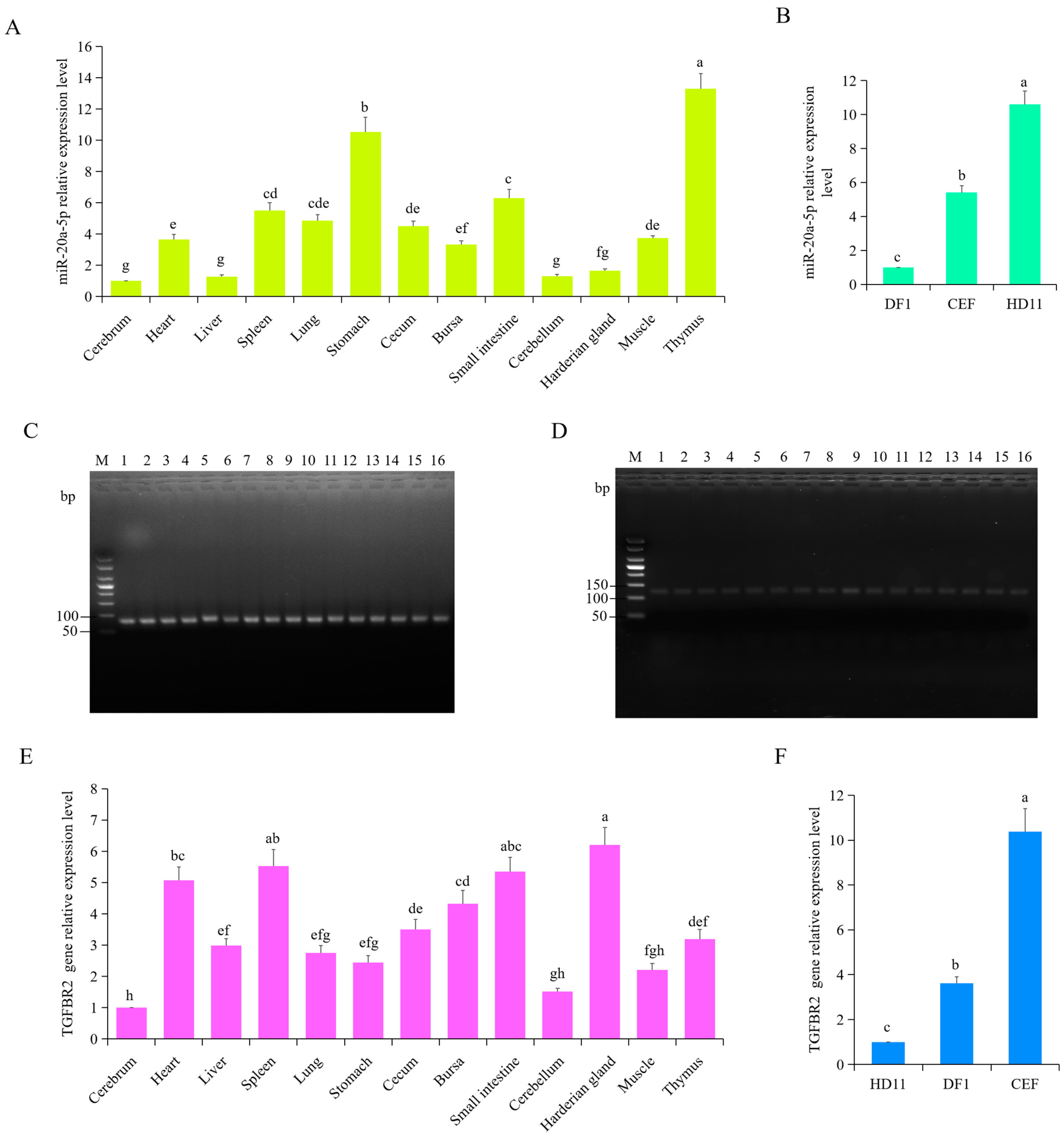
3.3. Expression Pattern of gga-miR-20a-5p and TGFBR2
3.4. Time- and Dose-Dependent Effects of APEC Infection on the Expression Levels of gga-miR-20a-5p and TGFBR2
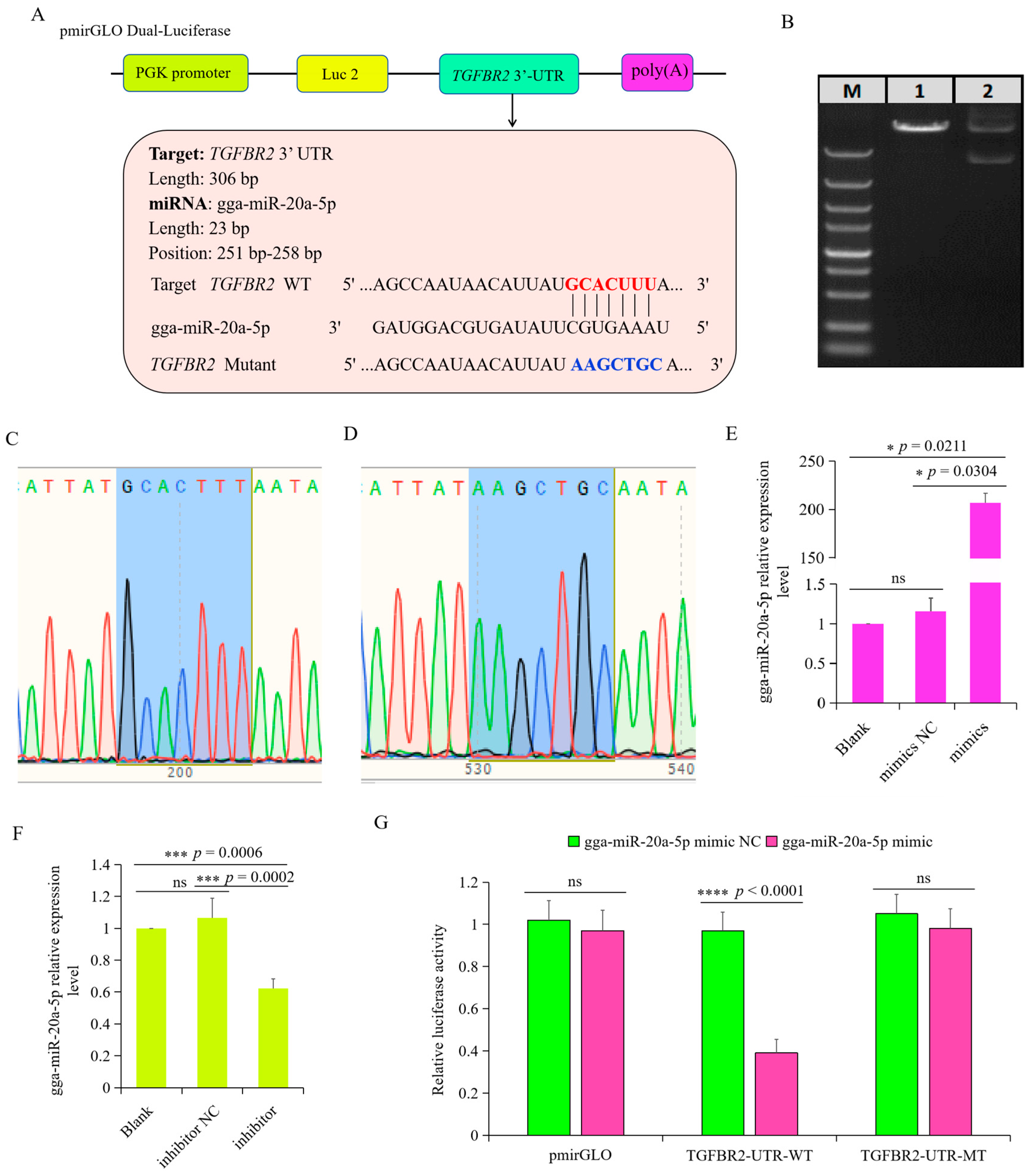
3.5. TGFBR2 Was the Target Gene of gga-miR-20a-5p
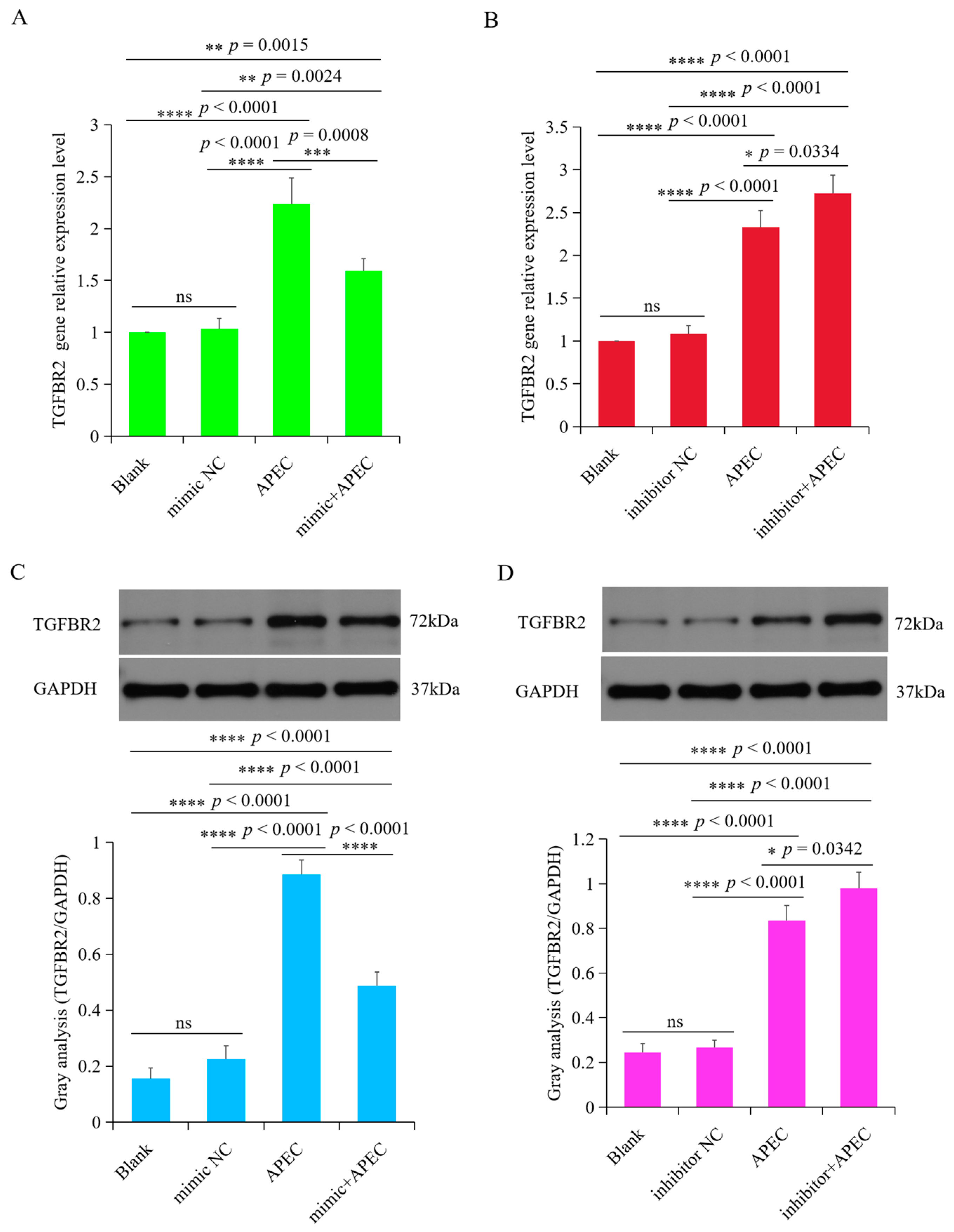
3.6. TGFBR2 Was Regulated by gga-miR-20a-5p upon APEC Infection
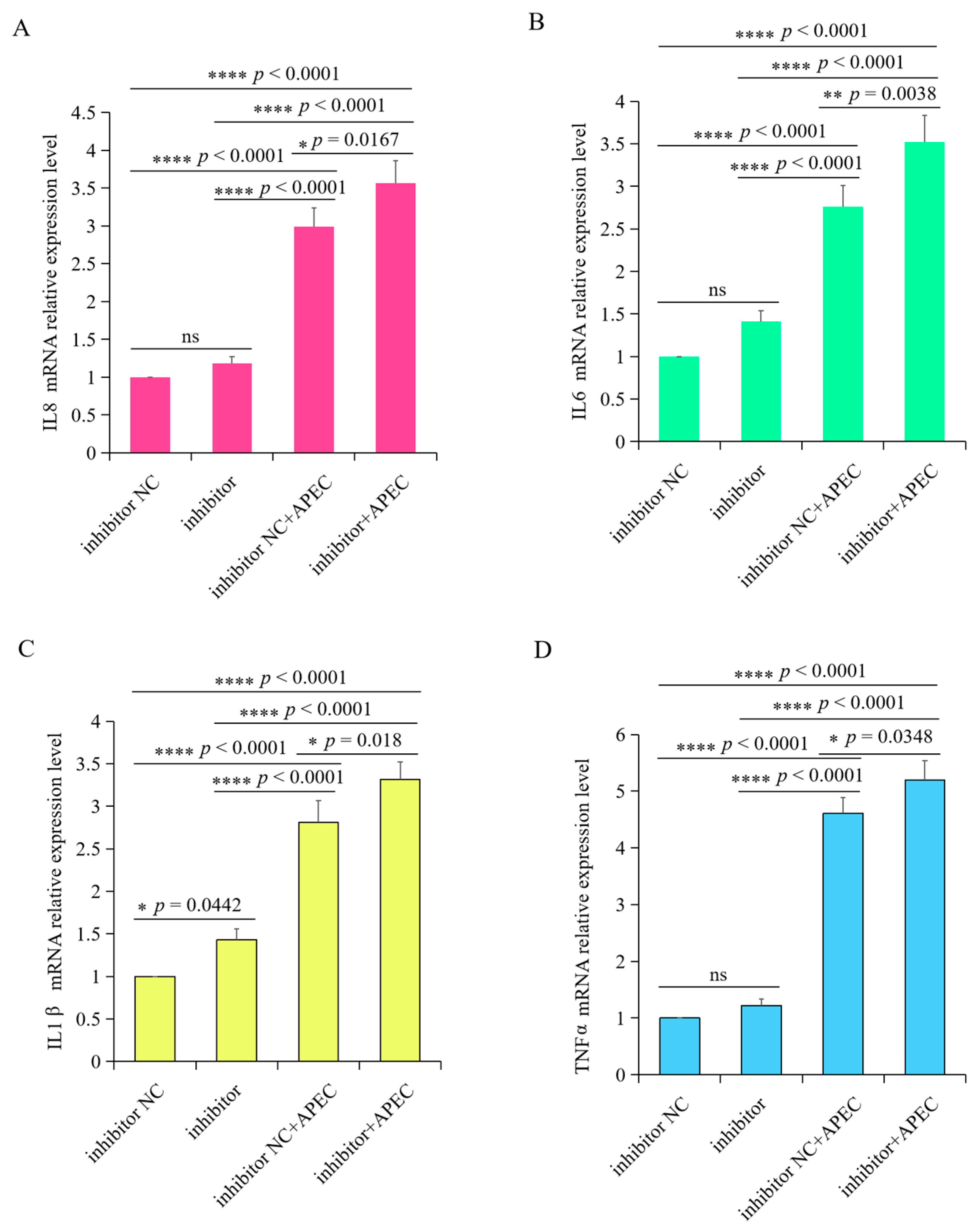
3.7. gga-miR-20a-5p Regulated the Expression of Downstream Inflammatory Mediators of TGFBR2 during APEC Infection
3.8. gga-miR-20a-5p Influenced Cell Viability and NO Production upon APEC Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dziva, F.; Stevens, M.P. Colibacillosis in Poultry: Unravelling the Molecular Basis of Virulence of Avian Pathogenic Escherichia coli in Their Natural Hosts. Avian Pathol. 2008, 37, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Guabiraba, R.; Schouler, C. Avian Colibacillosis: Still Many Black Holes. FEMS Microbiol. Lett. 2015, 362, fnv118. [Google Scholar] [CrossRef] [PubMed]
- Nolan, L.K.; Barnes, H.J.; Vaillancourt, J.-P.; Abdul-Aziz, T.; Logue, C.M. Colibacillosis. In Diseases of Poultry; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 751–805. [Google Scholar]
- Azam, M.; Mohsin, M.; Johnson, T.J.; Smith, E.A.; Johnson, A.; Umair, M.; Saleemi, M.K.; Sajjad-ur-Rahman. Genomic Landscape of Multi-Drug Resistant Avian Pathogenic Escherichia coli Recovered from Broilers. Vet. Microbiol. 2020, 247, 108766. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Thuan, N.K.; Tam, N.T.; Huyen Trang, C.T.; Khanh, N.P.; Bich, T.N.; Taniguchi, T.; Hayashidani, H.; Lien Khai, L.T. Prevalence and Genetic Relationship of Predominant Escherichia coli Serotypes Isolated from Poultry, Wild Animals, and Environment in the Mekong Delta, Vietnam. Vet. Med. Int. 2021, 2021, 6504648. [Google Scholar] [CrossRef] [PubMed]
- Monson, M.S.; Lamont, S.J. Genetic Resistance to Avian Pathogenic Escherichia coli (APEC): Current Status and Opportunities. Avian Pathol. 2021, 50, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Wu, Y.; Tian, Y.; Han, J.; Wang, Q.; Liu, Y.; Man, C. Circulating MiR-155, a Potential Regulator of Immune Responses to Different Vaccines in Chicken. Res. Vet. Sci. 2022, 152, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, L.; Liu, X.; Zhang, M.; Li, X. Correlation between MiRNAs and Target Genes in Response to Campylobacter Jejuni Inoculation in Chicken. Poult. Sci. 2018, 97, 485–493. [Google Scholar] [CrossRef]
- Sun, H.; Cao, Y.; Yang, Y.; Li, H.; Qu, L. Analysis of MiRNA Expression Profiling of RIP2 Knockdown in Chicken HD11 Cells When Infected with Avian Pathogenic E. coli (APEC). Int. J. Mol. Sci. 2022, 23, 7319. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Y.; Zhou, Y.; Sun, H.; Ma, Y.; Tan, J.; Li, N.; Li, H. Identification and Characterization of MicroRNAs, Especially ggamiR-181b-5p, in Chicken Macrophages Associated with Avian Pathogenic E. coli Infection. Avian Pathol. 2023, 52, 185–198. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, H.; Zhang, Q.; Liu, Z.; Wang, T.; Wu, Z.; Wu, W. CAF-Released Exosomal MiR-20a-5p Facilitates HCC Progression via the LIMA1-Mediated β-Catenin Pathway. Cells 2022, 11, 3857. [Google Scholar] [CrossRef]
- Shi, L.; Song, Z.; Li, Y.; Huang, J.; Zhao, F.; Luo, Y.; Wang, J.; Deng, F.; Shadekejiang, H.; Zhang, M. MiR-20a-5p Alleviates Kidney Ischemia/Reperfusion Injury by Targeting ACSL4-Dependent Ferroptosis. Am. J. Transplant. 2023, 23, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Heo, J.; Kang, S.; Vu, T.H.; Lillehoj, H.S.; Hong, Y.H. Exosome-Mediated Delivery of Gga-MiR-20a-5p Regulates Immune Response of Chicken Macrophages by Targeting IFNGR2, MAPK1, MAP3K5, and MAP3K14. Anim. Biosci. 2023, 36, 851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, X.; Wang, W.; Cai, Y.; Li, S.; Chen, Q.; Liao, M.; Zhang, M.; Zeng, G.; Zhou, B. Down-Regulation of MiR-20a-5p Triggers Cell Apoptosis to Facilitate Mycobacterial Clearance through Targeting JNK2 in Human Macrophages. Cell Cycle 2016, 15, 2527–2538. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, Q.; Han, J.; Wen, J.; Wu, Y.; Man, C. Stress-Induced Immunosuppression Affecting Avian Influenza Virus Vaccine Immune Response through MiR-20a-5p/NR4A3 Pathway in Chicken. Vet. Microbiol. 2022, 273, 109546. [Google Scholar] [CrossRef] [PubMed]
- Javelaud, D.; Mauviel, A. Mammalian Transforming Growth Factor-Βs: Smad Signaling and Physio-Pathological Roles. Int. J. Biochem. Cell Biol. 2004, 36, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGF-β Signal Transduction. Annu. Rev. Biochem. 1998, 67, 753–791. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, Y.; Cao, Y.; Li, H.; Qu, L.; Lamont, S.J. Gene Expression Profiling of RIP2-Knockdown in HD11 Macrophages—Elucidation of Potential Pathways (Gene Network) When Challenged with Avian Pathogenic E. coli (APEC). BMC Genom. 2022, 23, 341. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Zheng, L.; Yao, L.; Lei, L.; Huang, Y.; Zeng, Z.; Fang, N. Exosomes from Adipose-Derived Mesenchymal Stem Cells Improve Liver Fibrosis by Regulating the MiR-20a-5p/TGFBR2 Axis to Affect the p38 MAPK/NF-kappaB Pathway. Cytokine 2023, 172, 156386. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, P.; Nolan, L.K.; Lamont, S.J. Avian Pathogenic Escherichia coli (APEC) Infection Alters Bone Marrow Transcriptome in Chickens. BMC Genom. 2015, 16, 690. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server; Humana Press: Totowa, NJ, USA, 2005; ISBN 1588293432. [Google Scholar]
- Petersen, T.N.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating Signal Peptides from Transmembrane Regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Möller, S.; Croning, M.D.R.; Apweiler, R. Evaluation of Methods for the Prediction of Membrane Spanning Regions. Bioinformatics 2001, 17, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Blom, N.; Gammeltoft, S.; Brunak, S. Sequence and Structure-Based Prediction of Eukaryotic Protein Phosphorylation Sites. J. Mol. Biol. 1999, 294, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B.; Marcos-Silva, L. Precision Mapping of the Human O-GalNAc Glycoproteome through SimpleCell Technology. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X. MiRDB: An Online Database for Prediction of Functional MicroRNA Targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting Effective MicroRNA Target Sites in Mammalian MRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Eulalio, A.; Schulte, L.; Vogel, J. The Mammalian MicroRNA Response to Bacterial Infections. RNA Biol. 2012, 9, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.; Cruz, A.R.; Rodrigues Lopes, I.; Maudet, C.; Sunkavalli, U.; Silva, R.J.; Sharan, M.; Lisowski, C.; Zaldívar-López, S.; Garrido, J.J. Functional Screenings Reveal Different Requirements for Host MicroRNAs in Salmonella and Shigella Infection. Nat. Microbiol. 2020, 5, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Nie, Q.; Zhang, X.; Nolan, L.K.; Lamont, S.J. Novel MicroRNA Involved in Host Response to Avian Pathogenic Escherichia coli Identified by Deep Sequencing and Integration Analysis. Infect. Immun. 2017, 85, e00688-16. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Yang, J.; Wang, X.; Liu, X.; Wu, J.; Li, C.; Xu, D.; Hu, Y. MiR-200c-3p Inhibits LPS-Induced M1 Polarization of BV2 Cells by Targeting RIP2. Genes Genom. 2022, 44, 477–486. [Google Scholar] [CrossRef]
- Su, A.; Guo, Y.; Tian, H.; Zhou, Y.; Li, W.; Tian, Y.; Li, K.; Sun, G.; Jiang, R.; Yan, F. Analysis of MiRNA and MRNA Reveals Core Interaction Networks and Pathways of Dexamethasone-Induced Immunosuppression in Chicken Bursa of Fabricius. Mol. Immunol. 2021, 134, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Su, A.; Zhou, Y.; Guo, Y.; Yang, X.; Zhang, Y.; Li, W.; Tian, Y.; Li, K.; Sun, G.; Jiang, R. Identification and Expression Analysis of MicroRNAs in Chicken Spleen in a Corticosterone-Induced Stress Model. Res. Vet. Sci. 2021, 136, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, R.; Yu, Q.; Dong, L.; Bi, Y.; Liu, G. Metabolic Reprogramming of Macrophages during Infection and Cancer. Cancer Lett. 2019, 452, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Bao, Q.; Li, Y. Regulation of M1 Type and M2 Type Macrophage Polarization in RAW264.7 Cells by Galectin 9. Mol. Med. Rep. 2017, 16, 9111–9119. [Google Scholar] [CrossRef] [PubMed]
- Chendrimada, T.P.; Finn, K.J.; Ji, X.; Baillat, D.; Gregory, R.I.; Liebhaber, S.A.; Pasquinelli, A.E.; Shiekhattar, R. MicroRNA Silencing through RISC Recruitment of EIF6. Nature 2007, 447, 823–828. [Google Scholar] [CrossRef]
- Su, S.; Cao, J.; Meng, X.; Liu, R.; Vander Ark, A.; Woodford, E.; Zhang, R.; Stiver, I.; Zhang, X.; Madaj, Z.B. Enzalutamide-Induced and PTH1R-Mediated TGFBR2 Degradation in Osteoblasts Confers Resistance in Prostate Cancer Bone Metastases. Cancer Lett. 2022, 525, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Oku, K.; Kondo, H. Familial Exudative Vitreoretinopathy with TGFBR2 Mutation without Signs of Loeys-Dietz Syndrome. Ophthalmic Genet. 2021, 42, 637–640. [Google Scholar] [CrossRef]
- Tomela, K.; Karolak, J.A.; Ginter-Matuszewska, B.; Kabza, M.; Gajecka, M. Influence of TGFBR2, TGFB3, DNMT1, and DNMT3A Knockdowns on CTGF, TGFBR2, and DNMT3A in Neonatal and Adult Human Dermal Fibroblasts Cell Lines. Curr. Issues Mol. Biol. 2021, 43, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Pollack, J.L.; Rudolph, J.; Dash, S.; Abushawish, M.; Lee, T.; Jahchan, N.S.; Canaday, P.; Lu, E.; Norng, M. Targeting TREM2 on Tumor-Associated Macrophages Enhances Immunotherapy. Cell Rep. 2021, 37, 109844. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhang, C.; Zhang, X.; Chen, Y.; Zhang, H. MiR-145 Negatively Regulates TGFBR2 Signaling Responsible for Sepsis-Induced Acute Lung Injury. Biomed. Pharmacother. 2019, 111, 852–858. [Google Scholar] [CrossRef]
- Yang, P.; Han, J.; Li, S.; Luo, S.; Tu, X.; Ye, Z. MiR-128-3p Inhibits Apoptosis and Inflammation in LPS-Induced Sepsis by Targeting TGFBR2. Open Med. 2021, 16, 274–283. [Google Scholar] [CrossRef] [PubMed]





| Name | Accession Number | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|---|
| TGFBR2 | XM_046910484.1 | TCTTGTCCCTTTATTGGTG | TTATGTTTCTTGGGCTTGA |
| β-actin | NM_205518.2 | CAGCCAGCCATGGATGATGA | ACCAACCATCACACCCTGAT |
| IL1β | XM_046931582.1 | GCCGAGGAGCAGGGACTTT | ACTGTGAGCGGGTGTAGCG |
| IL8 | NM_205018.2 | GAGTTCACTGACCACCCT | TGCCTGAGCCATACCTTT |
| IL6 | NM_204628.2 | TTATGGAGAAGACCGTGAG | GTGGCAGATTGGTAACAGA |
| TNFα | XM_046900549.1 | CGTTCGGGAGTGGGCTTTA | TTGTGGGACAGGGTAGGG |
| gga-miR-20a-5p | NR_031405.1 | TAAAGTGCTTATAGTGCAGGTAG | CAGTGCGTGTCGTGGAGT |
| U6 | XM_025154275.3 | CAAGGACCCATCGTTCCACA | CCATTGGACACGCAGAATGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Ge, J.; Ma, Y.; Li, H.; Han, W.; Lamont, S.J.; Sun, H. MiR-20a-5p Targeting the TGFBR2 Gene Regulates Inflammatory Response of Chicken Macrophages Infected with Avian Pathogenic E. coli. Animals 2024, 14, 2277. https://doi.org/10.3390/ani14152277
Cao X, Ge J, Ma Y, Li H, Han W, Lamont SJ, Sun H. MiR-20a-5p Targeting the TGFBR2 Gene Regulates Inflammatory Response of Chicken Macrophages Infected with Avian Pathogenic E. coli. Animals. 2024; 14(15):2277. https://doi.org/10.3390/ani14152277
Chicago/Turabian StyleCao, Xinqi, Jiayi Ge, Yuyi Ma, Huan Li, Wei Han, Susan J Lamont, and Hongyan Sun. 2024. "MiR-20a-5p Targeting the TGFBR2 Gene Regulates Inflammatory Response of Chicken Macrophages Infected with Avian Pathogenic E. coli" Animals 14, no. 15: 2277. https://doi.org/10.3390/ani14152277
APA StyleCao, X., Ge, J., Ma, Y., Li, H., Han, W., Lamont, S. J., & Sun, H. (2024). MiR-20a-5p Targeting the TGFBR2 Gene Regulates Inflammatory Response of Chicken Macrophages Infected with Avian Pathogenic E. coli. Animals, 14(15), 2277. https://doi.org/10.3390/ani14152277




