The Effects of Lactobacillus plantarum and Lactobacillus buchneri on the Fermentation Quality, In Vitro Digestibility, and Aerobic Stability of Silphium perfoliatum L. Silage
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Design
2.2. Fermentation Quality Analysis and Microbial Counting
2.3. Chemical Composition and Energy Analysis
2.4. Aerobic Stability Analysis
2.5. In Vitro Fermentation Parameter Analysis
2.6. Statistical Analysis
3. Results
3.1. Fermentation Quality of Silphium perfoliatum L. Silage
3.2. Chemical Composition of Silphium perfoliatum L. Silage
3.3. Energy of Silphium perfoliatum L. Silage
3.4. In Vitro Digestibility of Silphium perfoliatum L. Silage
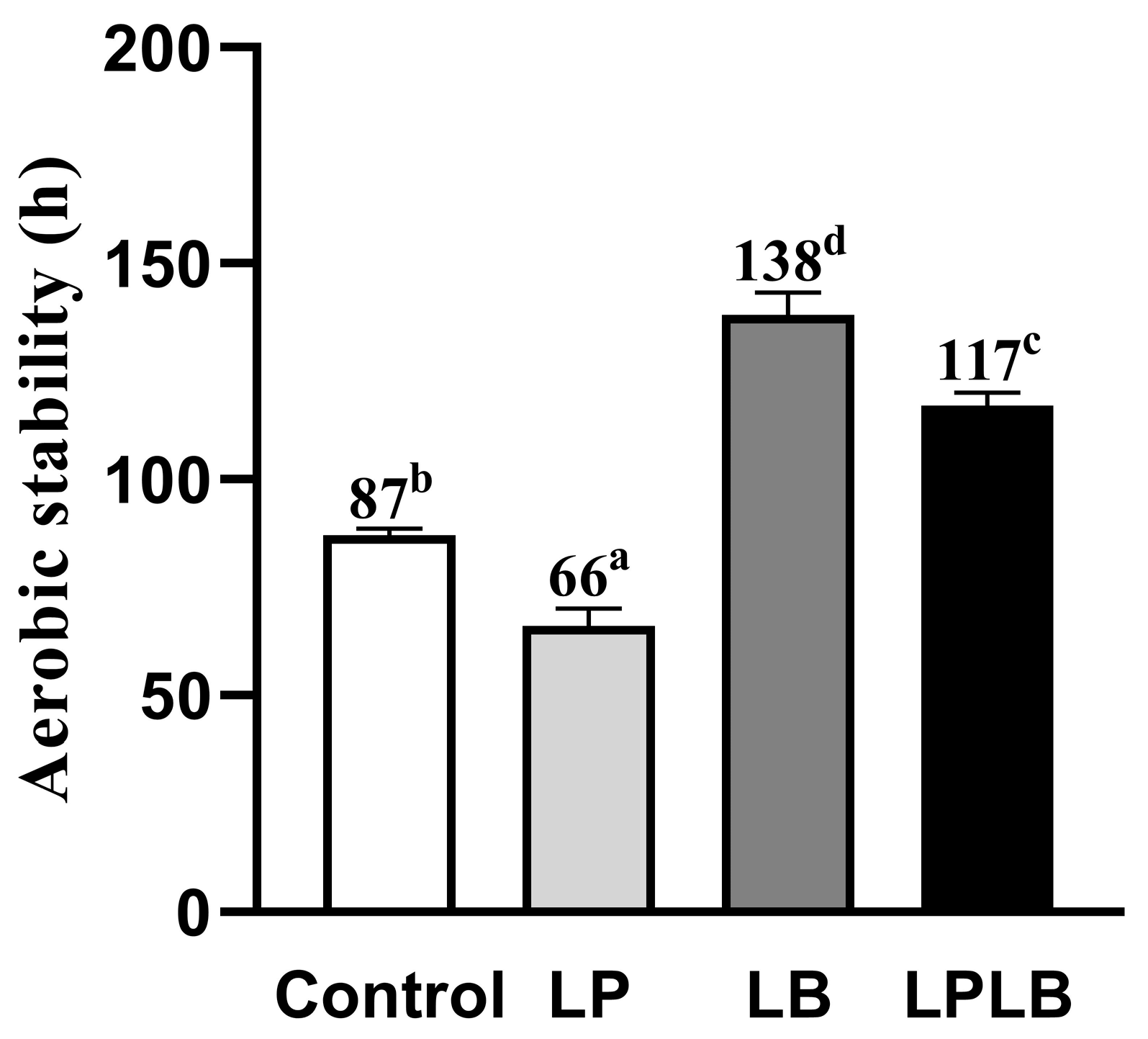
3.5. Aerobic Stability of Silphium perfoliatum L. Silage
4. Discussion
4.1. Effect of Different Fermenting Lactic Acid Bacteria on the Fermentation Quality of Silphium perfoliatum L. Silage
4.2. Effect of Different Fermenting Lactic Acid Bacteria on the Energy and Nutritional Value of Silphium perfoliatum L. Silage
4.3. Effect of Different Fermenting Lactic Acid Bacteria on the In Vitro Digestibility of Silphium perfoliatum L. Silage
4.4. Effect of Different Fermenting Lactic Acid Bacteria on the Aerobic Stability of Silphium perfoliatum L. Silage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Tassel, D.L.; Albrecht, K.A.; Bever, J.D.; Boe, A.A.; Brandvain, Y.; Crews, T.E.; Gansberger, M.; Gerstberger, P.; González-Paleo, L.; Hulke, B.S.; et al. Accelerating Silphium domestication: An opportunity to develop new crop ideotypes and breeding strategies informed by multiple disciplines. Crop Sci. 2017, 57, 1274–1284. [Google Scholar] [CrossRef]
- Kowalska, G.; Pankiewicz, U.; Kowalski, R. Evaluation of chemical composition of some Silphium L. species as alternative raw materials. Agriculture 2020, 10, 132. [Google Scholar] [CrossRef]
- El-Sayed, N.H.; Wojcińska, M.; Drost-Karbowska, K.; Matławska, I.; Williams, J.; Mabry, T.J. Kaempferol triosides from Silphium perfoliatum. Phytochemistry 2002, 60, 835–838. [Google Scholar] [CrossRef]
- Xie, Y.; Bao, J.; Li, W.; Sun, Z.; Gao, R.; Wu, Z.; Yu, Z. Effects of applying lactic acid bacteria and molasses on the fermentation quality, protein fractions and in vitro digestibility of baled alfalfa silage. Agronomy 2021, 11, 91. [Google Scholar] [CrossRef]
- Ren, H.; Feng, Y.; Pei, J.; Li, J.; Wang, Z.; Fu, S.; Zheng, Y.; Li, Z.; Peng, Z. Effects of Lactobacillus plantarum additive and temperature on the ensiling quality and microbial community dynamics of cauliflower leaf silages. Bioresour. Technol. 2020, 307, 123238. [Google Scholar] [CrossRef]
- Filya, I. The effect of Lactobacillus buchneri and Lactobacillus plantarum on the fermentation, aerobic stability, and ruminal degradability of low dry matter corn and sorghum silages. J. Dairy Sci. 2003, 86, 3575–3581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, Z.; Yang, H.; Na, R.S. The effects of stage of growth and additives with or without cellulase on fermentation and in vitro degradation characteristics of Leymus chinensis silage. Grass Forage Sci. 2016, 71, 595–606. [Google Scholar] [CrossRef]
- Xu, D.; Ding, W.; Ke, W.; Li, F.; Zhang, P.; Guo, X. Modulation of metabolome and bacterial community in whole crop corn silage by inoculating homofermentative Lactobacillus plantarum and heterofermentative Lactobacillus buchneri. Front. Microbiol. 2019, 9, 3299. [Google Scholar] [CrossRef]
- Woolford, M.K. The detrimental effects of air on silage. J. Appl. Bacteriol. 1990, 68, 101–116. [Google Scholar] [CrossRef]
- Xu, S.; Yang, J.; Qi, M.; Smiley, B.; Rutherford, W.; Wang, Y.; McAllister, T.A. Impact of saccharomyces cerevisiae and Lactobacillus buchneri on microbial communities during ensiling and aerobic spoilage of corn silage. J. Anim. Sci. 2019, 97, 1273–1285. [Google Scholar] [CrossRef]
- Zhang, T.; Li, L.; Wang, X.; Zeng, Z.; Hu, Y.; Cui, Z. Effects of Lactobacillus buchneri and Lactobacillus plantarum on fermentation, aerobic stability, bacteria diversity and ruminal degradability of alfalfa silage. World J. Microbiol. Biotechnol. 2009, 25, 965–971. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Cai, Y.; Hirakubo, T.; Fukui, H.; Matsuyama, H. Fermentation characteristics and microorganism composition of total mixed ration silage with local food by-products in different seasons. Anim. Sci. J. 2011, 82, 259–266. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC Int.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Deriaz, R.E. Routine analysis of carbohydrates and lignin in herbage. J. Sci. Food Agric. 1961, 12, 152–160. [Google Scholar] [CrossRef]
- Playne, M.J.; McDonald, P. The buffering constituents of herbage and of silage. J. Sci. Food Agric. 1966, 17, 264–268. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Si, R.; Chen, X.B. Feeding of Cattle, Sheep and Goats, 3rd ed.; China Agricultural University Press: Beijing, China, 2013; pp. 140–154. [Google Scholar]
- Kung, L.J.; Sheperd, A.C.; Smagala, A.M.; Endres, K.M.; Bessett, C.A.; Ranjit, N.K.; Glancey, J. The effect of preservatives based on propionic acid on the fermentation and aerobic stability of corn silage and a total mixed ration. J. Dairy Sci. 1998, 81, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, W.; Wang, Y.; Wang, C.; Chen, X.; Zhang, Q. Effect of applying lactic acid bacteria and cellulase on the fermentation quality, nutritive value, tannins profile and in vitro digestibility of Neolamarckia cadamba leaves silage. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1429–1436. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, G.; Chen, L.; Li, J.; Yuan, X.; Yu, C.; Shimojo, M.; Shao, T. Effect of applying lactic acid bacteria and propionic acid on fermentation quality and aerobic stability of oats-common vetch mixed silage on the T ibetan plateau. Anim. Sci. J. 2015, 86, 595–602. [Google Scholar] [CrossRef]
- Bai, J.; Ding, Z.; Ke, W.; Xu, D.; Wang, M.; Huang, W.; Zhang, Y.; Liu, F.; Guo, X. Different lactic acid bacteria and their combinations regulated the fermentation process of ensiled alfalfa: Ensiling characteristics, dynamics of bacterial community and their functional shifts. Microb. Biotechnol. 2021, 14, 1171–1182. [Google Scholar] [CrossRef]
- Xu, D.; Wang, N.; Rinne, M.; Ke, W.; Weinberg, Z.G.; Da, M.; Bai, J.; Zhang, Y.; Li, F.; Guo, X. The bacterial community and metabolome dynamics and their interactions modulate fermentation process of whole crop corn silage prepared with or without inoculants. Microb. Biotechnol. 2021, 14, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Tharangani, R.M.H.; Yakun, C.; Zhao, L.S.; Shen, Y.F.; Ma, L.; Bu, D.P. Proposal and validation of integrated alfalfa silage quality index (ASQI) method for the quality assessment of alfalfa silage for lactating dairy cows. Anim. Feed Sci. Technol. 2022, 289, 115339. [Google Scholar] [CrossRef]
- Soundharrajan, I.; Park, H.S.; Rengasamy, S.; Sivanesan, R.; Choi, K.C. Application and future prospective of lactic acid bacteria as natural additives for silage production—A review. Appl. Sci. 2021, 11, 8127. [Google Scholar] [CrossRef]
- Okoye, C.O.; Wang, Y.; Gao, L.; Wu, Y.; Li, X.; Sun, J.; Jiang, J. The performance of lactic acid bacteria in silage production: A review of modern biotechnology for silage improvement. Microbiol. Res. 2023, 266, 127212. [Google Scholar] [CrossRef] [PubMed]
- Teixeira Franco, R.; Buffière, P.; Bayard, R. Ensiling for biogas production: Critical parameters. A review. Biomass Bioenergy 2016, 94, 94–104. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Zhao, S.; Wang, Y. Lactobacillus plantarum inoculants delay spoilage of high moisture alfalfa silages by regulating bacterial community composition. Front. Microbiol. 2020, 11, 1989. [Google Scholar] [CrossRef]
- Romero, J.J.; Zhao, Y.; Balseca-Paredes, M.A.; Tiezzi, F.; Gutierrez-Rodriguez, E.; Castillo, M.S. Laboratory silo type and inoculation effects on nutritional composition, fermentation, and bacterial and fungal communities of oat silage. J. Dairy Sci. 2017, 100, 1812–1828. [Google Scholar] [CrossRef] [PubMed]
- Winters, A.L.; Cockburn, J.E.; Dhanoa, M.S.; Merry, R.J. Effects of lactic acid bacteria in inoculants on changes in amino acid composition during ensilage of sterile and non-sterile ryegrass. J. Appl. Microbiol. 2000, 89, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Benno, Y.; Ogawa, M.; Kumai, S. Effect of applying lactic acid bacteria isolated from forage crops on fermentation characteristics and aerobic deterioration of silage. J. Dairy Sci. 1999, 82, 520–526. [Google Scholar] [CrossRef]
- Blajman, J.E.; Vinderola, G.; Páez, R.B.; Signorini, M.L. The role of homofermentative and heterofermentative lactic acid bacteria for alfalfa silage: A meta-analysis. J. Agric. Sci. 2020, 158, 107–118. [Google Scholar] [CrossRef]
- Filya, I.; Sucu, E.; Karabulut, A. Effect of Lactobacillus buchneri on the fermentation, aerobic stability and ruminal degradability of maize silage. J. Appl. Microbiol. 2006, 101, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Govea, F.E.; Muck, R.E.; Broderick, G.A.; Weimer, P.J. Lactobacillus plantarum effects on silage fermentation and in vitro microbial yield. Anim. Feed Sci. Technol. 2013, 179, 61–68. [Google Scholar] [CrossRef]
- Weinberg, Z.; Ashbell, G.; Hen, Y.; Azrieli, A.; Szakacs, G.; Filya, I. Ensiling whole-crop wheat and corn in large containers with Lactobacillus plantarum and Lactobacillus buchneri. J. Ind. Microbiol. Biotechnol. 2002, 28, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Reich, L.J.; Kung, L. Effects of combining Lactobacillus buchneri 40788 with various lactic acid bacteria on the fermentation and aerobic stability of corn silage. Anim. Feed Sci. Technol. 2010, 159, 105–109. [Google Scholar] [CrossRef]
- Kung, L.; Carmean, B.R.; Tung, R.S. Microbial inoculation or cellulase enzyme treatment of barley and vetch silage harvested at three maturities. J. Dairy Sci. 1990, 73, 1304–1311. [Google Scholar] [CrossRef]
- Yi, Q.X.; Wang, P.; Tang, H.Y.; Zhao, T.Y.; Sheng, Z.Y.; Luo, H.L. Fermentation quality, in vitro digestibility, and aerobic stability of ensiling spent mushroom substrate with microbial additives. Animals 2023, 13, 920. [Google Scholar] [CrossRef]
- Nsereko, V.L.; Smiley, B.K.; Rutherford, W.M.; Spielbauer, A.; Forrester, K.J.; Hettinger, G.H.; Harman, E.K.; Harman, B.R. Influence of inoculating forage with lactic acid bacterial strains that produce ferulate esterase on ensilage and ruminal degradation of fiber. Anim. Feed Sci. Technol. 2008, 145, 122–135. [Google Scholar] [CrossRef]
- Madsen, J.; Hvelplund, T.; Weisbjerg, M.R. Appropriate methods for the evaluation of tropical feeds for ruminants. Anim. Feed Sci. Technol. 1997, 69, 53–66. [Google Scholar] [CrossRef]
- Mertens, D.R. Creating a system for meeting the fiber requirements of dairy cows. J. Dairy Sci. 1997, 80, 1463–1481. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Yu, Y.; Yu, Z.; Shao, T.; Na, R.; Zhao, M. Effects of lactic acid bacteria inoculants and cellulase on fermentation quality and in vitro digestibility of Leymus chinensis silage. Grassl. Sci. 2014, 60, 199–205. [Google Scholar] [CrossRef]
- Wan, J.C.; Xie, K.Y.; Wang, Y.X.; Liu, L.; Yu, Z.; Wang, B. Effects of wilting and additives on the ensiling quality and rumen fermentation characteristics of sudangrass silage. Anim. Biosci. 2021, 34, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Nkosi, B.D.; Meeske, R.; Palic, D.; Langa, T.; Leeuw, K.; Groenewald, I.B. Effects of ensiling whole crop maize with bacterial inoculants on the fermentation, aerobic stability, and growth performance of lambs. Anim. Feed Sci. Technol. 2009, 154, 193–203. [Google Scholar] [CrossRef]
- Kung, L.; Taylor, C.C.; Lynch, M.P.; Neylon, J.M. The effect of treating alfalfa with Lactobacillus buchneri 40788 on silage fermentation, aerobic stability, and nutritive value for lactating dairy cows. J. Dairy Sci. 2003, 86, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Addah, W.; Baah, J.; Okine, E.K.; McAllister, T.A. A third-generation esterase inoculant alters fermentation pattern and improves aerobic stability of barley silage and the efficiency of body weight gain of growing feedlot cattle. J. Anim. Sci. 2012, 90, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Hou, K.; Zhao, J.; Wang, H. The probiotic properties of lactic acid bacteria and their applications in animal husbandry. Curr. Microbiol. 2022, 79, 22. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, Z.G.; Shatz, O.; Chen, Y.; Yosef, E.; Nikbahat, M.; Ben-Ghedalia, D.; Miron, J. Effect of lactic acid bacteria inoculants on in vitro digestibility of wheat and corn silages. J. Dairy Sci. 2007, 90, 4754–4762. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.W.; Adesogan, A.T.; Kim, S.C.; Lee, S.S. Effects of an esterase-producing inoculant on fermentation, aerobic stability, and neutral detergent fiber digestibility of corn silage. J. Dairy Sci. 2009, 92, 732–738. [Google Scholar] [CrossRef]
- Hu, W.; Schmidt, R.J.; McDonell, E.E.; Klingerman, C.M.; Kung, L. The effect of Lactobacillus buchneri 40788 or Lactobacillus plantarum MTD-1 on the fermentation and aerobic stability of corn silages ensiled at two dry matter contents. J. Dairy Sci. 2009, 92, 3907–3914. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, C.; Jia, L.; Yu, K. Effect of Lactobacillus buchneri and Lactobacillus plantarum on the fermentation characteristics and aerobic stability of whipgrass silage in laboratory silos. Grassl. Sci. 2014, 60, 233–239. [Google Scholar] [CrossRef]
- Adesogan, A.T.; Salawu, M.B. The effect of different additives on the fermentation quality, aerobic stability and in vitro digestibility of pea/wheat bi-crop silages containing contrasting pea to wheat ratios. Grass Forage Sci. 2002, 57, 25–32. [Google Scholar] [CrossRef]
- Zhang, F.; Miao, F.; Wang, X.; Lu, W.; Ma, C. Effects of homo- and hetero-fermentative lactic acid bacteria on the quality and aerobic stability of corn silage. Can. J. Anim. Sci. 2021, 101, 761–770. [Google Scholar] [CrossRef]
- Kung, L.; Ranjit, N.K. The effect of Lactobacillus buchneri and other additives on the fermentation and aerobic stability of barley silage. J. Dairy Sci. 2001, 84, 1149–1155. [Google Scholar] [CrossRef] [PubMed]

| Items ‡ | Silphium perfoliatum L. |
|---|---|
| Chemical composition and buffering capacity | |
| Dry matter (% FW) | 31.43 |
| Organic matter (% DM) | 82.26 |
| Crude protein (% DM) | 22.97 |
| Neutral detergent fiber (% DM) | 61.50 |
| Acid detergent fiber (% DM) | 26.18 |
| Acid detergent lignin (% DM) | 9.43 |
| Water-soluble carbohydrate (% DM) | 4.68 |
| Buffering capacity (mEq kg−1 DM) | 242.21 |
| Energy | |
| GE (MJ kg−1 DM) | 15.24 |
| DE (MJ kg−1 DM) | 11.70 |
| ME (MJ kg−1 DM) | 8.72 |
| NEm (M Jkg−1 DM) | 6.24 |
| NEl (MJ kg−1 DM) | 5.33 |
| NEf (MJ kg−1 DM) | 4.61 |
| Microbial counts | |
| Lactic acid bacteria (log10 cfu g−1 FW) | 4.31 |
| Yeasts (log10 cfu g−1 FW) | 3.06 |
| Moulds (log10 cfu g−1 FW) | 1.29 |
| Items ‡ | Additives † | SEM | p-Value | Significance of Main Effects and Interactions | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | LP | LB | LPLB | LP | LB | LP × LB | |||
| pH | 4.64 c | 4.22 a | 4.44 b | 4.33 a b | 0.071 | 0.002 | 0.001 | 0.399 | 0.014 |
| LA (%DM) | 2.63 a | 4.92 c | 4.17 b | 5.51 c | 0.279 | <0.001 | <0.001 | 0.001 | 0.044 |
| AA (%DM) | 0.45 a | 0.47 a | 0.90 b | 0.86 b | 0.049 | <0.001 | 0.676 | <0.001 | 0.437 |
| PA (%DM) | 0.01 | 0.02 | 0.03 | 0.02 | 0.008 | 0.064 | 0.780 | 0.062 | 0.078 |
| BA (%DM) | 0.49 | 0.25 | 0.33 | 0.32 | 0.138 | 0.403 | 0.235 | 0.680 | 0.259 |
| NH3-N (%TN) | 2.96 b | 1.60 a | 1.76 a | 1.47 a | 0.284 | 0.003 | 0.003 | 0.011 | 0.029 |
| Items ‡ | Additives † | SEM | p-Value | Significance of Main Effects and Interactions | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | LP | LB | LPLB | LP | LB | LP × LB | |||
| DM (%FW) | 24.68 a | 26.69 b | 27.38 b | 29.47 c | 0.525 | <0.001 | 0.001 | <0.001 | 0.924 |
| OM (%DM) | 77.36 a | 78.06 a b | 78.34 b | 78.42 b | 0.320 | 0.038 | 0.123 | 0.018 | 0.207 |
| CP (%DM) | 16.76 a | 18.21 b | 21.03 c | 21.79 d | 0.310 | <0.001 | 0.001 | <0.001 | 0.162 |
| NDF (%DM) | 60.55 b | 57.40 a b | 54.58 a | 55.59 a | 1.799 | 0.045 | 0.424 | 0.016 | 0.141 |
| ADF (%DM) | 24.81 c | 24.22 b c | 22.61 a | 23.70 b | 0.283 | <0.001 | 0.247 | <0.001 | 0.003 |
| ADL (%DM) | 7.39 b | 6.67 a b | 6.10 a | 6.08 a | 0.371 | 0.023 | 0.200 | 0.007 | 0.219 |
| Items ‡ | Additives † | SEM | p-Value | Significance of Main Effects and Interactions | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | LP | LB | LPLB | LP | LB | LP × LB | |||
| GE (MJ kg −1DM) | 15.74 a | 15.77 a | 16.24 b | 16.22 b | 0.068 | <0.001 | 0.919 | <0.001 | 0.570 |
| DE (MJ kg −1 DM) | 11.85 a | 12.07 a | 12.52 b | 12.64 b | 0.168 | 0.005 | 0.184 | 0.001 | 0.666 |
| ME (MJ kg −1 DM) | 9.47 a | 9.61 a b | 9.98 c | 9.90 b c | 0.136 | 0.017 | 0.726 | 0.003 | 0.294 |
| NEm (MJ kg −1 DM) | 6.88 a | 7.01 a b | 7.29 b | 7.22 b | 0.118 | 0.029 | 0.701 | 0.006 | 0.266 |
| NEl (MJ kg −1 DM) | 5.75 a | 5.86 a b | 6.04 b | 6.10 b | 0.100 | 0.030 | 0.272 | 0.006 | 0.715 |
| NEf (MJ kg −1 DM) | 4.49 | 4.64 | 4.85 | 4.78 | 0.116 | 0.063 | 0.653 | 0.167 | 0.237 |
| Items ‡ | Additives † | SEM | p-Value | Significance of Main Effects and Interactions | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | LP | LB | LPLB | LP | LB | LP × LB | |||
| IVDMD (%DM) | 63.47 a | 64.45 b | 64.33 b | 65.51 c | 0.304 | 0.001 | 0.001 | 0.002 | 0.643 |
| IVOMD (%DM) | 67.32 | 68.19 | 68.14 | 68.45 | 0.465 | 0.164 | 0.112 | 0.136 | 0.428 |
| IVCPD (%DM) | 60.13 a | 61.69 b | 64.19 c | 64.98 d | 0.240 | <0.001 | <0.001 | <0.001 | 0.059 |
| IVNDFD (%DM) | 52.65 a | 54.45 b | 55.44 b c | 56.14 c | 0.654 | 0.004 | 0.272 | 0.001 | 0.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Wang, P.; Li, F.; Yu, M.; Du, J.; Zhao, T.; Yi, Q.; Tang, H.; Yuan, B. The Effects of Lactobacillus plantarum and Lactobacillus buchneri on the Fermentation Quality, In Vitro Digestibility, and Aerobic Stability of Silphium perfoliatum L. Silage. Animals 2024, 14, 2279. https://doi.org/10.3390/ani14152279
Jin Y, Wang P, Li F, Yu M, Du J, Zhao T, Yi Q, Tang H, Yuan B. The Effects of Lactobacillus plantarum and Lactobacillus buchneri on the Fermentation Quality, In Vitro Digestibility, and Aerobic Stability of Silphium perfoliatum L. Silage. Animals. 2024; 14(15):2279. https://doi.org/10.3390/ani14152279
Chicago/Turabian StyleJin, Yitong, Peng Wang, Fuhou Li, Meng Yu, Jiarui Du, Tianyue Zhao, Qixuan Yi, Hongyu Tang, and Bao Yuan. 2024. "The Effects of Lactobacillus plantarum and Lactobacillus buchneri on the Fermentation Quality, In Vitro Digestibility, and Aerobic Stability of Silphium perfoliatum L. Silage" Animals 14, no. 15: 2279. https://doi.org/10.3390/ani14152279





