Simple Summary
Domestic cats have spread worldwide, and their populations on islands have a significant impact on biodiversity. Particularly on small inhabited islands of tourist importance, cats can reach high densities. To evaluate cat impacts and plan cat population management, it is essential to know their population size and spatial distribution. This study examines the cat population on the small island of Tabarca (40 ha), near the Spanish Mediterranean coast, which includes a small village. Tabarca is included in the Natura 2000 Network due to its environmental value and bird populations. The overall cat density is among the highest reported (308 cats/km2), varying between the urban area (1084 cats/km2) and the uninhabited scrubland area (27 cats/km2). The home ranges of urban cats are much smaller (average 0.38 ha or 1.25 ha, depending on the estimation method) than those of cats in the scrubland (average 9.53 ha). These findings indicate that the urban area is a source of cats that colonize the scrubland. Despite the majority of cats being sterilized by the study’s end (89.5% of males and 91.7% of females), the population decline will be slow, taking many years to reach acceptable levels. Therefore, additional management measures are recommended to mitigate the cat population’s impact on biodiversity.
Abstract
There is growing concern about effectively controlling cat populations due to their impact on biodiversity, especially on islands. To plan this management, it is essential to know the cat population size, sterilization rates, and space they use. Small inhabited islands can have very high cat densities; thus, this study aimed to evaluate cat density and home range on a small tourist island in the Spanish Mediterranean. Surveys in the urban area identified individual cats using a photographic catalog, and camera trapping was conducted in the scrubland area. GPS devices were fitted on three urban cats. The overall cat density was estimated to be 308 cats/km2, varying between the urban area (1084 cats/km2) and the uninhabited scrubland (27 cats/km2). Urban cats had smaller average home ranges (0.38 ha or 1.25 ha, depending on the estimation method) compared to scrubland cats (9.53 ha). Penetration of scrubland cats into the urban area was not detected. These results indicate that the urban area acts as a source of cats for the scrubland. Although the total sterilization rate was high (90.3%), the large cat population implies that the density would take over a decade to decrease to acceptable levels. Therefore, complementary measures for managing this cat population are recommended.
Keywords:
free-roaming cats; cat density; cat home range; small islands; Tabarca; trap-neuter-return 1. Introduction
The domestic cat (Felis catus) is one of the most widespread and abundant carnivores in the world [1]. They have dispersed worldwide with the help of humans, reaching all continents [2,3,4,5] and also islands [6,7,8]. Their generalist predatory behavior [9,10,11] causes them to prey on other species of mammals, reptiles, birds, and insects [12] in both urban and suburban habitats, as well as in uninhabited areas [2], posing a significant challenge for biodiversity conservation worldwide [13,14,15].
It is known that domestic cats exist in high densities in urban areas [16,17,18,19], which are also an important habitat for wild species [20,21]. The abnormally high presence of cats in urban areas and their feeding by humans in public places prevents their populations from fluctuating according to prey densities. This means that cats have the potential to drive prey species populations to extinction [22]. In many areas, the density of free-roaming cats exceeds that of other urban carnivores, such as the fox (Vulpes vulpes) and the badger (Meles meles). One study estimated that a territory that would normally be occupied by one or two pairs of native predators could support up to 35 cats in a peri-urban area [23]. Therefore, the impacts they have on urban wildlife are often greater than the impacts that native predators may generate [24].
In island environments, the domestic cat is one of the most widely introduced predators [8,25]. The presence of cats in these environments causes severe disturbances. These habitats are often more vulnerable to biological invasion, as the species that inhabit them are more susceptible to non-native predators, competitors, pathogens, and parasites [7,26,27,28]. The cat population on islands with small human populations can reach very high densities, as occurs on small Japanese islands [29]. Cats were originally introduced to islands to control rodents, but their diet also includes native bird species, especially during certain times of the year [30].
The generalization of the TNR (Trap-Neuter-Return) method for the ethical management of feral cat colonies has been proposed, but there is little rigorous evidence supporting the reduction of cat populations with this method [31,32,33]. Some studies have indicated that this method can lead to an increase in individuals in the colonies due to food provision, illegal abandonment of cats, reproduction, immigration, and/or attraction of cats from surrounding areas [34,35]. On some islands, the extraction and lethal control of stray cats is an effective conservation tool and is supported by conservation organizations [36]. However, social factors may hinder its implementation on inhabited islands [34,37]. Therefore, other management strategies, such as TNR, adoption programs, or the creation of sanctuaries, are more frequently accepted [38]. However, the TNR does not address critical issues such as predation by cats, especially in natural areas adjacent to feral cat colonies, risks related to zoonotic diseases and wildlife, public health, or the welfare of feral cats [35,39,40,41]. Another proposed mechanism to reduce potential cat predation involves establishing buffer zones around areas of high conservation value. Within these zones, housing development would be restricted, or ownership of domestic cats would be prohibited for residents living within a certain distance of the protected area [42,43,44].
Effective management plans for feral cat colonies must be based on a solid understanding of the community cat population and should be socially acceptable, promote feline welfare and public health, and protect wildlife. The first step in this approach is to obtain accurate estimates of cat population size and the factors that influence its dynamics. These factors include human ownership patterns [16], access to food [45,46], veterinary care [47], predator impact [23], and breeding opportunities [47]. Furthermore, maps of population density across urban and rural areas are vital for allocating scarce resources and guiding optimal interventions [38,48].
The small island of Tabarca is the only inhabited island in the Valencian Community and is included in the Natura 2000 Network, designated as a Special Protection Area (SPA, ES0000214) and Site of Community Importance (SCI, ES5213024). In addition to nesting bird species, it serves as a stopover point for migratory birds crossing the Mediterranean in spring and autumn. The island receives a large number of tourists annually and includes a small village that hosts a significant feral cat colony [49]. To assess the potential impact of this colony on the island’s biodiversity and to promote appropriate cat management strategies, it is necessary to know the abundance and density of cats and their use of space. Therefore, the objectives of this study were (1) to estimate the cat population and density on a small tourist island and determine whether there is a population associated with the urban area and another group of individuals living in the more natural area, and (2) to estimate the home ranges of individuals living in the urban area and those living in the scrubland area.
2. Materials and Methods
2.1. Study Area
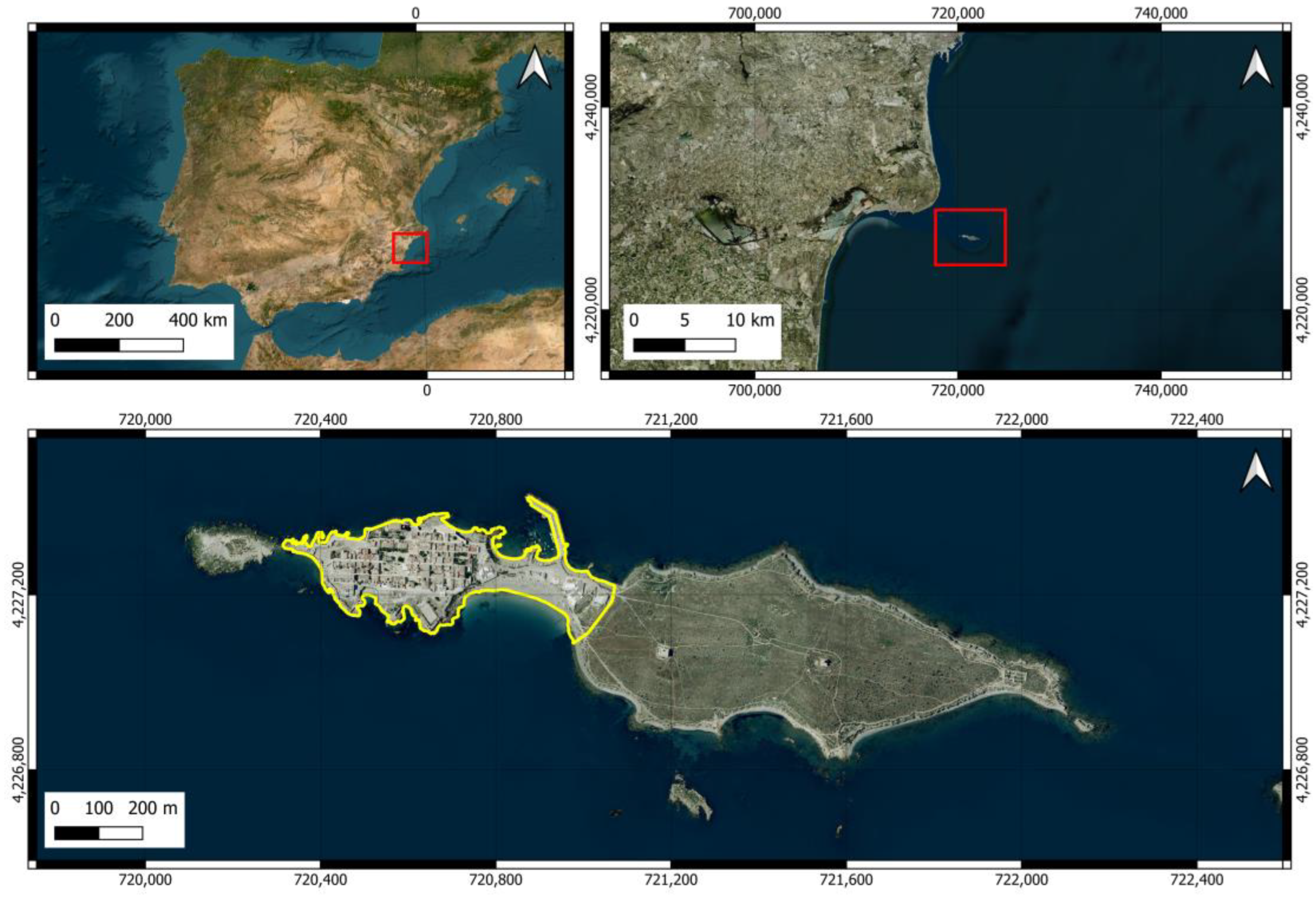
The island of Tabarca is the only inhabited island in the Valencian Community (Spain) and has a total area of approximately 40.2 hectares. It can be divided into two distinct areas: one located at the western end where the village is located (urban area covering 10.7 ha) and a larger area covered with scrubland and abandoned Opuntia crops of an extension of 29.5 (Figure 1). We have considered the urban area to include both the small village within the wall and the isthmus that connects the village with the shrubland area, as it includes various types of buildings (restaurants, official buildings, warehouses, and waste management facilities). Apart from birds, the island does not have abundant terrestrial fauna, but various species of arthropods, mollusks, and vertebrates can be observed [50]. The terrestrial flora is sparse due to the dry Mediterranean climate, strong winds, and high salinity. Although it lacks trees, it has various plant species, including the endemic Limonium furfuraceum, which is found only in the Valencian territory [51,52]. Some of the species of fauna and flora have some category of protection.
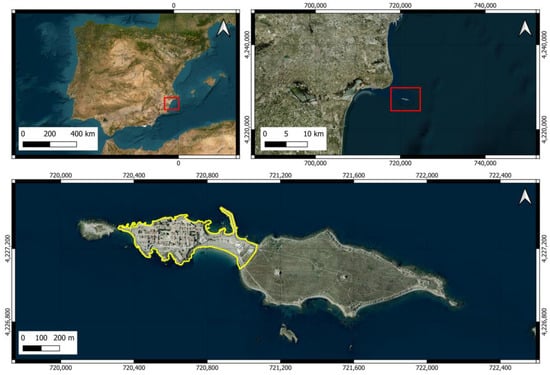
Figure 1.
Location of Tabarca Island. The area marked by the red rectangle in an image outlines the area that appears enlarged in the next one. The yellow line delineates the urban area of the island.
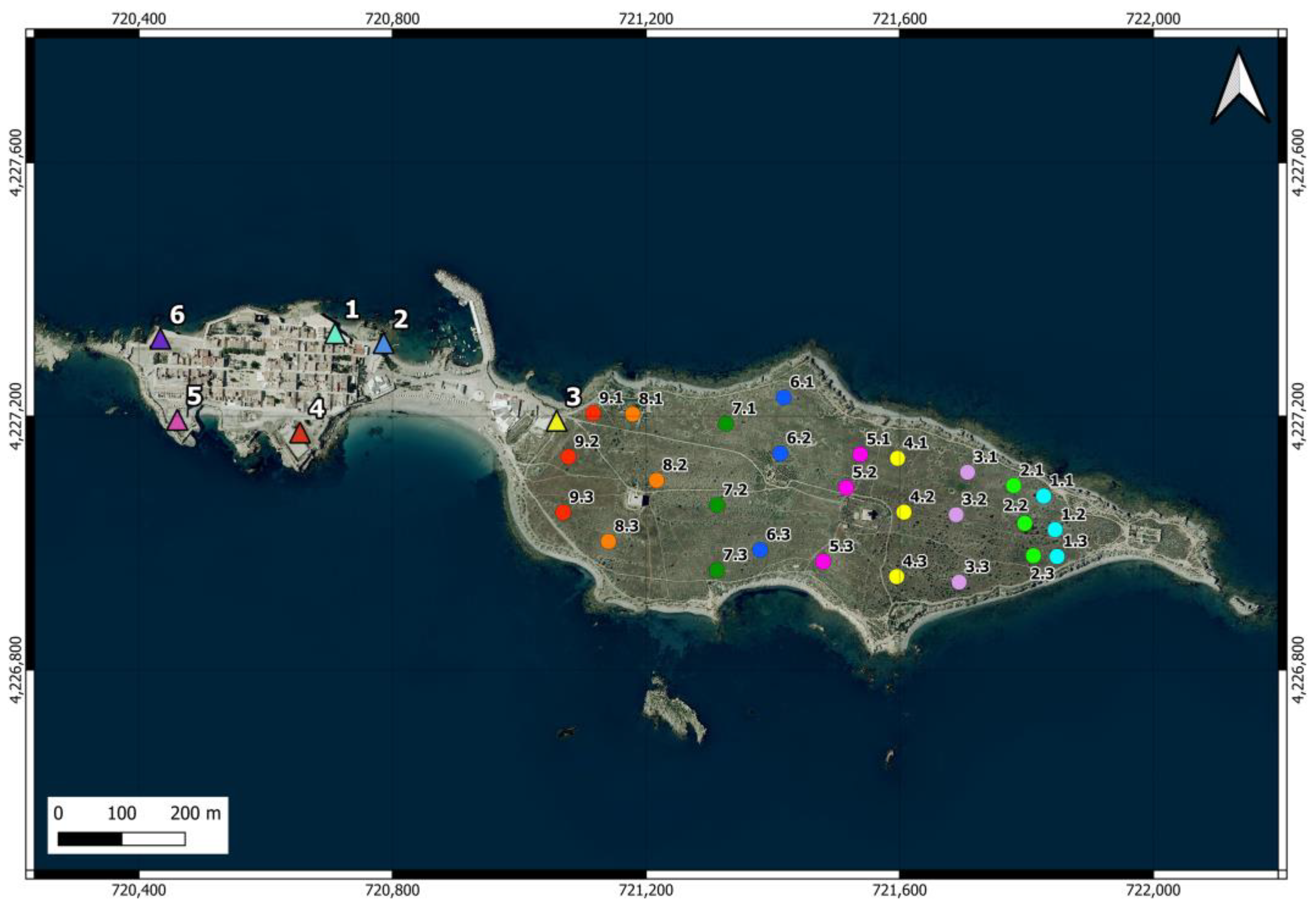
On the island of Tabarca, a community of cats has existed for decades, possibly playing a crucial role in controlling the rat population. Two caretakers are responsible for feeding and providing veterinary care to the cats. In the past, the population reached around 300 cats, many of which were unsterilized and in poor health. Between 2016 and 2017, an institutional neutering campaign widely publicized in the media neutered at least 160 cats; however, we have no record of whether other sterilizations were carried out by volunteers before that year. Despite logistical and financial limitations, the caretakers have managed the colony using the TNR method and established feeding stations. In 2021, the Alicante City Council proposed reducing feeding stations, generating opposition from caretakers. In 2023, several sterilization campaigns were carried out, sterilizing 18 cats, but some remained unsterilized.
There are 6 feeding stations on the island (one of them provisional for relocating the cats to another point) (Figure 2). These stations are equipped with plastic bottles and hoppers containing dry food and water, sheltered in structures to prevent access by other animals and provide protection. In some areas of the island, occasionally, residents and visitors also provide water, dry food, and food scraps to the cats, and some cats have even been observed feeding from garbage containers.
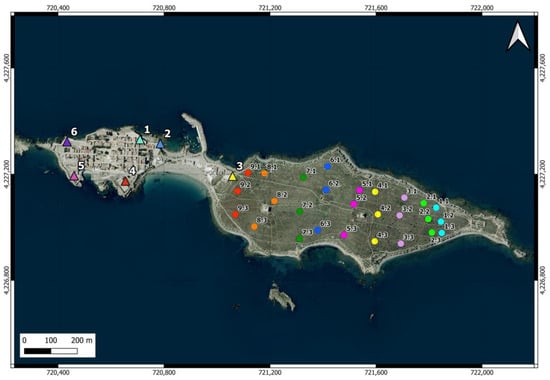
Figure 2.
Location of the feeding points of the cat colony (triangles in the urban area) and the 27 camera trap points distributed across 9 north-south oriented transects (circles in the scrubland area) on Tabarca Island. In the latter, the first number identifies the transect, and the second indicates the position. The points within the same transect, where the cameras operated simultaneously, are shown in the same color. The cameras were moved from east to west.
2.2. Counting of Urban Cats
In the urban area of the island periodic surveys were conducted at an average interval of 14.5 days, between 22 October 2022, and 18 April 2023. In total, 13 surveys were carried out, mostly during the day, except on 2 occasions when nighttime surveys were conducted. During each survey, the locations of the cats detected were georeferenced, and cats were photographed to create a database that would allow the identification of individuals based on their particular color characteristics. Neutered cats were identified by the presence of ear notches, which were placed in the left ear in females and in the right ear in males. Each survey focused on visiting as many feeding points as possible as well as the intermediate areas, especially those where the caretakers knew that the cats congregated most frequently.
2.3. GPS Monitoring of Urban Cats
Space use in the urban area was monitored by employing i-gotU GT-120 GPS data loggers (Mobile Action Technology Inc., Taipei, Taiwan) placed on cat collars with a safety mechanism. This type of device has been successfully used in several studies on cat movements [53]. Between January and February 2023, three devices were installed on neutered males, each from a different feeding point. The collars were placed by the caretaker of the feline colony on individuals, which allowed a close approach, using wet cat food as an incentive to attract them and facilitate installation without capturing the cat. No anomalous or rejection behavior toward the device was observed in the cats.
These data loggers are lightweight and affordable and have been proven to have high success rates and a positional error comparable to more expensive devices, supporting their use in studies of free-ranging animals [54,55,56]. The devices were programmed to record a position every 4 min, 24 h a day, allowing for a maximum battery life of 7 consecutive days, after which the devices were retrieved using the same food used for installation. The average time during which these devices collected data was 164.4 h (SD = 10.1, range = 153.9–174.1).
Cat 51 from feeding point No. 3 had the GPS device from 12 January 2023 to 19 January 2023. Cat 28 from point No. 5 had it from 28 January 2023 to 4 February 2023. Finally, cat 17 from point No. 1 had it from 4 February 2023 to 11 February 2023. Figure S1 shows the three individuals wearing the GPS.
2.4. Camera Traps in Scrubland Area
Camera traps were used to estimate the number of cats roaming in island scrublands [57,58,59,60]. Nine transects oriented north-south, thus perpendicular to the island’s east-west axis, were distributed in the scrubland area (Figure 2). Three cameras (1 Coolife H881 and 2 Sentinel Mini) were placed in the easternmost transect and were moved to the nearest transect in the westward direction every 14 days. The placement of the cameras started (27 December 2022) at the farthest end from the urban area to prevent the potential attraction of cats from the urban area toward the east. Camera trapping lasted until 2 May 2023. In each transect, a camera was located in the center of the island and the other two at points near the north and south coast. The distance between the two easternmost transects was 50 m, while the rest of the transects were spaced 100 m apart. The cameras were placed between 4 and 25 cm above the ground and oriented northward in locations that maximized the probability of capturing the entry or movement of the felines, avoiding pathways frequented by people and high-density vegetation. Approximately 50 g of tuna was used as bait [61].
In the first transect, the cameras were programmed with medium sensitivity to capture 3 consecutive photos with a 1-min delay between shots. After checking that the videos were more useful for identifying individual cats, cameras were programmed in the rest of the transects to record 10-s videos at medium sensitivity and with a 15-s delay. Cameras were always programmed to capture photos or videos 24 h a day.
In order to determine the number of cats using the feeding point closest to the scrubland area (Figure 2, number 3), a camera trap was installed within the infrastructure housing the food and drink containers. A Coolife H881 camera was used, positioned approximately two meters from the feeders. The camera was operated in two periods, during which it recorded 10-s videos at medium sensitivity 24 h a day. In the first period, between 27 and 28 February 2023, it was programmed with a 2 min delay, resulting in an excessive number of videos recorded over just one and a half days. In the second period, between 5 and 17 April 2023, it was programmed with a 15 min delay and recorded videos for 12 days.
2.5. Data Processing
To estimate home ranges, the minimum convex polygon (MCP) method [44,62,63] was used both in the urban and scrubland areas. MCPs were calculated in the urban zone only for cats with at least 5 records. The home range of the cats marked with GPS in the urban zone was also estimated using the kernel density estimation (KDE) method [43,64,65]. The home range area was estimated as the 95% kernel, and the core activity area as the 50% kernel. Before processing, the GPS-generated locations were filtered using the “SDLfilter” R package [66] to eliminate significant errors caused by poor signal reception or an insufficient number of satellites. The “adehabitatHR” R package [67] was used to estimate MCP and KDE. For KDE, href was used as an estimate for the bandwidth. When the home range estimated with KDE extended beyond the coastline, it was clipped using the island coastline polygon, and the areas reported were obtained after clipping. A heat map was created from all contacts obtained during the surveys to estimate the spatial variations in the intensity of cat use in the urban area. The radius used to calculate these maps (35.9 m) was derived as the mean distance between each location and the centroid of each individual, considering only individuals with at least three records. QGIS 3.22 Białowieża [68] was employed to generate the maps.
3. Results
3.1. Population Estimate
During the surveys in the urban area, 273 records were obtained, which allowed the identification of 116 individuals, consisting of 69 males (48 neutered and 21 intact) and 47 females (40 neutered and seven intact). The camera traps in the scrubland area recorded 264 images of cats, which made it possible to identify the individual in 87.8% of the cases. Using these images, eight individuals were identified (seven males: five neutered, and two intact; and one neutered female) (Figure S2) that were different from those in the urban area and were never detected there. The sex ratio was more balanced in the urban area, where the proportion of males was 59.5%, while in the scrubland area, 87.5% of the individuals were males.
In the westernmost scrubland camera trap transect, which was closest to the urban area and feeding point No. 3 (Figure 2), four additional individuals were detected that had been recorded previously during the surveys in the urban area but were not detected in the rest of the camera trap transects. Therefore, the cat population on the island has been estimated at 124 individuals (76 males and 48 females), with a total density of 308 cats/km2 (1084 cats/km2 in the urban area and 27 cats/km2 in the rural area).
At the start of the study, 94 cats were neutered (75.8%; 41 females and 53 males), and 30 were intact (24.2%; 7 females and 23 males). The caretakers of the cat colony, with the support of the Alicante City Council, conducted several capture sessions for neutering between 2, 16, and 29 March 2023. During these sessions, 18 individuals were neutered (16 from the urban area —13 males and three pregnant females—and two males from the scrubland area), increasing the neutering rate to 90.3% (89.5% of males and 91.7% of females). After these campaigns, 12 individuals remained unneutered, all from the urban area (eight males and four females). It was unknown whether some of the unneutered four females could be pregnant. During the study, the deaths of six individuals registered in the urban area were recorded (two neutered females, three neutered males, and one unneutered male), resulting in a population of 118 cats (72 males and 46 females) at the end of the study period.
3.2. Home Range
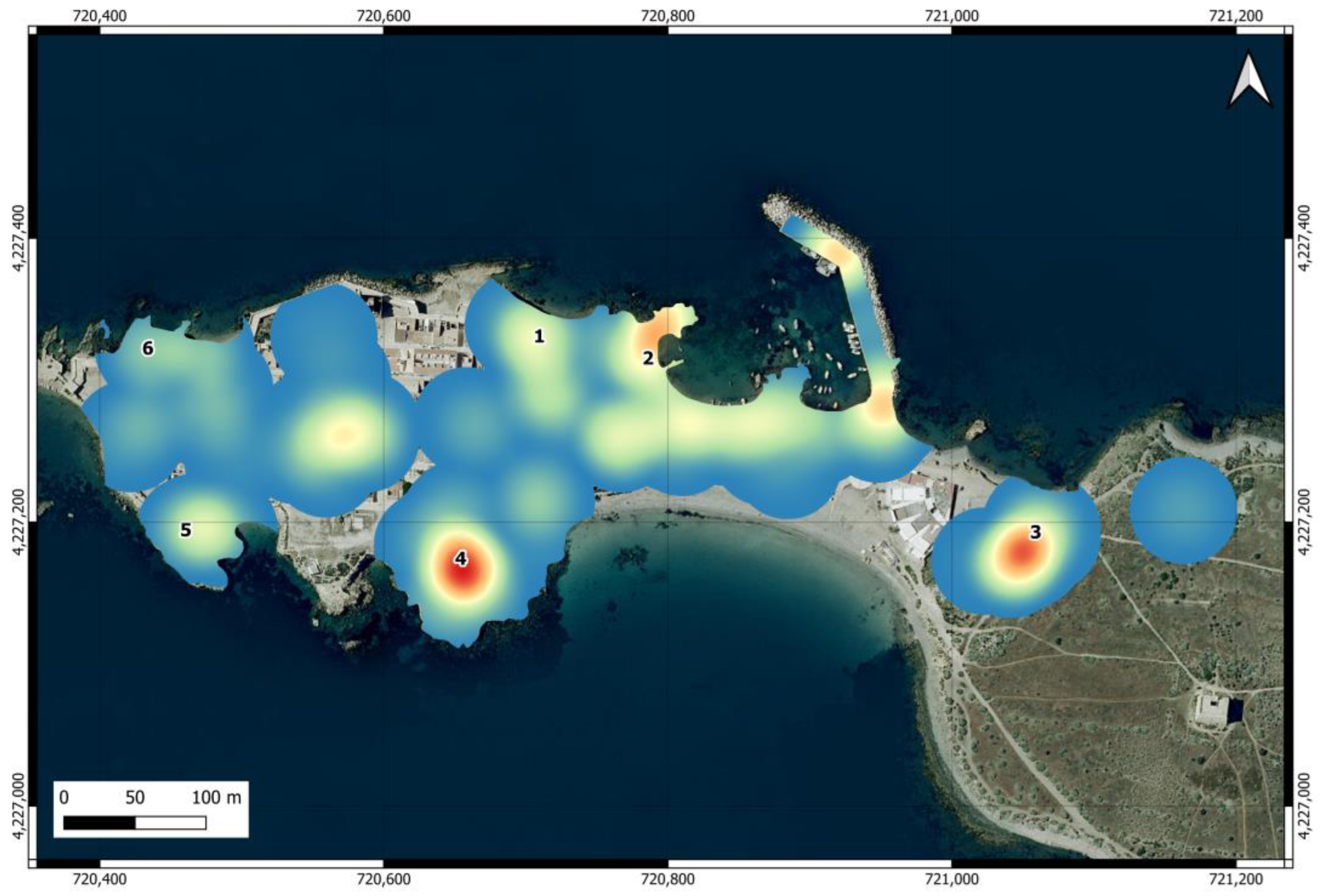
The map of the intensity of use of the urban area (Figure 3) shows that the areas near feeding points are among the most frequented by individuals, although the intensity of use varied between them and was particularly high at points 2, 3, and 4. Outside feeding points, there are other areas with high intensity of use, located at the island harbor, at the restaurants before entering the village, and at Plaza Grande, located in the center of the urban area, where cats also visit the restaurants.
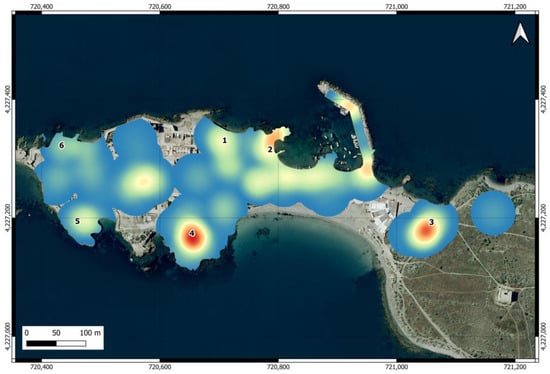
Figure 3.
Heat map of the intensity of use of the urban area of Tabarca by cats. The numbers indicate the locations of official feeding points.
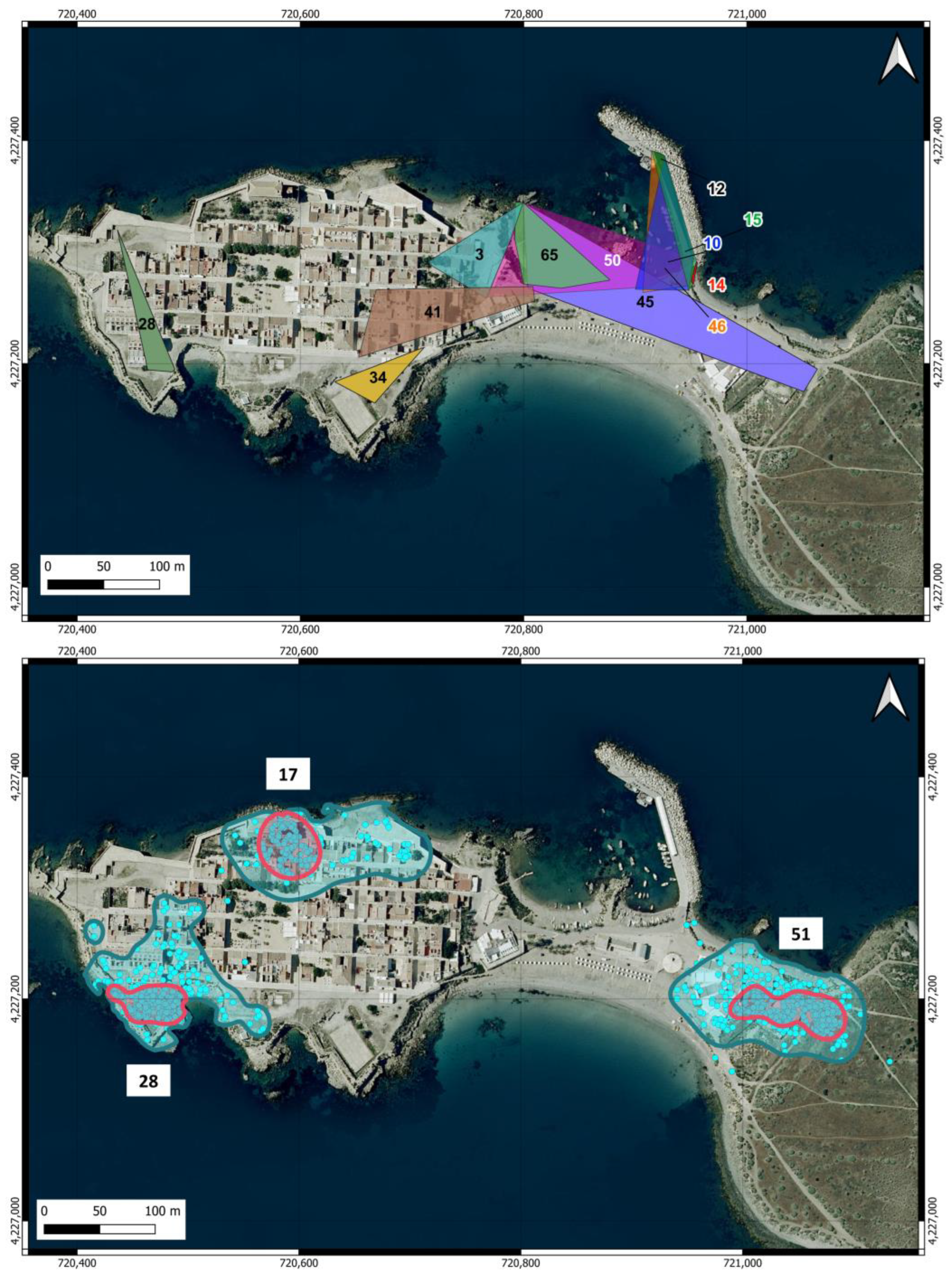
The home ranges estimated for the cats in the urban area are shown in Table 1 and Figure 4. The areas estimated from the sightings during the surveys covered a wide range (0.003–1.29 ha) and were not correlated with the number of records of the individuals (r = 0.059). The two individuals with the largest home ranges were males that were not neutered, although in 2 March 2023, one of them was neutered. The average home range estimated by this method yielded 0.38 ha (SD = 0.38) and was very similar for males (0.39 ha) and females (0.33 ha), although data from only two females were included.

Table 1.
Home range (ha) estimated using MCP (Minimum Convex Polygon) from the locations obtained during the surveys (MCP Survey, only cats detected in at least five surveys) and kernels (KDE 95% and KDE 50%) for individuals marked with GPS. For these individuals, Days show the number of days that the GPS recorded their position. MCP GPS is estimated using the GPS positions. N locs survey: number of locations obtained during the survey. N locs GPS: number of positions obtained by the GPS after filtering. Neutered: identifies cats that were neutered before the start of the study. An individual neutered during the study is marked with an asterisk.
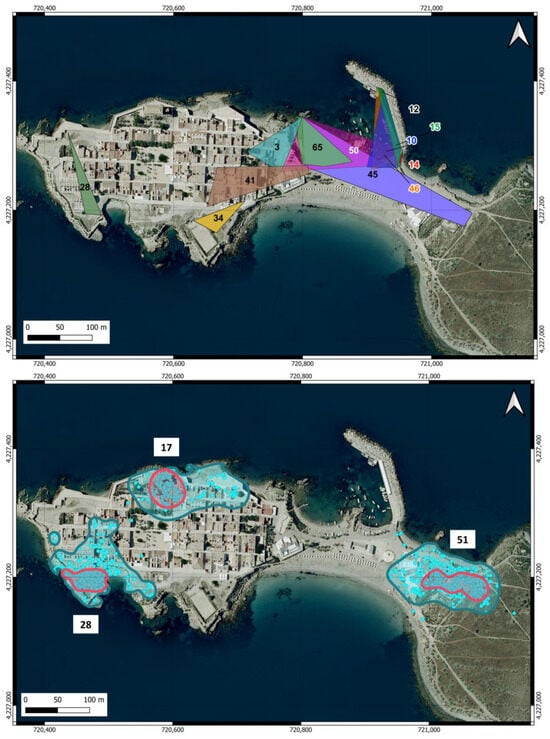
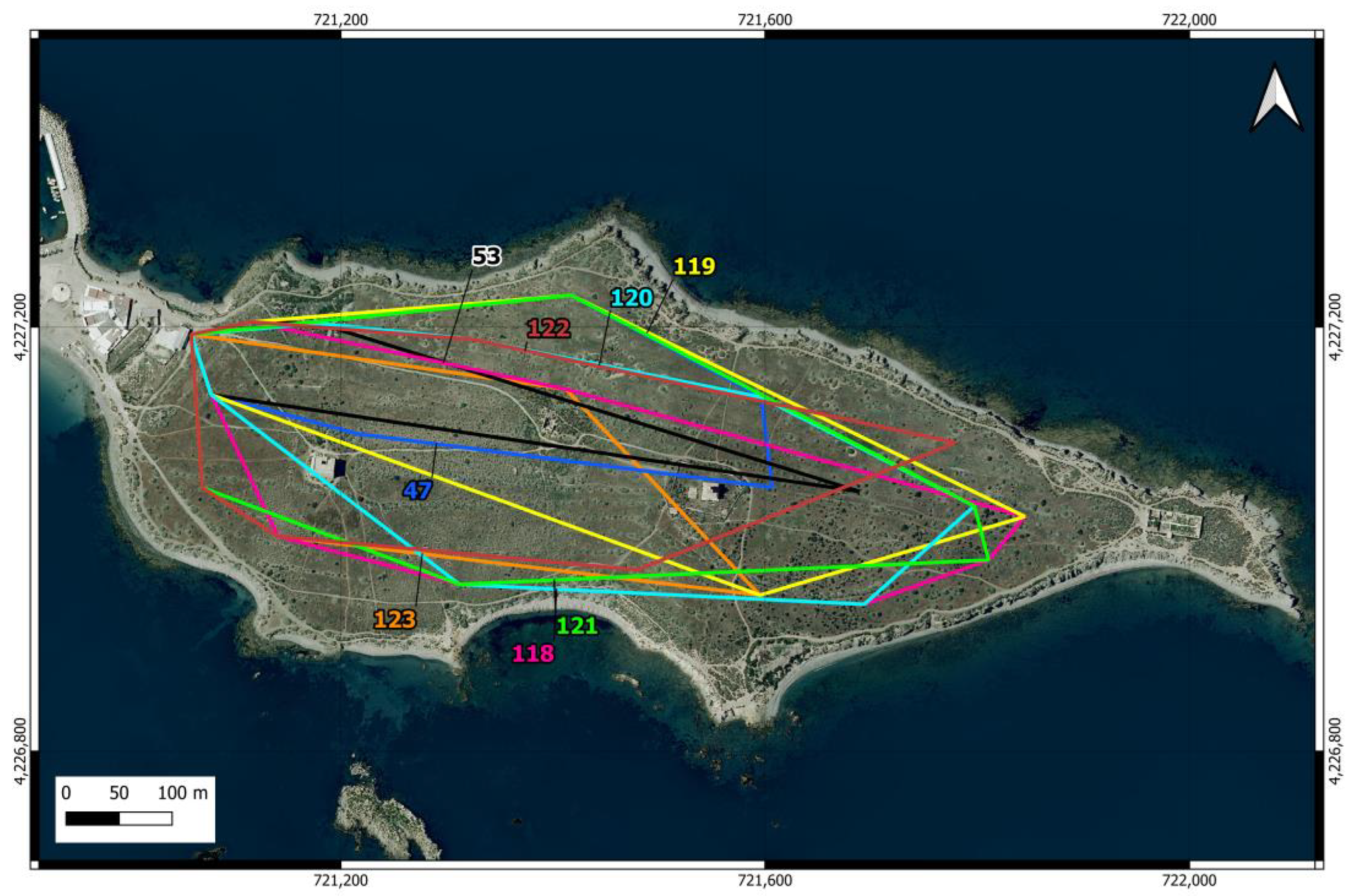
Figure 4.
Home ranges of cats in the urban area of Tabarca estimated using two methods. Upper map: MCP calculated from records of cats with at least five locations obtained during the surveys. Lower map: The home range was estimated as KDE 95% (blue line), and core areas were estimated as KDE 50% (pink line) for the three neutered male cats equipped with GPS. The blue dots show the locations used for the calculations. The numbers in both maps indicate the cats listed in Table 1.
The home ranges (KDE 95%) and core areas (KDE 50%) of the three neutered male cats equipped with GPS in the urban area are shown in Figure 4. The areas were very similar among the three individuals (Table 1) and were, on average, 1.25 ha (SD = 0.047) for the KDE 95% and 0.24 ha (SD = 0.051) for the KDE 50%. The average home range was remarkably similar to the maximum area estimated from the survey locations. There was no overlap between the home ranges of any individual, even though cats 28 and 17 moved in very close areas. The core area is located around the feeding points for cats 28 and 51, while for cat 17, it does not encompass the feeding point.
The home ranges of the eight individuals identified in the scrubland area were all larger than those estimated in the urban area, ranging from just under 3 ha to 13.9 ha (Table 2), with an average of 9.53 ha (SD = 3.65). The only female in this group was the individual with the largest home range. The individuals detected in the scrubland area covered nearly the entire available space up to the point on the island farthest from the urban area, and their home ranges overlapped extensively (Figure 5).

Table 2.
Home ranges of the cats identified in the scrubland area, estimated as the MCP of identification in camera traps. Neutered: Individuals that were neutered before the start of the study. The individuals who were sterilized during the study period are marked with an asterisk.
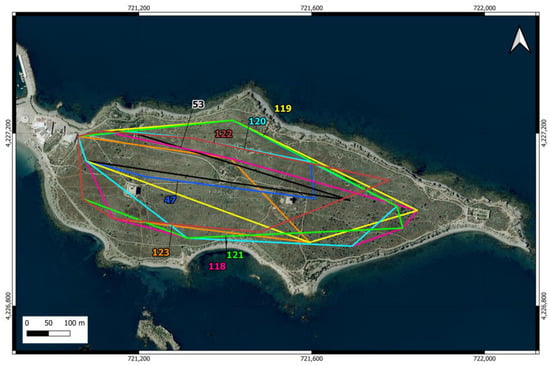
Figure 5.
Home ranges of cats in the scrubland area of Tabarca Island, estimated using MCP from locations obtained through camera trapping. Cats are identified by the same number as in Table 2.
The camera located at the feeding point near the scrubland area recorded 715 cat visits, in which the individual could be identified in 95.94% of them. Thirty individuals were identified (22 cats from the urban area and eight cats from the scrubland area). This feeding point was used by cats that moved through the eastern half of the urban area (Figure 5) and by all cats from the scrubland area. At this point, 56.6% (17/30) of the individuals were unneutered before the sterilization campaign, a percentage that decreased to 50% (6/12) after these campaigns.
4. Discussion
Our results show that Tabarca Island has one of the highest cat densities reported on islands (overall 308 cats/km2; urban zone 1084 cats/km2; scrubland zone 27 cats/km2). Due to its very small size, it has been possible to accurately estimate the density and total population of the two major habitat types present. Very few studies have estimated the cat density on islands by considering the entire area. Among the studies reviewed by Nogales et al. [69], apparently, only the study by Fitzgerald and Veitch [70] conducted on the small island of Herekopare (30 ha) in New Zealand estimated the total island population by capturing the entire population, and it reported a density of 120 cats/km2. Most studies on cat density in islands have been conducted on much larger islands, where cat density has been estimated by sampling specific areas within the islands. However, as shown by the difference in density between the two habitats in Tabarca, it is possible that densities estimated by sampling specific habitats may not be representative of total density. Many studies that have not covered the entire island report much lower densities than those found in Tabarca. For example, in the Ecological Park of Funchal on Madeira Island, the density was 1.4 cats/km2 [71], and in an area within the National Park of Réunion Island, the density was 0.25 cats/km2 [72]. In certain areas of Guadalcanal and Kolombangara (Solomon Islands), densities ranged between 0.31 and 2.65 cats/km2 and 0.65 cats/km2, respectively [73]. In their review of 27 islands where cats have been eradicated, Nogales et al. [69] found a maximum density of 243.3 cats/km2 on the small Cousine Island (30 ha) in Seychelles, although most islands had much lower densities (overall average 33.41 cats/km2). In some areas of Kangaroo Island, South Australia, the average density was 0.37 cats/km2 (0.06–3.27 cats/km2) [74]. On a peninsula at the northern tip of Auckland Island, south of New Zealand, a density between 0.7–1 cats/km2 was estimated [75].
In urban areas, the density of cats has been found to be highly variable, although values reported are generally lower than those recorded in Tabarca. In Bristol, United Kingdom, density has been estimated as 229 domestic cats/km2 [76]; in Guelph and Windsor, Canada, 0.494 and 13.3 stray cats/km2 [77,78]. Sims et al. [16] estimated a density of 417 domestic cats/km2 (132–1580 cats/km2) in urban areas of Great Britain, and McDonald and Skillings [79] estimated between 1.9 and 57 cats/km2, with an average of 9.3 cats/km2, in five urban areas of the United Kingdom. In South Korea, across six selected districts, the average density was 186.8 (132–268) stray cats/km2 [80], and in a historic neighborhood of Puerto Rico, it was 360 stray cats/km2 [81]. On Corvo Island, densities varied between the uninhabited area (3.6; 2.5–5.4 cats/km2) and the inhabited area (73.4; 58.1–92.7 domestic cats/km2) [82]. Densely populated areas tend to have more stray cats [13,18,19,76,77,79,81], as reflected in the urban area of Tabarca, which may be a source of cats in the scrubland area. In summary, Tabarca has one of the highest cat densities reported in the literature, whether considering the island as a whole or just the urban area.
The sterilization rate at the end of the study was 90.3%. Before the sterilization campaigns, the percentages were similar in the urban and scrubland areas at 75.86% (88/116) and 75% (6/8), respectively. This suggests that the high number of unsterilized cats of both sexes in the urban area could originate new litter and that this area might act as a source of individuals for the scrubland area. Although there is controversy in the scientific literature regarding the effectiveness of the TNR method for controlling cat colonies [31,35,83,84,85,86], the total sterilization rate is close to the 100% needed for the effectiveness of TNR according to Levy et al. [87] and Foley et al. [88]. Gunther et al. [85] mention that more than 70% of sterilization reduces the population by approximately 7% annually. Therefore, if this prediction holds true in Tabarca, it would take about 10 years for the cat population to be halved, and in that case, its density would still be within the high range of densities of the islands reviewed by [69].
The higher proportion of males in the scrubland area, where only one individual was female, suggests a greater tendency for males to move to this area. This occurs even if the males are neutered since most of the males in the scrubland area were neutered before the start of the study. Various studies have shown that males have, on average, larger home ranges than females, both during and outside the breeding season [89,90,91,92,93,94,95], which could increase the likelihood of males from the urban area moving to the scrubland area and establishing their home range there compared to females. Cats from the scrubland area were not detected in the urban area, except at feeding point No. 3, which is located at the border. On the contrary, four cats from the urban area (three males and one female) were detected on the scrubland camera traps closest to the village, supporting the idea that there is a main flow of individuals from the urban area to the scrubland area.
During the surveys in the urban area, it was difficult to obtain sufficient observations of individual cats to reliably estimate their home ranges. Consequently, only 12 cats (10.3% of the population), most of them males, reached the threshold of five records required to calculate the MCP. The home ranges estimated in the urban area from these surveys were very small, and in the only individual where it was also estimated using KDE, it was seven times larger than the estimate with MCP. This strongly suggests that the spatial distribution of observations during the surveys underestimates the home range. It is possible that at least part of the difference is due to the fact that most of the contacts obtained during the surveys were collected during the day (97.1%), whereas the data obtained from GPS-tagged cats encompassed the entire daily cycle. It is also likely that most of the contacts obtained in these surveys occur in places where cats are more detectable or seek human proximity, such as feeding points or restaurants.
The home ranges of all cats in the scrubland area, estimated using MCP, were larger than those of cats moving in the urban area, estimated using GPS, even though estimates obtained by MCP from camera trap data are likely to underestimate the home range because the locations are limited by the position of the cameras. Choeur et al. [91] found a similar result on Réunion Island but compared unowned cats with owned cats, where the home range of the former was nearly four times larger than the latter. In our study, all cats were unowned, and the difference lies in the area they inhabit, such that in Tabarca, urban area cats behave like owned cats, as the village is very small with six feeding points and numerous restaurants where they often feed on food scraps, similar to observations on Réunion Island [91]. Cats in the scrubland area behave like unowned cats, and their home range is about seven times larger than that of urban area cats.
Compared to other studies, cat home ranges are smaller in Tabarca, largely due to the small size of the island (40.2 ha), and much smaller than the home ranges reported in numerous studies, which can be tens or hundreds of hectares. For example, Bengsen et al. [57] reported an average home range of 511 ha on Kangaroo Island. Goltz et al. [96] observed in Mauna Kea, Hawaii, that males had larger home ranges than females (1418–2050 ha for males and 772 ha for females). In New Zealand, Nottingham et al. [95] also recorded larger home ranges for males (22.1–3232 ha) than for females (9.6–2078 ha). On Rota Island in the Mariana Islands, Leo et al. [94] found that males had home ranges of approximately 132 ha, while females occupied areas of approximately 22 ha. On Socorro Island, in the Revillagigedo Archipelago, Ortiz-Alcaraz et al. [92] recorded average home ranges of 219.1 ha for males and 118.86 ha for females. In Hawaii, Smucker et al. [93] estimated that males occupied areas of 574 ± 273 ha, compared to 223 ± 44 ha for females. The extent of these home ranges exceeds by far the size of small islands, such as Tabarca, Herekopare, or Cousin Island, which range between 30 and 40 ha. This small area relative to the potential home range that the species can achieve likely influences their behavior, forcing smaller home ranges and reducing the differences between males and females. In our study, we did not obtain enough information to compare the home ranges of males and females, but the results do not support a significant difference between the sexes in Tabarca. Conversely, on the small Japanese island of Ainoshima (125 ha), which is three times larger than Tabarca and has a similar population (130 cats), the home range, estimated using a method similar to one we used in Tabarca (MCP from visual observations obtained during surveys), showed average values of 0.78 ha (non-estrous season) and 1.45 ha (estrous season) for males and 0.44 ha and 0.61 ha in those same seasons for females [29].
The areas near feeding points are the most frequented and show a higher intensity of use, which is in line with the findings of [61]. Additionally, some areas without feeding points also show a high intensity of use due to other food sources provided by residents, tourists, and local restaurants, which also contributes to maintaining feline populations and affects home ranges [91]. This was evidenced by the presence of containers with human food scraps in the study area and the observation of foraging behavior in dumpsters [97,98]. The home ranges of the GPS-collared cats did not overlap, as each individual was associated with a feeding point and stayed very close to it despite the short distances between them. At each feeding point, the individuals that used it had overlapping ranges, as inferred from the locations of their observations during surveys. In the scrubland area, the home ranges of all individuals overlapped extensively, and no territorial behaviors were observed. Several cats were even seen together during their movements through this area.
Domestic cats are generalist predators that capture a wide variety of wild prey [7,26], even when provided with food by their owners or caretakers [99]. The most significant impacts are observed on islands [8,100,101,102], where small mammals [76,103,104], birds [26,97], reptiles [104,105], invertebrates, and amphibians [2,7,106] are particularly vulnerable. The island of Tabarca, included in the network of Special Protection Areas for birds, is a breeding area for various seabird species, as well as passerines. It used to be a breeding ground for the Kentish plover (Charadrius alexandrinus), which has recently disappeared as a breeder [107]. Additionally, Tabarca is a stopover point for migratory birds [108] that frequently arrive on the island under poor physical conditions [109]. Cats can have a potential negative impact on the island’s fauna, as migratory birds and fledglings that spend a lot of time in low vegetation, characteristic of the entire island, are especially vulnerable to predation [110]. Furthermore, the presence of cats can have other effects, such as reduced reproductive success [22], by causing nest abandonment and increasing predation risk [111,112]. They can also cause avoidance of suitable habitats [113], increase vigilance, and decrease feeding rates [114] in both adults and chicks [111]. All these effects are intensified when cat density is high, and cats hunting for birds, mammals, and reptiles in Tabarca have been reported previously or observed by the authors. Therefore, management measures should be adopted to reduce the number of cats on the island. The legislation in Spain prohibits culling as a measure of controlling cat colony size, but not their relocation [115]. The legal conditions that allow for the relocation of community cats include negative impacts on biodiversity in protected natural areas and Natura 2000 sites as well as on protected wildlife, which would be applicable in this case. Since relocating a large group of cats, like the one in Tabarca, can pose a significant logistical challenge, we propose a solution in several steps that would be easier to implement. The greatest impact on biodiversity is expected in the shrubland area; thus, we believe it is more urgent to address the problem in this zone. Therefore, we propose that the environmental and local authorities declare the shrubland area of the island as a cat exclusion zone [43]. This would involve monitoring the area with camera traps, capturing newly detected individuals, and transferring them to an authorized site. Additionally, feeding point No. 3, located on the edge of the urban area, should be moved further inside the urban zone away from the shrubland to reduce the likelihood of urban cats using this point to move into the shrubland area. Finally, given the difficulties of neutering the entire population solely through the efforts of volunteers, it is recommended that local institutions engage specialized companies for the complete sterilization of the entire urban cat population.
5. Conclusions
The island of Tabarca has one of the highest densities of cats reported in the literature, which we have estimated to be 308 cats/km2 for the entire island, reaching 1084 cats/km2 in the small urban area. Our data support that this urban area acts as a source of cats for the scrubland zone, where we estimated a density of 27 cats/km2. The home ranges of cats in Tabarca are very small compared to those estimated on larger islands, most likely due to the small size of the study island. Additionally, there are differences in the size of the home ranges of cats depending on the zone they occupy. In the urban area, cats have a much smaller home range than those in the scrubland zone. In both areas, there is extensive overlap of home ranges among different individuals, and no territorial behavior is observed, likely due to the high percentage of sterilized cats. Cats have been observed hunting birds, mammals, and reptiles in Tabarca. Given the high density of cats, measures should be taken to reduce their population, especially considering that the island is included in the Natura 2000 Network as a Special Protection Area (SPA).
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ani14162288/s1, Supplementary Figure S1. Photographs of the three cats with the GPS devices attached (from left to right, cats 51, Mía, 28, Negrito and 17, Pascual). Supplementary Figure S2. Images of the cats from the scrubland area extracted from the camera trap videos (the only female is identified with an asterisk).
Author Contributions
Conceptualization, G.M.L.-I. and S.M.-B.; methodology, G.M.L.-I. and S.M.-B.; formal analysis: S.M.-B. and G.M.L.-I.; investigation, S.M.-B. and G.M.L.-I.; data curation, S.M.-B.; writing the original draft, S.M.-B. and G.M.L.-I.; writing, reviewing, and editing, S.M.-B. and G.M.L.-I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because no cats were captured. The cats that carried GPS devices were docile individuals, which allowed the colony caretakers to touch them, and both the GPS device and the collar holding it were sold for domestic use with pets, allowing cat owners to track their pets’ movements.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
We would like to thank the caretakers of the cat colony on Tabarca Island, as this study would not have been possible without their help and advice. However, it is important to note that the discussion presented here reflects only the authors’ perspectives regarding the issues of the cat colony. We are also grateful to the Department of the Environment of the Alicante City Council for allowing us to stay at the Environmental Education Center (CEAM) in Tabarca.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Serpell, J.A. Companion Animals. In Anthrozoology: Human-Animal Interactions in Domesticated and Wild Animals; Hosey, G., Melfi, V., Eds.; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Woods, M.; Mcdonald, R.A.; Harris, S. Predation of wildlife by domestic cats Felis catus in Great Britain. Mammal Rev. 2003, 33, 174–188. [Google Scholar] [CrossRef]
- Loss, S.R.; Will, T.; Marra, P.P. The impact of free-ranging domestic cats on wildlife of the United States. Nat. Commun. 2013, 4, 1396. [Google Scholar] [CrossRef] [PubMed]
- Woinarski, J.C.Z.; Murphy, B.P.; Legge, S.M.; Garnett, S.T.; Lawes, M.J.; Comer, S.; Dickman, C.R.; Doherty, T.S.; Edwards, G.; Nankivell, A.; et al. How many birds are killed by cats in Australia? Biol. Conserv. 2017, 214, 76–87. [Google Scholar] [CrossRef]
- Mori, E.; Menchetti, M.; Camporesi, A.; Cavigioli, L.; Tabarelli De Fatis, K.; Girardello, M. License to Kill? Domestic Cats Affect a Wide Range of Native Fauna in a Highly Biodiverse Mediterranean Country. Front. Ecol. Evol. 2019, 7, 477. [Google Scholar] [CrossRef]
- Nogales, M.; Rodríguez-Luengo, J.L.; Marrero, P. Ecological effects and distribution of invasive non-native mammals on the Canary Islands. Mammal Rev. 2006, 36, 49–65. [Google Scholar] [CrossRef]
- Bonnaud, E.; Medina, F.M.; Vidal, E.; Nogales, M.; Tershy, B.; Zavaleta, E.; Donlan, C.J.; Keitt, B.; Le Corre, M.; Horwath, S.V. The diet of feral cats on islands: A review and a call for more studies. Biol. Invasions 2011, 13, 581–603. [Google Scholar] [CrossRef]
- Medina, F.M.; Bonnaud, E.; Vidal, E.; Tershy, B.R.; Zavaleta, E.S.; Josh Donlan, C.; Keitt, B.S.; Corre, M.; Horwath, S.V.; Nogales, M. A global review of the impacts of invasive cats on island endangered vertebrates. Glob. Chang. Biol. 2011, 17, 3503–3510. [Google Scholar] [CrossRef]
- Coman, B.J.; Brunner, H. Food Habits of the Feral House Cat in Victoria. J. Wildl. Manag. 1972, 36, 848. [Google Scholar] [CrossRef]
- Konecny, M.J. Food Habits and Energetics of Feral House Cats in the Galápagos Islands. Oikos 1987, 50, 24. [Google Scholar] [CrossRef]
- Loyd, K.A.T.; Hernandez, S.M.; Carroll, J.P.; Abernathy, K.J.; Marshall, G.J. Quantifying free-roaming domestic cat predation using animal-borne video cameras. Biol. Conserv. 2013, 160, 183–189. [Google Scholar] [CrossRef]
- Fitzgerald, B.M. Diet of Domestic Cats and Their Impact on Prey Populations. In The Domestic Cat: The Biology of Its Behaviour; Turner, D.C., Bateson, P., Eds.; Cambridge University Press: Cambridge, UK, 1988; pp. 123–144. [Google Scholar]
- Lepczyk, C.A.; Mertig, A.G.; Liu, J. Landowners and cat predation across rural-to-urban landscapes. Biol. Conserv. 2004, 115, 191–201. [Google Scholar] [CrossRef]
- Lohr, C.A.; Lepczyk, C.A. Desires and Management Preferences of Stakeholders Regarding Feral Cats in the Hawaiian Islands. Conserv. Biol. 2014, 28, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Wald, D.M.; Jacobson, S.K. Factors Affecting Student Tolerance for Free-Roaming Cats. Hum. Dimens. Wildl. 2013, 18, 263–278. [Google Scholar] [CrossRef]
- Sims, V.; Evans, K.L.; Newson, S.E.; Tratalos, J.A.; Gaston, K.J. Avian assemblage structure and domestic cat densities in urban environments. Divers. Distrib. 2008, 14, 387–399. [Google Scholar] [CrossRef]
- Baker, P.J.; Molony, S.E.; Stone, E.; Cuthill, I.C.; Harris, S. Cats about town: Is predation by free-ranging pet cats Felis catus likely to affect urban bird populations? Ibis 2008, 150, 86–99. [Google Scholar] [CrossRef]
- Levy, J.K.; Woods, J.E.; Turick, S.L.; Etheridge, D.L. Number of unowned free-roaming cats in a college community in the southern United States and characteristics of community residents who feed them. J. Am. Vet. Med. Assoc. 2003, 223, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Natoli, E.; Maragliano, L.; Cariola, G.; Faini, A.; Bonanni, R.; Cafazzo, S.; Fantini, C. Management of feral domestic cats in the urban environment of Rome (Italy). Prev. Vet. Med. 2006, 77, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Belaire, J.A.; Whelan, C.J.; Minor, E. S Having our yards and sharing them too: The collective effects of yards on native bird species in an urban landscape. Ecol. Appl. 2014, 24, 2132–2143. [Google Scholar] [CrossRef] [PubMed]
- Lepczyk, C.A.; Warren, P.S. (Eds.) Urban Bird Ecology and Conservation; University of California Press: Berkeley, CA, USA, 2012. [Google Scholar]
- Beckerman, A.P.; Boots, M.; Gaston, K.J. Urban bird declines and the fear of cats. Anim. Conserv. 2007, 10, 320–325. [Google Scholar] [CrossRef]
- Crooks, K.R.; Soulé, M.E. Mesopredator release and avifaunal extinctions in a fragmented system. Nature 1999, 400, 563–566. [Google Scholar] [CrossRef]
- Trouwborst, A.; McCormack, P.C.; Martínez Camacho, E. Domestic cats and their impacts on biodiversity: A blind spot in the application of nature conservation law. People Nat. 2020, 2, 235–250. [Google Scholar] [CrossRef]
- Courchamp, F.; Chapuis, J.; Pascal, M. Mammal invaders on islands: Impact, control and control impact. Biol. Rev. 2003, 78, 347–383. [Google Scholar] [CrossRef]
- Nogales, M.; Medina, F.M. Trophic ecology of feral cats (Felis silvestris f. catus) in the main environments of an oceanic archipelago (Canary Islands): An updated approach. Mamm. Biol. 2009, 74, 169–181. [Google Scholar]
- Hilton, G.M.; Cuthbert, R.J. Review article: The catastrophic impact of invasive mammalian predators on birds of the UK Overseas Territories: A review and synthesis. Ibis 2010, 152, 443–458. [Google Scholar] [CrossRef]
- Doherty, T.S.; Glen, A.S.; Nimmo, D.G.; Ritchie, E.G.; Dickman, C.R. Invasive predators and global biodiversity loss. Proc. Natl. Acad. Sci. USA 2016, 113, 11261–11265. [Google Scholar] [CrossRef] [PubMed]
- Yamane, A.; Ono, Y.; Doi, T. Home Range Size and Spacing Pattern of a Feral Cat Population on a Small Island. J. Mammal. Soc. Jpn. 1994, 19, 9–20. [Google Scholar]
- Azumi, S.; Watari, Y.; Oka, N.; Miyashita, T. Seasonal and spatial shifts in feral cat predation on native seabirds vs. non-native rats on Mikura Island, Japan. Mammal Res. 2021, 66, 75–82. [Google Scholar] [CrossRef]
- Longcore, T.; Rich, C.; Sullivan, L.M. Critical Assessment of Claims Regarding Management of Feral Cats by Trap–Neuter–Return. Conserv. Biol. 2009, 23, 887–894. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Dauphiné, N.; Bird, D.M.; Conant, S.; Cooper, R.J.; Duffy, D.C.; Hatley, P.J.; Marra, P.P.; Stone, E.; Temple, S.A. What Conservation Biologists Can Do to Counter Trap-Neuter-Return: Response to Longcore et al. Conserv. Biol. 2010, 24, 627–629. [Google Scholar] [CrossRef]
- Hostetler, M.; Wisely, S.M.; Johnson, S.; Pienaar, E.; Main, M. How Effective and Humane Is Trap-Neuter-Release (TNR) for Feral Cats? WEC423/UW468 03/2020. EDIS 2020, 2020, 8. [Google Scholar] [CrossRef]
- Gunther, I.; Finkler, H.; Terkel, J. Demographic differences between urban feeding groups of neutered and sexually intact free-roaming cats following a trap-neuter-return procedure. J. Am. Vet. Med. Assoc. 2011, 238, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Castillo, D.; Clarke, A.L. Trap/Neuter/Release Methods Ineffective in Controlling Domestic Cat “Colonies” on Public Lands. Nat. Areas J. 2003, 23, 247–253. [Google Scholar]
- Farnworth, M.J.; Watson, H.; Adams, N.J. Understanding Attitudes Toward the Control of Nonnative Wild and Feral Mammals: Similarities and Differences in the Opinions of the General Public, Animal Protectionists, and Conservationists in New Zealand (Aotearoa). J. Appl. Anim. Welf. Sci. 2014, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Parkes, J.; Fisher, P.; Robinson, S.; Aguirre-Muñoz, A. Eradication of feral cats from large islands: An assessment of the effort required for success. N. Z. J. Ecol. 2014, 38, 307–314. [Google Scholar]
- Loyd, K.A.T.; DeVore, J.L. An Evaluation of Feral Cat Management Options Using a Decision Analysis Network. Ecol. Soc. 2010, 15, 10. [Google Scholar] [CrossRef]
- Gerhold, R.W.; Jessup, D.A. Zoonotic Diseases Associated with Free-Roaming Cats. Zoonoses Public Health 2013, 60, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Lepczyk, C.A.; Lohr, C.A.; Duffy, D.C. A review of cat behavior in relation to disease risk and management options. Appl. Anim. Behav. Sci. 2015, 173, 29–39. [Google Scholar] [CrossRef]
- Domínguez Carrillo, H.; Barrios Gonzales, V.; Pérez Fernandez, Y. Algunas Zoonosis Asociadas a Los Felinos Domésticos; Universidad de Matanzas: Matanzas, Cuba, 2009. Available online: https://www.researchgate.net/publication/328925474_ALGUNAS_ZOONOSIS_ASOCIADAS_A_LOS_FELINOS_DOMESTICOS (accessed on 23 May 2024).
- Lunney, D.; Munn, A.; Meikle, W. (Eds.) Too Close for Comfort: Contentious Issues in Human-Wildlife Encounters; Royal Zoological Society of New South Wales: Mosman, Australia, 2008. [Google Scholar]
- Metsers, E.M.; Seddon, P.J.; Van Heezik, Y.M. Cat-exclusion zones in rural and urban-fringe landscapes: How large would they have to be? Wildl. Res. 2010, 37, 47. [Google Scholar] [CrossRef]
- Thomas, R.L.; Baker, P.J.; Fellowes, M.D.E. Ranging characteristics of the domestic cat (Felis catus) in an urban environment. Urban Ecosyst. 2014, 17, 911–921. [Google Scholar] [CrossRef]
- Calhoon, R.E.; Haspel, C. Urban Cat Populations Compared by Season, Subhabitat and Supplemental Feeding. J. Anim. Ecol. 1989, 58, 321. [Google Scholar] [CrossRef]
- Mirmovitch, V. Spatial Organisation of Urban Feral Cats (Felis catus) in Jerusalem. Wildl. Res. 1995, 22, 299. [Google Scholar] [CrossRef]
- Finkler, H.; Hatna, E.; Terkel, J. The influence of neighbourhood socio-demographic factors on densities of free-roaming cat populations in an urban ecosystem in Israel. Wildl. Res. 2011, 38, 235. [Google Scholar] [CrossRef]
- Stoskopf, M.K.; Nutter, F.B. Analyzing approaches to feral cat management--one size does not fit all. J. Am. Vet. Med. Assoc. 2004, 225, 1361–1964. [Google Scholar] [CrossRef] [PubMed]
- Pérez Burgos, J.M. Nueva Tabarca. Patrimonio integral en el horizonte marítimo; Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente (España): Madrid, España, 2017. [Google Scholar]
- Belenguer Barrionuevo, R. El ecosistema terrestre tabarquino: Avatares e incertidumbres. Canelobre 2012, 60, 334–345. [Google Scholar]
- Visor Cartogràfic de la Generalitat. Available online: https://visor.gva.es/visor/?idioma=es (accessed on 24 June 2024).
- Tabarca—Paisajes Turísticos Valencianos, Valiosos y Valorados. Available online: https://paisajesturisticosvalencianos.com/paisajes/tabarca/ (accessed on 23 May 2024).
- Kays, R.; Dunn, R.R.; Parsons, A.W.; Mcdonald, B.; Perkins, T.; Powers, S.A.; Shell, L.; McDonald, J.L.; Cole, H.; Kikillus, H.; et al. The small home ranges and large local ecological impacts of pet cats. Anim. Conserv. 2020, 23, 516–523. [Google Scholar] [CrossRef]
- Forin-Wiart, M.-A.; Hubert, P.; Sirguey, P.; Poulle, M.-L. Performance and Accuracy of Lightweight and Low-Cost GPS Data Loggers According to Antenna Positions, Fix Intervals, Habitats and Animal Movements. PLoS ONE 2015, 10, e0129271. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Conner, L.M. Assessment of accuracy, fix success rate, and use of estimated horizontal position error (EHPE) to filter inaccurate data collected by a common commercially available GPS logger. PLoS ONE 2017, 12, e0189020. [Google Scholar] [CrossRef] [PubMed]
- Bischof, R.; Gjevestad, J.G.O.; Ordiz, A.; Eldegard, K.; Milleret, C. High frequency GPS bursts and path-level analysis reveal linear feature tracking by red foxes. Sci. Rep. 2019, 9, 8849. [Google Scholar] [CrossRef] [PubMed]
- Bengsen, A.J.; Butler, J.A.; Masters, P. Applying home-range and landscape-use data to design effective feral-cat control programs. Wildl. Res. 2012, 39, 258. [Google Scholar] [CrossRef]
- Jones, A.L.; Downs, C.T. Managing Feral Cats on a University’s Campuses: How Many Are There and Is Sterilization Having an Effect? J. Appl. Anim. Welf. Sci. 2011, 14, 304–320. [Google Scholar] [CrossRef]
- Glen, A.; Warburton, B.; Cruz, J.; Coleman, M. Comparison of camera traps and kill traps for detecting mammalian predators: A field trial. N. Z. J. Zool. 2014, 41, 155–160. [Google Scholar] [CrossRef]
- McGregor, H.W.; Legge, S.; Potts, J.; Jones, M.E.; Johnson, C.N. Density and home range of feral cats in north-western Australia. Wildl. Res. 2015, 42, 223. [Google Scholar] [CrossRef]
- Elizondo, E.C.; Loss, S.R. Using trail cameras to estimate free-ranging domestic cat abundance in urban areas. Wildl. Biol. 2016, 22, 246–252. [Google Scholar] [CrossRef]
- Seaman, D.E.; Millspaugh, J.J.; Kernohan, B.J.; Brundige, G.C.; Raedeke, K.J.; Gitzen, R.A. Effects of Sample Size on Kernel Home Range Estimates. J. Wildl. Manag. 1999, 63, 739. [Google Scholar] [CrossRef]
- Tennent, J.; Downs, C.T. Abundance and home ranges of feral cats in an urban conservancy where there is supplemental feeding: A case study from South Africa. Afr. Zool. 2008, 43, 218–229. [Google Scholar] [CrossRef]
- Börger, L.; Franconi, N.; De Michele, G.; Gantz, A.; Meschi, F.; Manica, A.; Lovari, S.; Coulson, T. Effects of sampling regime on the mean and variance of home range size estimates. J. Anim. Ecol. 2006, 75, 1393–1405. [Google Scholar] [CrossRef]
- Laver, P.N.; Kelly, M.J. A Critical Review of Home Range Studies. J. Wildl. Manag. 2008, 72, 290–298. [Google Scholar] [CrossRef]
- Shimada, T.; Jones, R.; Limpus, C.; Hamann, M. Improving data retention and home range estimates by data-driven screening. Mar. Ecol. Prog. Ser. 2012, 457, 171–180. [Google Scholar] [CrossRef]
- Calenge, C. The package “adehabitat” for the R software: A tool for the analysis of space and habitat use by animals. Ecol. Model. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- QGIS.org. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2021. Available online: http://qgis.org (accessed on 15 April 2024).
- Nogales, M.; Martín, A.; Tershy, B.R.; Donlan, C.J.; Veitch, D.; Puerta, N.; Wood, B.; Alonso, J. A Review of Feral Cat Eradication on Islands. Conserv. Biol. 2004, 18, 310–319. [Google Scholar] [CrossRef]
- Fitzgerald, B.M.; Veitch, C.R. The cats of Herekopare Island, New Zealand; their history, ecology and affects on birdlife. N. Z. J. Zool. 1985, 12, 319–330. [Google Scholar] [CrossRef]
- Soto, E.J.; Nunes, J.; Nóbrega, E.; Palmeirim, A.F.; Rocha, R. Density and ecological drivers of free-ranging cat abundance and activity in Madeira Island, Macaronesia. Conserv. Sci. Pract. 2023, 5, e13040. [Google Scholar] [CrossRef]
- Juhasz, C.-C.; Avargues, N.; Humeau, L.; Ringler, D.; Pinet, P.; Hollinger, C.; Beaulieu, R.; Faulquier, L.; Choeur, A.; Bureau, S.; et al. Application of genetic and Spatially Explicit Capture-Recapture analyses to design adaptive feral cat control in a large inhabited island. NeoBiota 2022, 79, 51–85. [Google Scholar] [CrossRef]
- Lavery, T.H.; Alabai, M.; Holland, P.; Qaqara, C.; Vatohi, N. Feral cat abundance, density and activity in tropical island rainforests. Wildl. Res. 2020, 47, 660. [Google Scholar] [CrossRef]
- Hohnen, R.; Berris, K.; Hodgens, P.; Mulvaney, J.; Florence, B.; Murphy, B.P.; Legge, S.M.; Dickman, C.R.; Woinarski, J.C.Z. Pre-eradication assessment of feral cat density and population size across Kangaroo Island, South Australia. Wildl. Res. 2020, 47, 669. [Google Scholar] [CrossRef]
- Glen, A.; Sagar, R.L.; Brav-Cubitt, T.; Jacques, P.M. Monitoring and detection of feral cats on Auckland Island. N. Z. J. Ecol. 2022, 46, 3494. [Google Scholar] [CrossRef]
- Baker, P.J.; Bentley, A.J.; Ansell, R.J.; Harris, S. Impact of predation by domestic cats Felis catus in an urban area. Mammal Rev. 2005, 35, 302–312. [Google Scholar] [CrossRef]
- Flockhart, D.T.T.; Norris, D.R.; Coe, J.B. Predicting free-roaming cat population densities in urban areas. Anim. Conserv. 2016, 19, 472–483. [Google Scholar] [CrossRef]
- Hand, A. Estimating feral cat densities using distance sampling in an urban environment. Ecol. Evol. 2019, 9, 2699–2705. [Google Scholar] [CrossRef]
- McDonald, J.L.; Skillings, E. Human influences shape the first spatially explicit national estimate of urban unowned cat abundance. Sci. Rep. 2021, 11, 20216. [Google Scholar] [CrossRef]
- Hwang, J.; Gottdenker, N.L.; Oh, D.-H.; Nam, H.-W.; Lee, H.; Chun, M.-S. Disentangling the link between supplemental feeding, population density, and the prevalence of pathogens in urban stray cats. PeerJ 2018, 6, e4988. [Google Scholar] [CrossRef] [PubMed]
- Castro-Prieto, J.; Andrade Núñez, M. Health and Ecological Aspects of Stray Cats in Old San Juan, Puerto Rico: Baseline Information to Develop an Effective Control Program. Puerto Rico Health Sci. J. 2018, 37, 110–114. [Google Scholar]
- Oppel, S.; Hervías, S.; Oliveira, N.; Pipa, T.; Cowen, H.; Silva, C.; Geraldes, P. Estimating feral cat density on Corvo Island, Azores, to assess the feasibility of feral cat eradication. Airo 2012, 22, 3–11. [Google Scholar]
- Crawford, H.M.; Calver, M.C.; Fleming, P.A. A Case of Letting the Cat out of The Bag—Why Trap-Neuter-Return Is Not an Ethical Solution for Stray Cat (Felis catus) Management. Animals 2019, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Nutter, F.B. Evaluation of a Trap-Neuter-Return Management Program for Feral Cat Colonies: Population Dynamics, Home Ranges, and Potentially Zoonotic Diseases. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2006. [Google Scholar]
- Gunther, I.; Hawlena, H.; Azriel, L.; Gibor, D.; Berke, O.; Klement, E. Reduction of free-roaming cat population requires high-intensity neutering in spatial contiguity to mitigate compensatory effects. Proc. Natl. Acad. Sci. USA 2022, 119, e2119000119. [Google Scholar] [CrossRef]
- Boone, J.D.; Miller, P.S.; Briggs, J.R.; Benka, V.A.W.; Lawler, D.F.; Slater, M.; Levy, J.K.; Zawistowski, S. A Long-Term Lens: Cumulative Impacts of Free-Roaming Cat Management Strategy and Intensity on Preventable Cat Mortalities. Front. Vet. Sci. 2019, 6, 238. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.K.; Isaza, N.M.; Scott, K.C. Effect of high-impact targeted trap-neuter-return and adoption of community cats on cat intake to a shelter. Vet. J. 2014, 201, 269–274. [Google Scholar] [CrossRef]
- Foley, P.; Foley, J.E.; Levy, J.K.; Paik, T. Analysis of the impact of trap-neuter-return programs on populations of feral cats. J. Am. Vet. Med. Assoc. 2005, 227, 1775–1781. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Ullah, S.; Chen, L.; Ning, S.; Lu, L.; Lin, W.; Li, Z. Home Range and Activity Patterns of Free-Ranging Cats: A Case Study from a Chinese University Campus. Animals 2022, 12, 1141. [Google Scholar] [CrossRef]
- Bischof, R.; Hansen, N.R.; Nyheim, Ø.S.; Kisen, A.; Prestmoen, L.; Haugaasen, T. Mapping the “Catscape” Formed by a Population of Pet Cats with Outdoor Access. Sci. Rep. 2022, 12, 5964. [Google Scholar] [CrossRef]
- Choeur, A.; Faulquier, L.; Orlowski, S.; Dijoux, J.; Potin, G.; Bureau, S.; Guilhaumon, F.; Le Corre, M. Impacts and management of unowned and owned cats at a seabird colony on Reunion Island (Western Indian Ocean). Biol. Invasions 2022, 24, 2365–2382. [Google Scholar] [CrossRef]
- Ortiz-Alcaraz, A.; Arnaud, G.; Aguirre-Muñoz, A.; Galina-Tessaro, P.; Méndez-Sánchez, F.; Ortega-Rubio, A. Diet and home-range of the feral cat, Felis catus (Carnivora: Felidae) on Socorro Island, Revillagigedo Archipelago, Mexico. Acta Zool. Mex. 2017, 33, 482–489. [Google Scholar] [CrossRef]
- Smucker, T.D.; Lindsey, G.D.; Mosher, S.M. Home range and diet of feral cats in Hawaii forests. Pac. Conserv. Biol. 2000, 6, 229–237. [Google Scholar] [CrossRef]
- Leo, B.T.; Anderson, J.J.; Brand Phillips, R.; Ha, R.R. Home Range Estimates of Feral Cats (Felis catus) on Rota Island and Determining Asymptotic Convergence. Pac. Sci. 2016, 70, 323–331. [Google Scholar] [CrossRef]
- Nottingham, C.; Buckley, H.; Case, B.; Glen, A.; Stanley, M. Factors affecting home range size of feral cats: A meta-analysis. N. Z. J. Ecol. 2022, 46, 3476. [Google Scholar] [CrossRef]
- Goltz, D.M.; Hess, S.C.; Brinck, K.W.; Banko, P.C.; Danner, R.M. Home Range and Movements of Feral Cats on Mauna Kea, Hawai’i. Pac. Conserv. Biol. 2008, 14, 177–184. [Google Scholar] [CrossRef]
- Flores Ravelo, A.J.; Rando Reyes, J.C. Trophic Ecology of Cats (Felis catus) in Montaña de Guaza: Implications for the Conservation of the Critically Endangered Giant Lizard of Tenerife (Gallotia intermedia). Sci. Insul. Rev. Cienc. Nat. En Islas 2021, 4, 63–80. [Google Scholar] [CrossRef]
- Cove, M.V.; Gardner, B.; Simons, T.R.; Kays, R.; O’Connell, A.F. Free-ranging domestic cats (Felis catus) on public lands: Estimating density, activity, and diet in the Florida Keys. Biol. Invasions 2018, 20, 333–344. [Google Scholar] [CrossRef]
- Hernandez, S.M.; Loyd, K.A.T.; Newton, A.N.; ‘Chip’ Gallagher, M.; Carswell, B.L.; Abernathy, K.J. Activity patterns and interspecific interactions of free-roaming, domestic cats in managed Trap-Neuter-Return colonies. Appl. Anim. Behav. Sci. 2018, 202, 63–68. [Google Scholar] [CrossRef]
- Nogales, M.; Vidal, E.; Medina, F.M.; Bonnaud, E.; Tershy, B.R.; Campbell, K.J.; Zavaleta, E.S. Feral Cats and Biodiversity Conservation: The Urgent Prioritization of Island Management. BioScience 2013, 63, 804–810. [Google Scholar] [CrossRef]
- Ratcliffe, N.; Bell, M.; Pelembe, T.; Boyle, D.; Benjamin, R.; White, R.; Godley, B.; Stevenson, J.; Sanders, S. The Eradication of Feral Cats from Ascension Island and Its Subsequent Recolonization by Seabirds. Oryx 2010, 44, 20–29. [Google Scholar] [CrossRef]
- Gómez-Alceste, M.; Rando, J.C. Shifts in the trophic ecology of feral cats in the alpine ecosystem of an oceanic island: Implications for the conservation of native biodiversity. Mammal Res. 2024, 69, 1–8. [Google Scholar] [CrossRef]
- Krauze-Gryz, D.; Żmihorski, M.; Gryz, J. Annual variation in prey composition of domestic cats in rural and urban environment. Urban Ecosyst. 2017, 20, 945–952. [Google Scholar] [CrossRef]
- Galán, P. Depredación de gato doméstico sobre reptiles en Galicia. Bol. Asoc. Herpetológica Esp. 2013, 24, 103–107. [Google Scholar]
- Read, J.; Bowen, Z. Population dynamics, diet and aspects of the biology of feral cats and foxes in arid South Australia. Wildl. Res. 2001, 28, 195. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Fantle-Lepczyk, J.E.; Dunham, K.D.; Bonnaud, E.; Lindner, J.; Doherty, T.S.; Woinarski, J.C.Z. A global synthesis and assessment of free-ranging domestic cat diet. Nat. Commun. 2023, 14, 7809. [Google Scholar] [CrossRef] [PubMed]
- Bañuls Patiño, A. Chorlitejo Patinegro (Charadrius alexandrinus). In Atlas de aves nidificantes en la provincia de Alicante; López Iborra, G.M., Zaragozí Llenes, A., Sala Bernabeu, J., Izquierdo Rosique, A., Martínez Pérez, J.E., Ramos Sánchez, J., Bañuls Patiño, D., Arroyo Morcillo, S., Sánchez Zapata, J.A., Campos Roig, B., et al., Eds.; Publicaciones de la Universidad de Alicante: Alicante, España, 2015; pp. 200–201. [Google Scholar]
- López-Iborra, G.M.; Bañuls, A.; Castany, J.; Escandell, R.; Sallent, Á.; Suárez, M. Drivers of migrant passerine composition at stopover islands in the western Mediterranean. Sci. Rep. 2022, 12, 2943. [Google Scholar] [CrossRef] [PubMed]
- Gargallo, G.; Barriocanal, C.; Castany, J.; Clarabuch, O.; Escandell, R.; López-Iborra, G.; Rguibi-Idrissi, H.; Robson, D.; Suárez, M. Spring Migration in the Western Mediterranean and NW Africa: The Results of 16 Years of the Piccole Isole Project; Institut Català d’Ornitologia: Barcelona, Spain, 2013. [Google Scholar]
- Pavisse, R.; Vangeluwe, D.; Clergeau, P. Domestic Cat Predation on Garden Birds: An Analysis from European Ringing Programmes. Ardea 2019, 107, 103. [Google Scholar] [CrossRef]
- Bonnington, C.; Gaston, K.J.; Evans, K.L. Fearing the feline: Domestic cats reduce avian fecundity through trait-mediated indirect effects that increase nest predation by other species. J. Appl. Ecol. 2013, 50, 15–240. [Google Scholar] [CrossRef]
- Langston, R.H.W.; Liley, D.; Murison, G.; Woodfield, E.; Clarke, R.T. What effects do walkers and dogs have on the distribution and productivity of breeding European Nightjar Caprimulgus europaeus? Ibis 2007, 149, 27–36. [Google Scholar] [CrossRef]
- Cresswell, W. Age-dependent choice of redshank (Tringa totanus) feeding location: Profitability or risk? J. Anim. Ecol. 1994, 63, 589. [Google Scholar] [CrossRef]
- MacLeod, R.; Lind, J.; Clark, J.; Cresswell, W. Mass regulation in response to predation risk can indicate population declines. Ecol. Lett. 2007, 10, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Ley 7/2023, de 28 de Marzo, de Protección de los Derechos y el Bienestar de los Animales. Boletín Oficial del Estado, 75, de 29 de marzo de 2023. Available online: https://www.boe.es/eli/es/l/2023/03/28/7 (accessed on 26 July 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).