Predicting Conservation Status of Testudoformes under Climate Change Using Habitat Models
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Distribution Models
2.2. Occurrence Records
2.3. Environmental Predictors
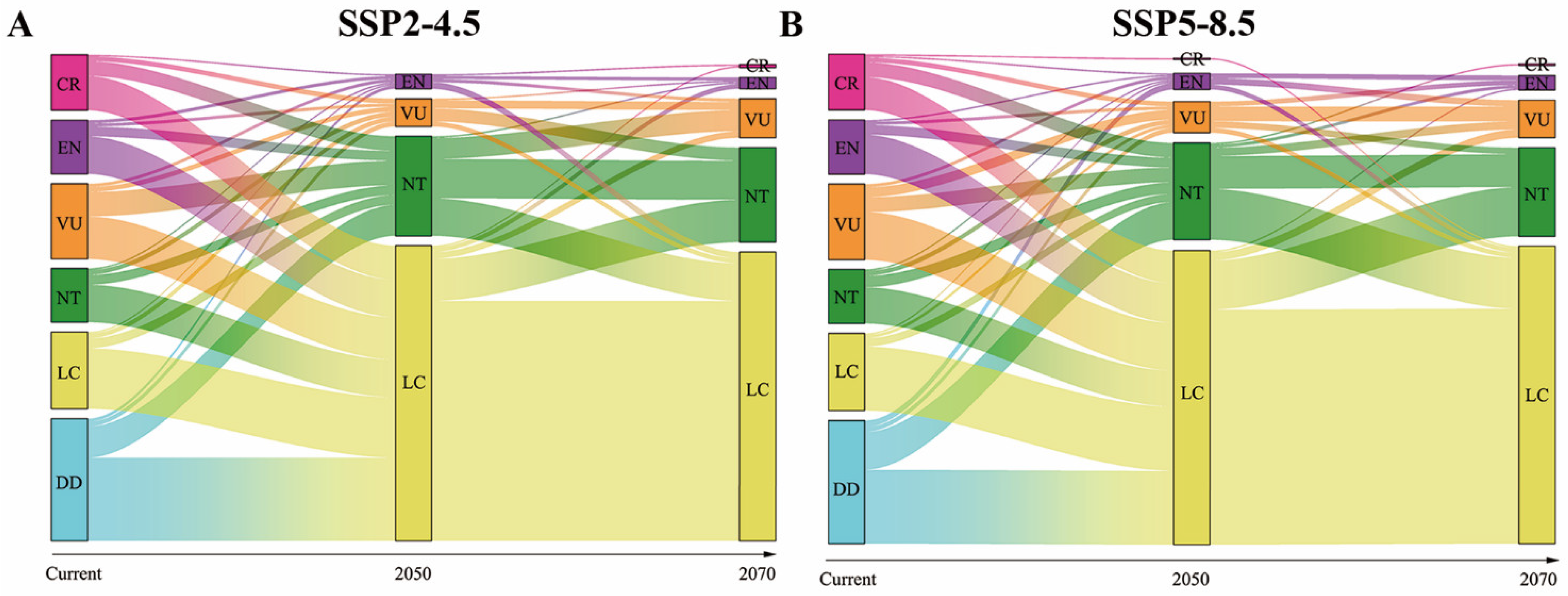
2.4. Endangerment Estimate
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lancaster, L.; Morrison, G.; Fitt, R. Life history trade-offs, the intensity of competition, and coexistence in novel and evolving communities under climate change. Philos. Trans. R. Soc. B Biol. Sci. 2017, 1712, 20160046. [Google Scholar] [CrossRef] [PubMed]
- Pecl, G.; Araújo, M.; Bell, J.; Blachard, J.; Bonebrake, T.; Chen, I.; Clark, T.; Colwell, R.; Danielsen, F.; Evengard, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 6332, eaai9214. [Google Scholar] [CrossRef] [PubMed]
- Sinervo, B.; Méndez-de-la-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Cruz, M.V.-S.; Lara-Resendiz, R.; Martínez-Méndez, N.; Calderón-Espinosa, M.L.; Meza-Lázaro, R.N.; et al. Erosion of lizard diversity by climate change and altered thermal niches. Science 2010, 328, 894899. [Google Scholar] [CrossRef]
- Xiong, Y.; Hao, Y.; Cheng, Y.; Fan, L.; Song, G.; Li, D.; Qu, Y.F.; Lei, F.M. Comparative transcriptomic and metabolomic analysis reveals pectoralis highland adaptation across altitudinal songbirds. Integr. Zool. 2022, 17, 1162–1178. [Google Scholar] [CrossRef]
- Jin, L.; Liao, W.B.; Merila, J. Genomic evidence for adaptive differentiation among Microhyla fissipes populations: Implications for conservation. Divers. Distrib. 2023, 28, 2665–2680. [Google Scholar] [CrossRef]
- Ren, Y.; Jia, T.; Zhang, H.; Zhu, W.; Wang, Z. Population genomics provides insights into the evolution and adaptation of tree shrews (Tupaia belangeri) in China. Integr. Zool. 2023, 18, 45–62. [Google Scholar] [CrossRef]
- Jin, L.; Zheng, Y.C.; Luan, X.F.; Liao, W.B. Genomic differentiation with isolation by distance along a latitudinal gradient in the spotted-leg treefrog Polypedates megacephalus. Integr. Zool. 2023, 18, 569–580. [Google Scholar] [CrossRef]
- Lei, Z.X.; Yan, C.C.; Jin, L.; Li, J.T.; Yan, C.Z.; Liao, W.B. Genomic evidence for climatic adaptation in Fejervarya multistriata. Divers. Distrib. 2024, 30, e13796. [Google Scholar] [CrossRef]
- Imakando, C.I.; Fernandez-Grandon, G.M.; Singleton, G.R.; Belmain, S.R. Impact of fertility versus mortality control on the demographics of Mastomys natalensis in maize fields. Integr. Zool. 2022, 17, 1028–1040. [Google Scholar] [CrossRef]
- Zamora-camacho, F.J. Sex and habitat differences in size and coloration of an amphibian’s poison glands match differential predator pressures. Integr. Zool. 2022, 17, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Van Linden, L.; Stoops, K.; Dumba, L.C.C.S.; Cozzuol, M.A.; Maclaren, J.A. Sagittal crest morphology decoupled from relative bite performance in Pleistocene tapirs (Perissodactyla: Tapiridae). Integr. Zool. 2023, 18, 254–277. [Google Scholar] [CrossRef] [PubMed]
- Cowie, R.H.; Bouchet, P.; Fontaine, B. The sixth mass extinction: Fact, fiction or speculation? Biol. Rev. 2022, 97, 640–663. [Google Scholar] [CrossRef] [PubMed]
- Espunyes, J.; Serrano, E.; Chaves, S.; Bartolome, J.; Menaut, P.; Albanell, E.; Marchand, P.; Foulche, K.; Garel, M. Positive effect of spring advance on the diet quality of an alpine herbivore. Integr. Zool. 2022, 17, 78–92. [Google Scholar] [CrossRef]
- Pacifici, M.; Foden, W.B.; Visconti, P.; Watson, J.E.; Butchart, S.H.; Kovacs, K.M.; Scheffers, B.R.; Hole, D.G.; Martin, T.G.; Resit Akçakaya, H.; et al. Assessing species vulnerability to climate change. Nat. Clim. Chang. 2015, 5, 215–224. [Google Scholar] [CrossRef]
- Sharnuud, R.; Ameca, E.I. Taxonomy, distribution, and contemporary exposure of terrestrial mammals to floods and human pressure across different areas for biodiversity conservation in China. Integr. Zool. 2024, 19, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Ehrlén, J.; Morris, W.F. Predicting changes in the distribution and abundance of species under environmental change. Ecol. Lett. 2015, 18, 303–314. [Google Scholar] [CrossRef]
- Tourinho, L.; Vale, M.M. Choosing among correlative, mechanistic, and hybrid models of species’ niche and distribution. Integr. Zool. 2023, 18, 93–109. [Google Scholar] [CrossRef]
- Warren, R.; VanDerWal, J.; Price, J.; Welbergen, J.A.; Atkinson, I.; Ramirez-Villegas, J.; Osborn, T.J.; Jarvis, A.; Shoo, L.P.; Williams, S.E.; et al. Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nat. Clim. Chang. 2013, 3, 678682. [Google Scholar] [CrossRef]
- Tranquillo, C.; Wauters, L.A.; Santicchia, F.; Preatoni, D.; Martinoli, A. Living on the edge: Morphological and behavioral adaptations to a marginal high-elevation habitat in an arboreal mammal. Integr. Zool. 2023, 18, 746–761. [Google Scholar] [CrossRef]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, G.; Herrel, A.; Chaverri, G.; Brown, R.P.; Russo, D.; Scaravelli, D.; Meloro, C. Functional correlates of skull shape in Chiroptera: Feeding and echolocation adaptations. Integr. Zool. 2022, 17, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L. Climate disruption and biodiversity. Curr. Biol. 2009, 19, R595–R601. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Phommachanh, C.; Ngoprasert, D.; Steinmetz, R.; Savini, T.; Gale, G.A. Habitat use of the Saola Pseudoryx nghetinhensis (Mammalia; Bovidae) based on local sightings in the Northern Annamite Mountains of Lao PDR. Trop. Conserv. Sci. 2017, 10, 1940082917713014. [Google Scholar] [CrossRef]
- Andreychev, A.; Kuznetsov, V.; Lapshin, A. Distribution and population density of the Russian desman (Desmana moschata L., Talpidae, Insectivora) in the Middle Volga of Russia. For. Study 2019, 71, 48–68. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Luan, X.F.; Liao, W.B. Anuran brain size predicts food availability-driven population density. Sci. China Life Sci. 2022, 66, 415–417. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Obregon, R.L.; Scolaro, J.A.; IbargÜengoytÍa, N.R.; Medina, M. Thermal biology and locomotor performance in Phymaturus calcogaster: Are Patagonian lizards vulnerable to climate change? Integr. Zool. 2021, 16, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Tape, K.D.; Gustine, D.D.; Ruess, R.W.; Adams, L.G.; Clark, J.A. Range expansion of moose in Arctic Alaska linked to warming and increased shrub habitat. PLoS ONE 2016, 11, e0152636. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shao, W.J.; Jiang, Y.; Yan, C.Z.; Liao, W.B. Assessing reptile conservation status under global climate change. Biology 2024, 13, 436. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Luo, T.; Wang, Y.; Wang, S.; Liu, T.; Xiao, N.; Zhou, J. Molecular phylogeny and historical biogeography of the cave fish genus Sinocyclocheilus (Cypriniformes: Cyprinidae) in southwest China. Integr. Zool. 2022, 17, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Sun, Y.; Wang, T.; Skidmore, A.K.; Ding, C.; Ye, X. Linking the past and present to predict the distribution of Asian crested ibis (Nipponia nippon) under global changes. Integr. Zool. 2022, 17, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.M.; Leadley, P.W.; Proença, V.; Alkemade, R.; Scharlemann, J.P.; Fernandez-Manjarrés, J.F.; Araújo, M.B.; Balvanera, P.; Biggs, R.; Cheung, W.W.; et al. Scenarios for global biodiversity in the 21st century. Science 2010, 330, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Hinds, L.A.; Henry, S.; Van de Weyer, N.; Robinson, F.; Ruscoe, W.A.; Brown, P.R. Acute oral toxicity of zinc phosphide: An assessment for wild house mice (Mus musculus). Integr. Zool. 2023, 18, 63–75. [Google Scholar] [CrossRef]
- Yang, S.N.; Wang, X.Y.; Hu, J.H. Mountain frog species losing out to climate change around the Sichuan Basin. Sci. Total Environ. 2022, 806, 150605. [Google Scholar] [CrossRef]
- Simpkins, G. Protecting reptiles and amphibians. Nat. Rev. Earth Environ. 2023, 4, 359. [Google Scholar] [CrossRef]
- Mi, C.; Ma, L.; Yang, M.; Li, X.; Meiri, S.; Roll, U.; Oskyrko, O.; Pincheira, D.D.; Harvey, L.P.; Jablonski, D. Global protected areas as refuges for amphibians and reptiles under climate change. Nat. Commun. 2023, 14, 1389. [Google Scholar] [CrossRef]
- Whitfield, S.M.; Bell, K.E.; Philippi, T.; Sasa, M.; Bolaños, F.; Chaves, G.; Savage, J.M.; Donnelly, M.A. Amphibian and reptile declines over 35 years at La Selve, Costa Rica. Proc. Natl. Acad. Sci. USA 2007, 104, 352–8336. [Google Scholar] [CrossRef] [PubMed]
- Currie, D.J. Projected effects of climate change on patterns of vertebrate and tree species richness in the conterminous United States. Ecosystems 2001, 4, 216–225. [Google Scholar] [CrossRef]
- Hansen, A.J.; Neilson, R.P.; Dale, V.H.; Flather, C.H.; Iverson, L.R.; Currie, D.J.; Shafer, S.; Cook, R.; Bartlein, P.J. Global change in forests: Responses of species, communities and biomes. BioScience 2001, 51, 765–779. [Google Scholar] [CrossRef]
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Kubisch, E.L.; Fernández, J.B.; Ibargüengoytía, N.R. Thermophysiological plasticity could buffer the effects of global warming on a Patagonian lizard. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2023, 339, 590–601. [Google Scholar] [CrossRef]
- Song, X.Q.; Jiang, Y.; Zhao, L.; Jin, L.; Yan, C.Z.; Liao, W.B. Predicting the potential distribution of the Szechwan rat snake (Euprepiophis perlacea) and its response to climate change in the Yingjing area of the Giant Panda National Park. Animals 2023, 13, 3390. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhou, K.Y. Overview of systematic studies of the order. Chin. J. Zool. 1998, 6, 38–45. [Google Scholar]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Shi, H.T.; Zhao, E.M.; Wang, L.J. Hainan Amphibians and Reptile Chronicles; Science Press: Beijing, China, 2011. [Google Scholar]
- Shi, H.T.; Parham, J.; Lau, M.; Chen, T. Farming endangered turtles to extinction in China. Conserv. Biol. 2007, 21, 5–6. [Google Scholar]
- McNeely, J.A. Today’s protected areas: Supporting a more sustainable future for humanity. Integr. Zool. 2020, 15, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Buonincontri, M.P.; Bosso, L.; Smeraldo, S.; Chiusano, M.L.; Pasta, S.; Di Pasquale, G. Shedding light on the effects of climate and anthropogenic pressures on the disappearance of Fagus sylvatica in the Italian lowlands: Evidence from archaeo-anthracology and spatial analyses. Sci. Total Environ. 2023, 77, 162893. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, J.R.; Huettmann, F.; Leathwick, R.J.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Krasnov, B.R.; Surkova, E.N.; Shenbrot, G.I.; Khokhlova, I.S. Latitudinal gradients in body size and sexual size dimorphism in fleas: Males drive Bergmann’s pattern. Integr. Zool. 2023, 18, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Tibatá, J.; Salaman, P.; Graham, C.H. Effects of climate change on species distribution, community structure, and conservation of birds in protected areas in Colombia. Reg. Environ. Chang. 2013, 13, 235–248. [Google Scholar] [CrossRef]
- Gutiérrez, J.A.; Duivenvoorden, J.F. Can we expect to protect threatened species in protected areas? A case study of the genus Pinus in Mexico. Rev. Mex. Biodivers. 2010, 81, 875–882. [Google Scholar]
- Hannah, L.; Midgley, G.; Andelman, S.; Araújo, M.; Hughes, G.; Martinez-Meyer, E.; Pearson, R.; Williams, P. Protected area needs in a changing climate. Front. Ecol. Environ. 2007, 5, 131–138. [Google Scholar] [CrossRef]
- Bazzichetto, M.; Malavasi, M.; Bartak, V.; Acosta, A.T.R.; Rocchini, D.; Carranza, M.L. Plant invasion risk: A quest for invasive species distribution modelling in managing protected areas. Ecol. Indic. 2018, 95, 31–319. [Google Scholar] [CrossRef]
- Deb, C.R.; Jamir, N.S.; Kikon, Z.P. Distribution prediction model of a rare orchid species (Vanda bicolor Griff.) using small sample size. Am. J. Plant Sci. 2017, 8, 1388. [Google Scholar]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Naimi, B. USDM: Uncertainty Analysis for Species Distribution Models. R Package Version 1.1–15. R Documentation. 2015. Available online: http://www.rdocu-mentation.org/packages/usdm (accessed on 15 December 2022).
- McPherson, J.; Jetz, W.; Rogers, D.J. The effects of species’ range sizes on the accuracy of distribution models: Ecological phenomenon or statistical artefact? J. Appl. Ecol. 2014, 41, 811–823. [Google Scholar] [CrossRef]
- Gong, J.; Li, Y.; Wang, R.; Yu, C.; Fan, J.; Shi, K. MaxEnt modeling for predicting suitable habitats of snow leopard (Panthera uncia) in the mid-eastern Tianshan Mountains. J. Resour. Ecol. 2023, 14, 1075–1085. [Google Scholar]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J.; Gordon, A.; Kujala, H.; Lentini, P.E.; McCarthy, M.A.; Tingley, R.; Wintle, B.A. Is my species distribution model fit for purpose? Matching data and models to applications. Glob. Ecol. Biogeogr. 2015, 24, 276–292. [Google Scholar] [CrossRef]
- Kikstra, J.S.; Nicholls, Z.R.J.; Smith, C.J.; Lewis, J.; Lamboll, R.D.; Byers, E.; Sandstad, M.; Meinshausen, M.; Gidden, M.J.; Rogelj, J.; et al. The IPCC Sixth assessment report WGIII climate assessment of mitigation pathways: From emissions to global temperatures. Geosci. Model Dev. 2022, 15, 9075–9109. [Google Scholar] [CrossRef]
- Warner, D.A.; Shine, R. The adaptive significance of temperature-dependent sex determination in a reptile. Nature 2008, 451, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.L.; Peters, A.; Kearney, M.R.; Joseph, L.; Heinsohn, R. Declining body size: A third universal response to warming? Trends Ecol. Evol. 2011, 26, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, J.; Bickford, D. Shrinking body size as an ecological response to climate change. Nat. Clim. Chang. 2011, 1, 401–406. [Google Scholar] [CrossRef]
- Liao, W.B.; Jiang, Y.; Li, D.Y.; Jin, L.; Zhong, M.J.; Qi, Y.; Lüpold, S.; Kotrschal, A. Cognition contra camouflage: How the brain mediates predator-driven crypsis evolution. Sci. Adv. 2022, 8, eabq1878. [Google Scholar] [CrossRef]
- Rubalcaba, J.G.; Gouveia, S.F.; Villalobos, F.; Olalla-Tárraga, M.Á.; Sunday, J. Climate drives global functional trait variation in lizards. Nat. Ecol. Evol. 2023, 7, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Slavenko, A.; Feldman, A.; Allison, A.; Bauer, A.M.; Böhm, M.; Chirio, L.; Colli, G.R.; Das, I.; Doan, T.M.; LeBreton, M.; et al. Global patterns of body size evolution in squamate reptiles are not driven by climate. Glob. Ecol. Biogeogr. 2019, 28, 471–483. [Google Scholar] [CrossRef]
- He, Q.Q.; Yan, S.S.; Garber, P.A.; Ren, B.P.; Qi, X.M.; Zhou, J. Habitat restoration is the greatest challenge for population recovery of Hainan gibbons (Nomascus hainanus). Integr. Zool. 2023, 18, 630–646. [Google Scholar] [CrossRef] [PubMed]
- Jadwiszczak, P.; Svensson-Marcial, A.; Mors, T. An integrative insight into the synsacral canal of fossil and extant Antarctic penguins. Integr. Zool. 2023, 18, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Arponen, A. Prioritizing species for conservation planning. Biodivers. Conserv. 2012, 21, 875–893. [Google Scholar] [CrossRef]
- Tape, K.D.; Christie, K.; Carroll, G.; O’Donnell, J.A. Novel wildlife in the Arctic: The influence of changing riparian ecosystems and shrub habitat expansion on snowshoe hares. Glob. Chang. Biol. 2016, 22, 208–219. [Google Scholar] [CrossRef]
- Brambilla, M.; Pedrini, P.; Rolando, A.; Chamberlain, D.E. Climate change will increase the potential conflict between skiing and high-elevation bird species in the Alps. J. Biogeogr. 2016, 43, 2299–2309. [Google Scholar] [CrossRef]
- Ahmadi, M.; Nawaz, M.A.; Asadi, H.; Hemami, M.R.; Naderi, M.; Shafapourtehrany, M.; Shabani, F. Protecting alpine biodiversity in the Middle East from climate change: Implications for high-elevation birds. Divers. Distrib. 2024, 30, e13826. [Google Scholar] [CrossRef]
- Ahmadi, M.; Hemami, M.R.; Kaboli, M.; Malekian, M.; Zimmermann, N.E. Extinction risks of a Mediterranean neo-endemism complex of mountain vipers triggered by climate change. Sci. Rep. 2019, 9, 6332. [Google Scholar] [CrossRef]
- Vaissi, S. Potential changes in the distributions of near eastern fire salamander (Salamandra infraimmaculata) in response to historical, recent and future climate change in the near and Middle East: Implication for conservation and management. Glob. Ecol. Conserv. 2021, 29, e01730. [Google Scholar] [CrossRef]
- Niknaddaf, Z.; Hemami, M.R.; Pourmanafi, S.; Ahmadi, M. An integrative climate and land cover change detection unveils extensive range contraction in mountain newts. Glob. Ecol. Conserv. 2023, 48, e02739. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Xin, X.; Wu, T.; Zhang, J. Introduction of CMIP5 experiments carried out with the climate system models of Beijing climate center. Adv. Clim. Chang. Res. 2013, 4, 41–49. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Kraus, F.; Tingley, R.; Li, Y. Risk of biological invasions is concentrated in biodiversity hotspots. Front. Ecol. Environ. 2016, 14, 411–417. [Google Scholar] [CrossRef]
- Voldoire, A.; Sanchez-Gomez, E.; Melia, D.S.; Decharme, B.; Cassou, C.; Senesi, S.; Valcke, S.; Beau, I.; Alias, A.; Chevallier, M.; et al. The CNRM-CM5.1 global climate model: Description and basic evaluation. Clim. Dyn. 2013, 40, 2091–2121. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, C.; Liao, W.B. Anuran interorbital distance variation: The role of ecological and behavioral factors. Integr. Zool. 2022, 17, 777–786. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2022, 8, 1–16. [Google Scholar] [CrossRef]
- Kocmánková, E.; Trnka, M.; Juroch, J.; Dubrovský, M.; Semerádová, D.; Možný, M.; Žalud, Z. Impact of climate change on the occurrence and activity of harmful organisms. Plant Prot. Sci. 2009, 45, S48–S52. [Google Scholar] [CrossRef]
- Muthoni, F.K. Modelling the Spatial Distribution of Snake Species under Changing Climate Scenario in Spain. Master’s Thesis, University of Twente, Enschede, The Netherlands, 2010. [Google Scholar]
- Muñoz, I.; Rigarlsford, G.; I Canals, L.M.; King, H. Accounting for greenhouse gas emissions from the degradation of chemicals in the environment. Int. J. Life Cycle Assess. 2013, 18, 252–262. [Google Scholar] [CrossRef]
- Della, R.F.; Milanesi, P. Combining climate, land use change and dispersal to predict the distribution of endangered species with limited vagility. J. Biogeogr. 2020, 47, 1427–1438. [Google Scholar] [CrossRef]
- Powers, R.P.; Jetz, W. Global habitat loss and extinction risk of terrestrial vertebrates under future land-use-change scenarios. Nat. Clim. Chang. 2019, 9, 323–329. [Google Scholar] [CrossRef]
- Pounds, J.A.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.L.; Foster, P.N.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 2006, 439, 161–167. [Google Scholar] [CrossRef]
- Kong, F.; Tang, L.; He, H.; Yang, F.; Tao, J.; Wang, W. Assessing the impact of climate change on the distribution of Osmanthus fragrans using Maxent. Environ. Sci. Pollut. Res. 2021, 28, 34655–34663. [Google Scholar] [CrossRef]
- Thapa, A.; Wu, R.; Hu, Y.B.; Nie, Y.G.; Singh, P.B.; Khatiwada, J.R.; Yan, L.; Gu, X.D.; Wei, F.W. Predicting the potential distribution of the endangered red panda across its entire range using MaxEnt modeling. Ecol. Evol. 2018, 8, 10542–10554. [Google Scholar] [CrossRef]
- Liao, W.B.; Cao, L.S. Conservation and evolution of wildlife in the context of climate change and human population growth. Biology 2024, 13, 448. [Google Scholar] [CrossRef]
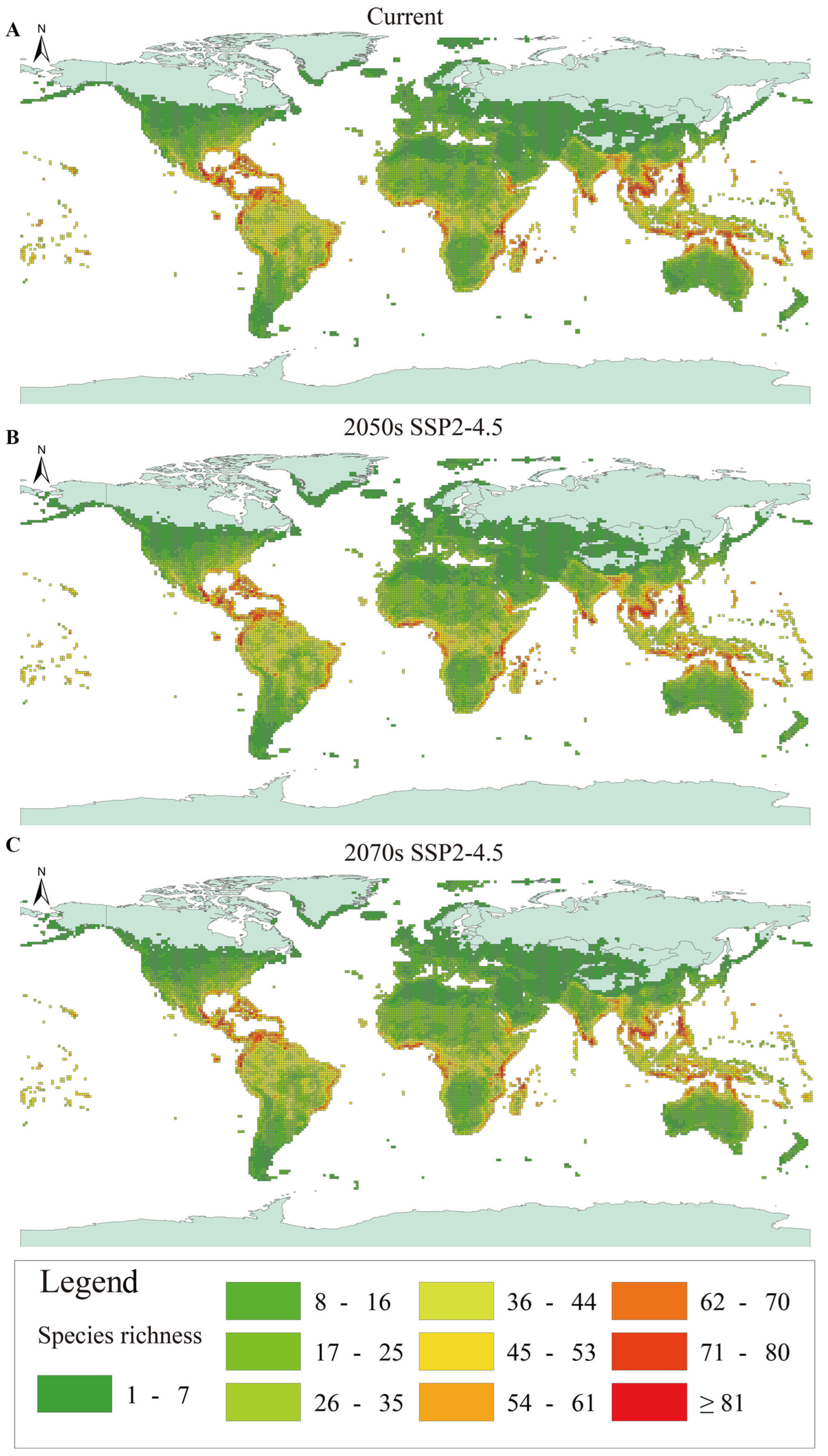

| Continent | Current | SSP2-4.5 | SSP5-8.5 | ||
|---|---|---|---|---|---|
| 2050s | 2070s | 2050s | 2070s | ||
| North America | 132 | 134 | 133 | 133 | 132 |
| South America | 49 | 49 | 50 | 51 | 51 |
| Asia | 103 | 102 | 102 | 101 | 104 |
| European | 43 | 42 | 41 | 38 | 42 |
| Oceania | 39 | 39 | 40 | 41 | 41 |
| Africa | 56 | 53 | 52 | 57 | 57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, W.; Cao, S.; Jiang, Y.; Shao, W.; Zhao, L.; Yan, C. Predicting Conservation Status of Testudoformes under Climate Change Using Habitat Models. Animals 2024, 14, 2300. https://doi.org/10.3390/ani14162300
Liao W, Cao S, Jiang Y, Shao W, Zhao L, Yan C. Predicting Conservation Status of Testudoformes under Climate Change Using Habitat Models. Animals. 2024; 14(16):2300. https://doi.org/10.3390/ani14162300
Chicago/Turabian StyleLiao, Wenbo, Shun Cao, Ying Jiang, Weijie Shao, Li Zhao, and Chengzhi Yan. 2024. "Predicting Conservation Status of Testudoformes under Climate Change Using Habitat Models" Animals 14, no. 16: 2300. https://doi.org/10.3390/ani14162300






