Identification of ActivinβA and Gonadotropin Regulation of the Activin System in the Ovary of Chinese Sturgeon Acipenser sinensis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Sample Collection
2.2. Histological Analysis
2.3. Full-Length cDNA Sequence Cloning of ActivinβA
2.4. Sequence Analysis
2.5. Tissue Distribution Analysis
2.6. Human ActivinβA and hCG Treatment with In Vitro Ovarian Cell Culture
2.7. Statistical Analysis
3. Results
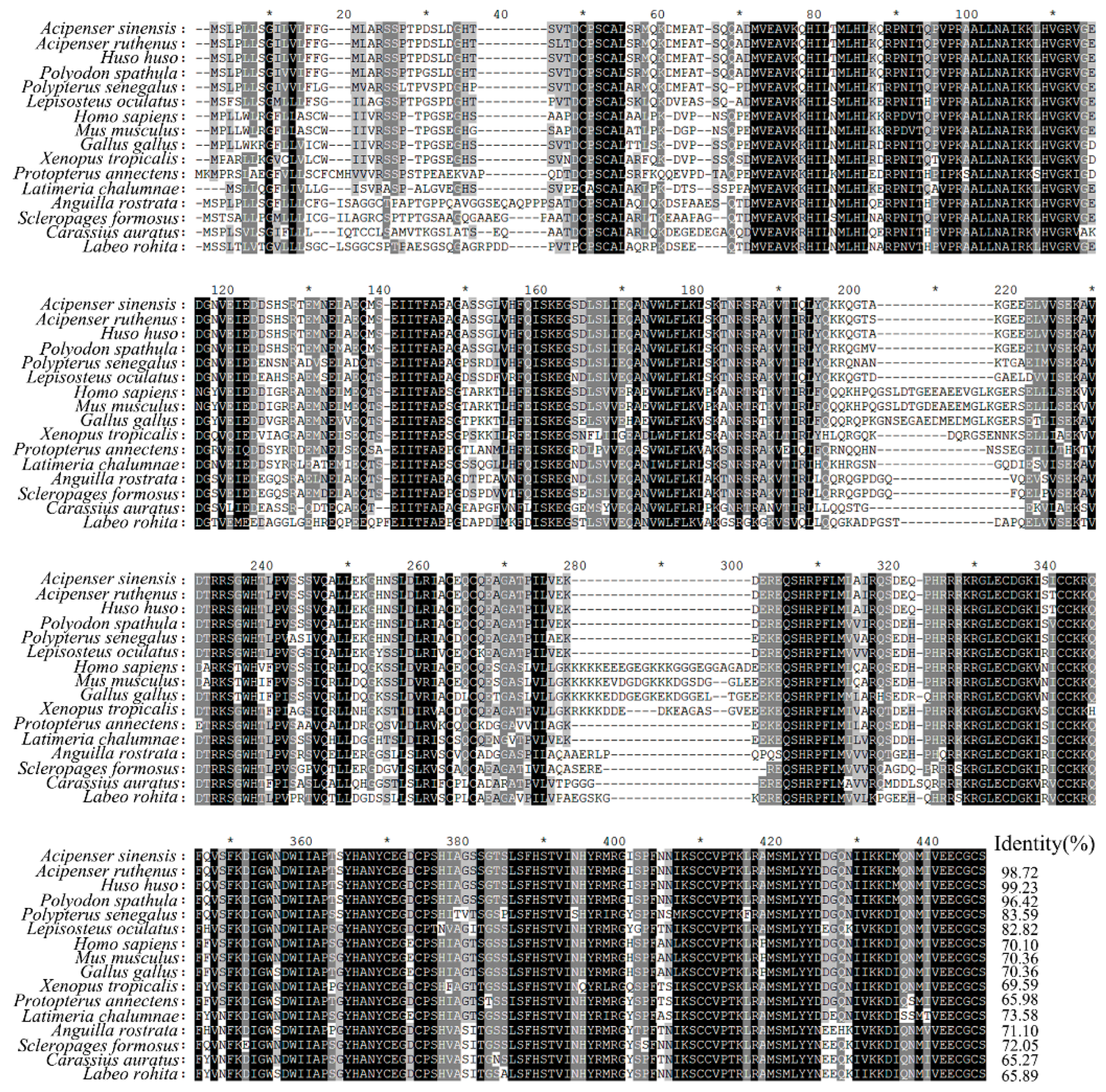
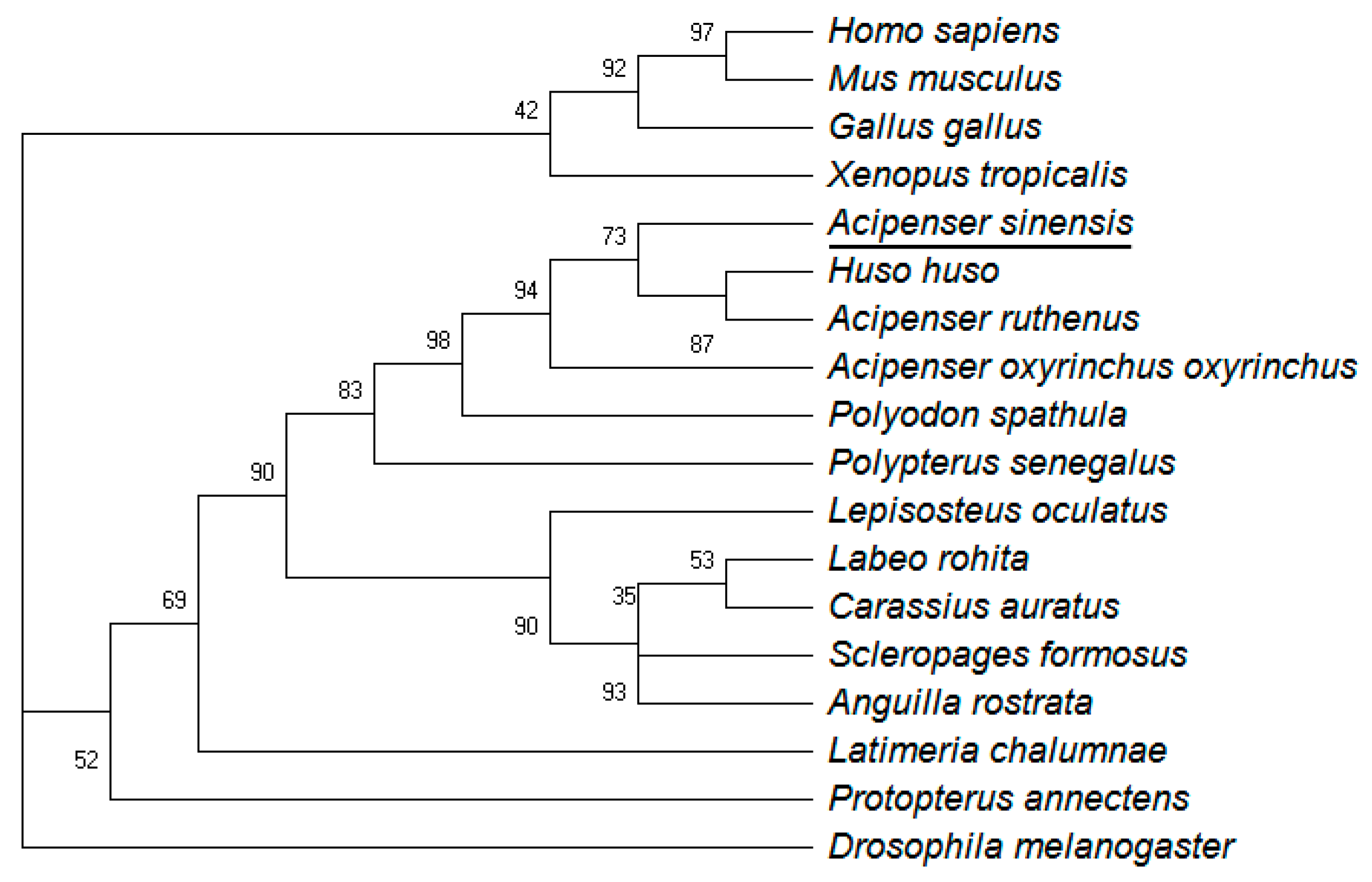
3.1. Molecular Characterization of ActivinβA in Chinese Sturgeon
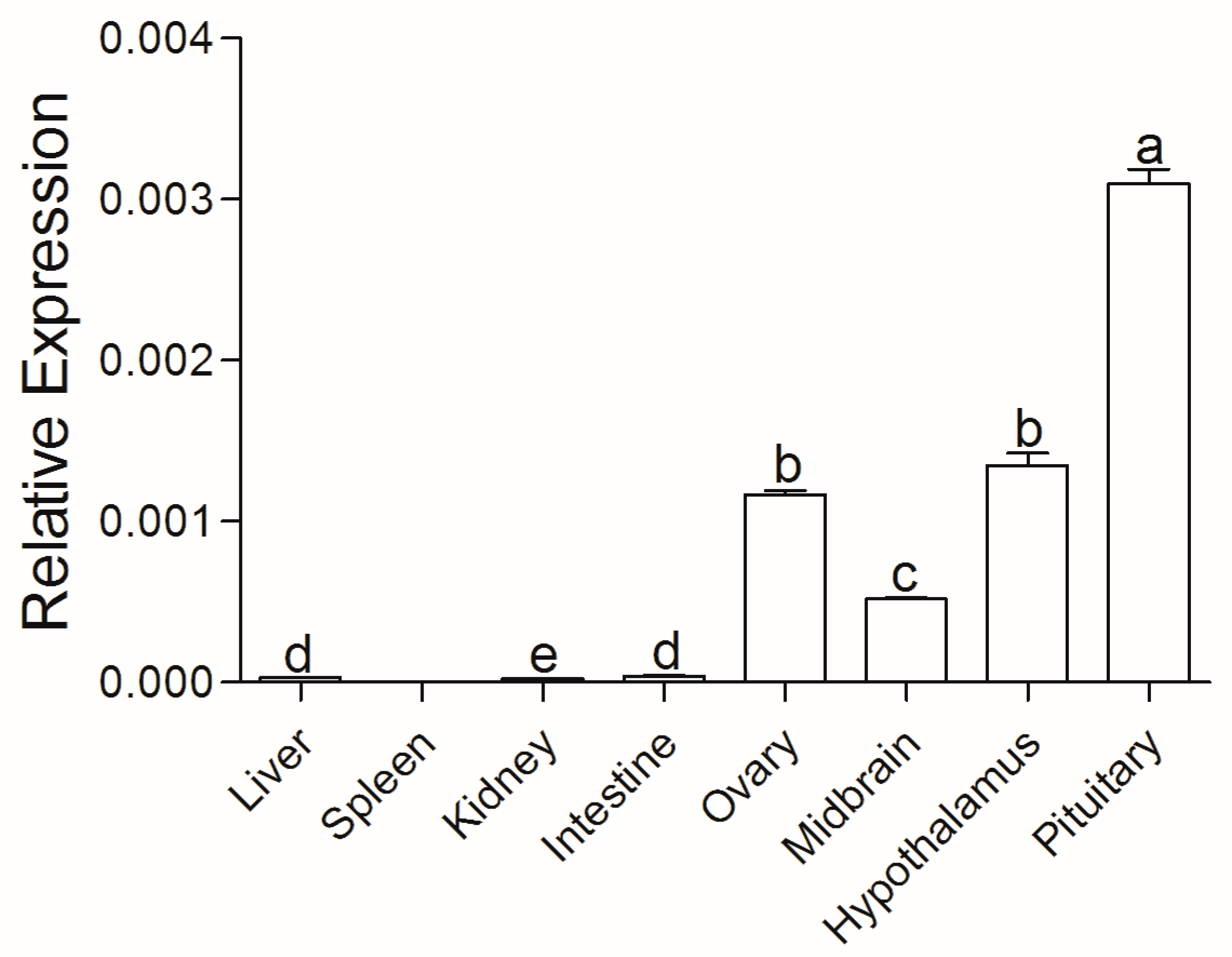
3.2. Tissue Distribution of ActivinβA in Chinese Sturgeon
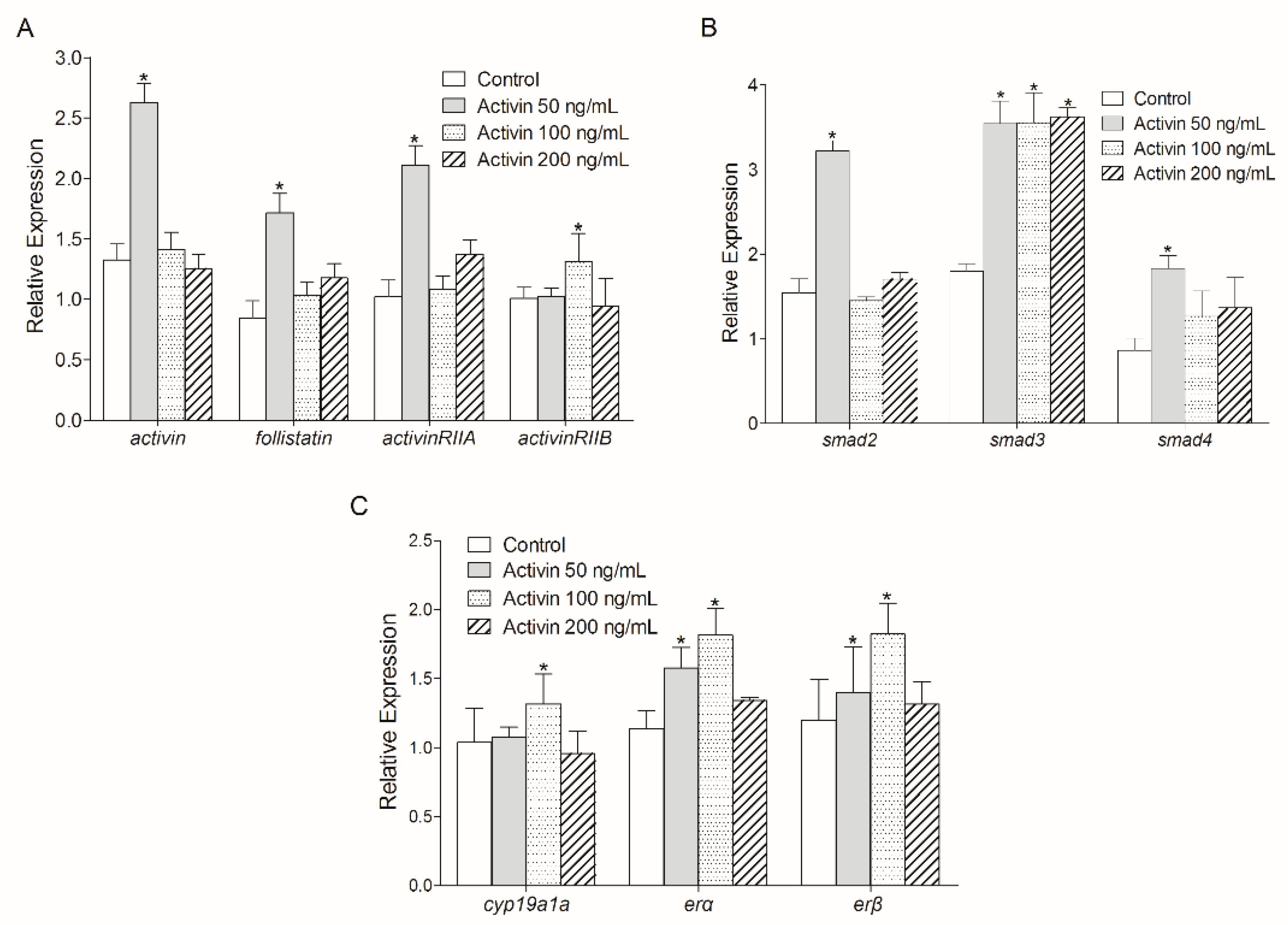
3.3. Effect of Human ActivinβA on Activin Signaling Pathway-Related Gene Transcription
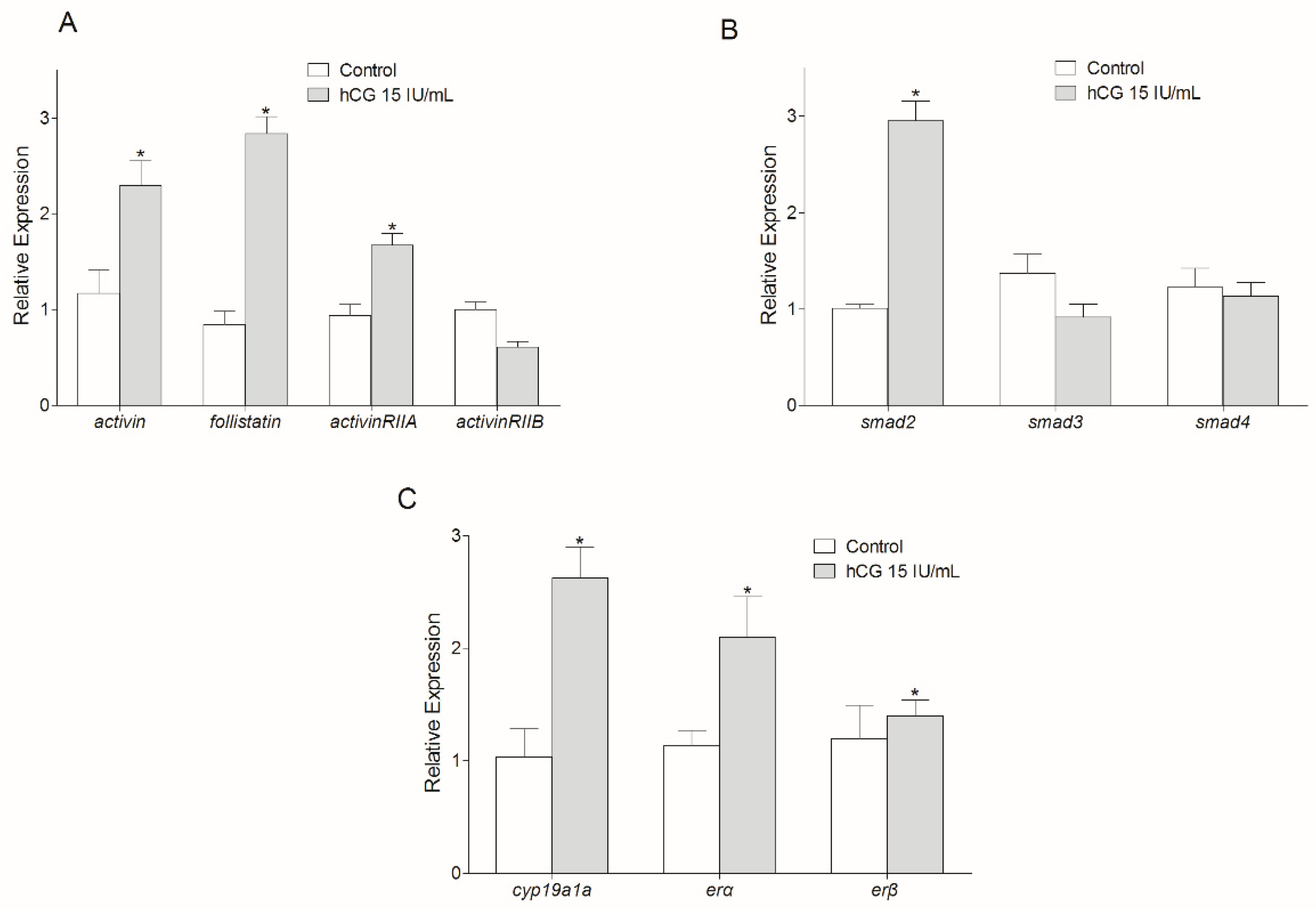
3.4. Regulation of Activin Signaling Pathway-Related Genes by Gonadotropin
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ling, N.; Ying, S.Y.; Ueno, N.; Shimasaki, S.; Esch, F.; Hotta, M.; Guillemin, R. A homodimer of the beta-subunits of inhibin A stimulates the secretion of pituitary follicle stimulating hormone. Biochem. Biophys. Res. Commun. 1986, 138, 1129–1137. [Google Scholar] [CrossRef]
- Vale, W.; Rivier, J.; Vaughan, J.; McClintock, R.; Corrigan, A.; Woo, W.; Karr, D.; Spiess, J. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature 1986, 321, 776–779. [Google Scholar] [CrossRef]
- Pangas, S.A.; Woodruff, T.K. Activin signal transduction pathways. Trends Endocrinol. Metab. 2000, 8, 1043–2760. [Google Scholar] [CrossRef]
- Abe, Y.; Minegishi, T.; Leung, P.C. Activin receptor signaling. Growth Factors 2004, 22, 105–110. [Google Scholar] [CrossRef]
- Wijayarathna, R.; de Kretser, D.M. Activins in reproductive biology and beyond. Hum. Reprod. Update 2016, 22, 342–357. [Google Scholar] [CrossRef]
- Maeshima, A.; Nojima, Y.; Kojima, I. Activin A: An autocrine regulator of cell growth and differentiation in renal proximal tubular cells. Kidney Int. 2002, 62, 446–454. [Google Scholar] [CrossRef]
- Shiozaki, S.; Tajima, T.; Zhang, Y.Q.; Furukawa, M.; Nakazato, Y.; Kojima, I. Impaired differentiation of endocrine and exocrine cells of the pancreas in transgenic mouse expressing the truncated type II activin receptor. Biochim. Biophys. Acta 1999, 1450, 1–11. [Google Scholar] [CrossRef]
- Hubner, G.; Hu, Q.; Smola, H.; Werner, S. Strong induction of activin expression after injury suggests an important role of activin in wound repair. Dev. Biol. 1996, 173, 490–498. [Google Scholar] [CrossRef]
- Wankell, M.; Munz, B.; Hubner, G.; Hans, W.; Wolf, E.; Goppelt, A.; Werner, S. Impaired wound healing in transgenic mice overexpressing the activin antagonist follistatin in the epidermis. EMBO J. 2001, 20, 5361–5372. [Google Scholar] [CrossRef]
- Sugatani, T.; Alvarez, U.M.; Hruska, K.A. Activin A stimulates IkappaB-alpha/NFkappaB and RANK expression for osteoclast differentiation, but not AKT survival pathway in osteoclast precursors. J. Cell Biochem. 2003, 90, 59–67. [Google Scholar] [CrossRef]
- Hedger, M.P.; Winnall, W.R.; Phillips, D.J.; de Kretser, D.M.; Vitam, H. The regulation and functions of activin and follistatin in inflammation and immunity. Vitam. Horm. 2011, 85, 255–297. [Google Scholar]
- Bilezikjian, L.M.; Justice, N.J.; Blackler, A.N.; Wiater, E.; Vale, W.W. Cell-type specific modulation of pituitary cells by activin, inhibin and follistatin. Mol. Cell. Endocrinol. 2012, 359, 43–52. [Google Scholar] [CrossRef]
- Hedger, M.; Winnall, W. Regulation of activin and inhibin in the adult testis and the evidence for functional roles in spermatogenesis and immunoregulation. Mol. Cell. Endocrinol. 2012, 359, 30–42. [Google Scholar] [CrossRef]
- Knight, P.G.; Satchell, L.; Glister, C. Intra-ovarian roles of activins and inhibins. Mol. Cell. Endocrinol. 2012, 359, 53–65. [Google Scholar] [CrossRef]
- Ge, W.; Miura, T.; Kobayashi, H.; Peter, R.E.; Nagahama, Y. Cloning of cDNA for goldfish activin beta B subunit, and the expression of its mRNA in gonadal and non-gonadal tissues. Mol. Endocrinol. 1997, 19, 37–45. [Google Scholar] [CrossRef]
- Tada, T.; Endo, M.; Hirono, I.; Takashima, F.; Aoki, T. Differential expression and cellular localization of activin and inhibin mRNA in the rainbow trout ovary and testis. Gen. Comp. Endocrinol. 2002, 125, 142–149. [Google Scholar] [CrossRef]
- Wang, Y.J.; Ge, W. Involvement of cyclic adenosine 3′,5′-monophosphate in the differertial regulation of activin βA and βB expression by gonadotropin in the zebrafish ovarian follicle cells. Endocrinology 2003, 144, 491–499. [Google Scholar] [CrossRef]
- Wang, Y.; Ge, W. Developmental profiles of activin βA, βB, and follistatin expression in the zebrafish ovary: Evidence for their differential roles during sexual maturation and ovulatory cycle. Biol. Reprod. 2004, 71, 2056–2064. [Google Scholar] [CrossRef]
- Yam, K.M.; Yu, K.L.; Ge, W. Cloning and characterization of goldfish activin betaA subunit. Mol. Cell. Endocrinol. 1999, 154, 45–54. [Google Scholar] [CrossRef]
- Ge, W. Intrafollicular paracrine communication in the zebrafish ovary: The state of the art of an emerging model for the study of vertebrate folliculogenesis. Mol. Cell. Endocrinol. 2005, 237, 1–10. [Google Scholar] [CrossRef]
- Li, C.W.; Ge, W. Regulation of the activin-inhibin-follistatin system by bone morphogenetic proteins in the zebrafish ovary. Biol. Reprod. 2013, 89, 55. [Google Scholar] [CrossRef]
- Wang, Y.J.; Ge, W. Spatial expression patterns of activin and its signaling system in the zebrafish ovarian follicle: Evidence for paracrine action of activin on the oocytes. Biol. Reprod. 2003, 69, 1998–2006. [Google Scholar] [CrossRef]
- Calp, M.K.; Matsumoto, J.A.; Van Der Kraak, G. Activin and transforming growth factor-β as local regulators of ovarian steroidogenesis in the goldfish. Gen. Comp. Endocrinol. 2003, 132, 142–150. [Google Scholar] [CrossRef]
- Pang, Y.; Ge, W. Activin stimulation of zebrafish oocyte maturation in vitro and its potential role in mediating gonadotropin-induced oocyte maturation. Biol. Reprod. 1999, 61, 987–992. [Google Scholar] [CrossRef]
- Wu, T.T.; Patel, H.; Mukai, S.; Melino, C.; Garg, R.; Ni, X.Y.; Chang, J.B.; Peng, C. Activin, inhibin, and follistatin in zebrafish ovary: Expression and role in oocyte maturation. Biol. Reprod. 2000, 62, 1585–1592. [Google Scholar] [CrossRef]
- Pang, Y.F.; Ge, W. Gonadotropin regulation of activin βA and activin type IIA receptor expression in the ovarian follicle cells of the zebrafish. Mol. Cell. Endocrnol. 2002, 188, 195–205. [Google Scholar] [CrossRef]
- Kwok, H.F.; So, W.K.; Wang, Y.J.; Ge, W. Zebrafish gonadotropins and their receptors: 1. Cloning and characterization of zebrafish follicle-stimulating hormone and luteinizing hormone receptors-evidence for their distinct functions in follicle development. Biol. Reprod. 2005, 72, 1370–1381. [Google Scholar] [CrossRef]
- Wei, Q.W.; Ke, F.E.; Zhang, J.M.; Zhuang, P.; Luo, J.D.; Zhou, R.Q.; Yang, W.H. Biology, fisheries, and conservation of sturgeons and paddlefish, in China. Environ. Biol. Fish. 1997, 48, 241–255. [Google Scholar] [CrossRef]
- Wei, Q.W. Conservation of Chinese sturgeon (Acipenser sinensis) based on its life history: Dilemma and breakthrough. J. Lake Sci. 2020, 5, 1297–1319. (In Chinese) [Google Scholar]
- Wei, Q.W.; Li, L.X.; Du, H.; Zhang, X.Y.; Xiong, W.; Zhang, H.; Shen, L.; Wu, J.M.; Zhang, S.H.; Wang, C.Y.; et al. Research on technology for controlled propagation of cultured Chinese sturgeon (Acipenser sinensis). J. Fish. Sci. Chin. 2013, 1, 1–11. (In Chinese) [Google Scholar] [CrossRef]
- Yue, H.M.; Li, C.J.; Du, H.; Zhang, S.H.; Wei, Q.W. Sequencing and De Novo Assembly of the Gonadal Transcriptome of the Endangered Chinese Sturgeon (Acipenser sinensis). PLoS ONE 2015, 10, e0127332. [Google Scholar] [CrossRef]
- Wu, M.B.; Ye, H.; Yue, H.M.; Ruan, R.; Du, H.; Zhou, C.L.; Xiang, H.; Li, C.J. Identification of reference genes for qRT-PCR in Dabry’s sturgeon, Acipenser dabryanus. J. Fish. Sci. Chin. 2020, 7, 759–770. (In Chinese) [Google Scholar]
- Yuen, C.W.; Ge, W. Follistatin suppresses FSHβ but increases LHβ expression in the goldfish: Evidence for an activin-mediated autocrine/paracrine system in fish pituitary. Gen. Comp. Endocrinol. 2004, 135, 108–115. [Google Scholar] [CrossRef]
- Yue, H.M.; Ye, H.; Ruan, R.; Du, H.; Wei, Q.W.; Li, C.J. Intraperitoneal injection of 17 beta-estradiol increases ovarian smad2/3 expression in Yangtze sturgeon Acipenser dabryanus. Aquac. Res. 2022, 53, 3059–3068. [Google Scholar] [CrossRef]
- Meunier, H.; Rivier, C.; Evans, R.M.; Vale, W. Gonadal and extragonadal expression of inhibin α, βA, and βB subunits in various tissues predicts diverse functions. Proc. Natl. Acad. Sci. USA 1988, 85, 247–251. [Google Scholar] [CrossRef]
- Bilezikjian, L.M.; Corrigan, A.Z.; Vale, W. Activin-A modulates growth hormone secretion from cultures of rat anterior pituitary cells. Endocrinology 1990, 126, 2369–2376. [Google Scholar] [CrossRef]
- Munz, B.; Tretter, Y.P.; Hertel, M.; Engelhardt, F.; Alzheimer, C.; Werner, S. The roles of activins in repair processes of the skin and the brain. Mol. Cell. Endocrinol. 2001, 180, 169–177. [Google Scholar] [CrossRef]
- Tada, T.; Hirono, I.; Aoki, T.; Takashima, F. Structure and expression of activin genes in rainbow trout. Mol. Mar. Biol. Biotechnol. 1998, 7, 72–77. [Google Scholar]
- DiMuccio, T.; Mukai, S.T.; Clelland, E.; Kohli, G.; Cuartero, M.; Wu, T.T.; Peng, C. Cloning of a second form of activin-βA cDNA and regulation of activin-βA subunits and activin type II receptor mRNA expression by gonadotropin in the zebrafish ovary. Gen. Comp. Endocr. 2005, 143, 287–299. [Google Scholar] [CrossRef]
- Fung, R.S.K.; Bai, J.; Yuen, K.W.Y.; Wong, A.O.L. Activin/follistatin system in grass carp pituitary cells: Regulation by local release of growth hormone and luteinizing hormone and its functional role in growth hormone synthesis and secretion. PLoS ONE 2017, 12, e0179789. [Google Scholar] [CrossRef]
- Bilezikjian, L.M.; Blount, A.L.; Leal, A.M.O.; Donaldson, C.J.; Fischer, W.H.; Vale, W.W. Autocrine/paracrine regulation of pituitary function by activin, inhibin and follistatin. Mol. Cell. Endocrinol. 2004, 225, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Tanaka, M.; Yoshikuni, M.; Eto, Y.; Nagahama, Y. Cloning and characterization of goldfish activin type IIB receptor. J. Mol. Endocrinol. 1997, 19, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Song, C.L.; Wang, X.Y.; Zhou, H. Molecular cloning of activin type I and type II receptors and differential regulation of their expression by activin in grass carp pituitary cells. Gen. Comp. Endocr. 2010, 166, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Yaron, Z.; Gur, G.; Melamed, P.; Rosenfeld, H.; Levavi-Sivan, B.; Elizur, A. Regulation of gonadotropin subunit genes in tilapia. Comp. Biochem. Physiol. B 2001, 129, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.K.; D’Agostino, J.; Schwartz, N.B.; Mayo, K.E. Dynamic changes in inhibin messenger RNAs in rat ovarian follicles during the reproductive cycle. Science 1988, 239, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Turner, I.M.; Saunders, P.T.; Shimasaki, S.; Hillier, S.G. Regulation of inhibin subunit gene expression by FSH and estradiol in cultured rat granulosa cells. Endocrinology 1989, 125, 2790–2792. [Google Scholar] [CrossRef]
- Eramaa, M.; Tuuri, T.; Hilden, K.; Ritvos, O. Regulation of inhibin alpha- and beta A-subunit messenger ribonucleic acid levels by chorionic gonadotropin and recombinant follicle-stimulating hormone in cultured human granulosa–luteal cells. J. Clin. Endocrinol. Metab. 1994, 79, 1670–1677. [Google Scholar]
| Primer | Sequence (5′-3′) |
|---|---|
| activin-F1 | ATGTCCTTGCCTCTGCTGAGTG |
| activin-F2 | GTCCTCTGGCACCTCTCTCTCCTT |
| activin-R1 | AGAGCAGCCACATTCCTCTACTATCA |
| activin-R2 | CATTCTGGAGAGGGCACAAGAGGGG |
| UPM | CTAATACGACTCACTATAGGGCAAGCAGTGGTAT CAACGCAGAGT |
| UPMS | CTAATACGACTCACTATAGGGC |
| activin-rtF | GGACAGAGATGAATGAGTTGGCT |
| activin-rtR | AGCTTGAGAAAGAGCCAGACGT |
| follistatin-rtF | ACCAATAATGCCTACTGCGTGA |
| follistatin-rtR | TCCCTCGTATGCCACTCCTATC |
| activinRIIA-rtF | ATGTTGGTCTTGCCCTCTTCC |
| activinRIIA-rtR | AGGTCATCTTTCCCTGTTCGT |
| activinRIIB-F | GGATGCCTTCCTCAGAATAGA |
| activinRIIB-R | ATGTTTCAGCCAGCAGTCCTT |
| smad4-rF | AGGATTGACATTACAAAGTTCTGCT |
| smad4-rR | GACTCTGAAGGCGGTAGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, H.; Ye, H.; Ruan, R.; Du, H.; Li, C. Identification of ActivinβA and Gonadotropin Regulation of the Activin System in the Ovary of Chinese Sturgeon Acipenser sinensis. Animals 2024, 14, 2314. https://doi.org/10.3390/ani14162314
Yue H, Ye H, Ruan R, Du H, Li C. Identification of ActivinβA and Gonadotropin Regulation of the Activin System in the Ovary of Chinese Sturgeon Acipenser sinensis. Animals. 2024; 14(16):2314. https://doi.org/10.3390/ani14162314
Chicago/Turabian StyleYue, Huamei, Huan Ye, Rui Ruan, Hao Du, and Chuangju Li. 2024. "Identification of ActivinβA and Gonadotropin Regulation of the Activin System in the Ovary of Chinese Sturgeon Acipenser sinensis" Animals 14, no. 16: 2314. https://doi.org/10.3390/ani14162314






