Nutrient Utilization and Gut Microbiota Composition in Giant Pandas of Different Age Groups
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Chemical Analysis and Apparent Digestibility Calculation
2.3. DNA Extraction and Amplicon Sequencing
2.4. Statistical Analysis
3. Results
3.1. Composition and Nutrient Level of GPs’ Diet
3.2. Apparent Nutrient Digestibility in GPs
3.3. Comparisons of Microbiota Diversity in Different Age of GPs
3.4. Comparisons of Microbiota Community Composition in Different Age Groups of GPs
3.5. Comparisons of Microbiota Function in Different Age Groups of GPs
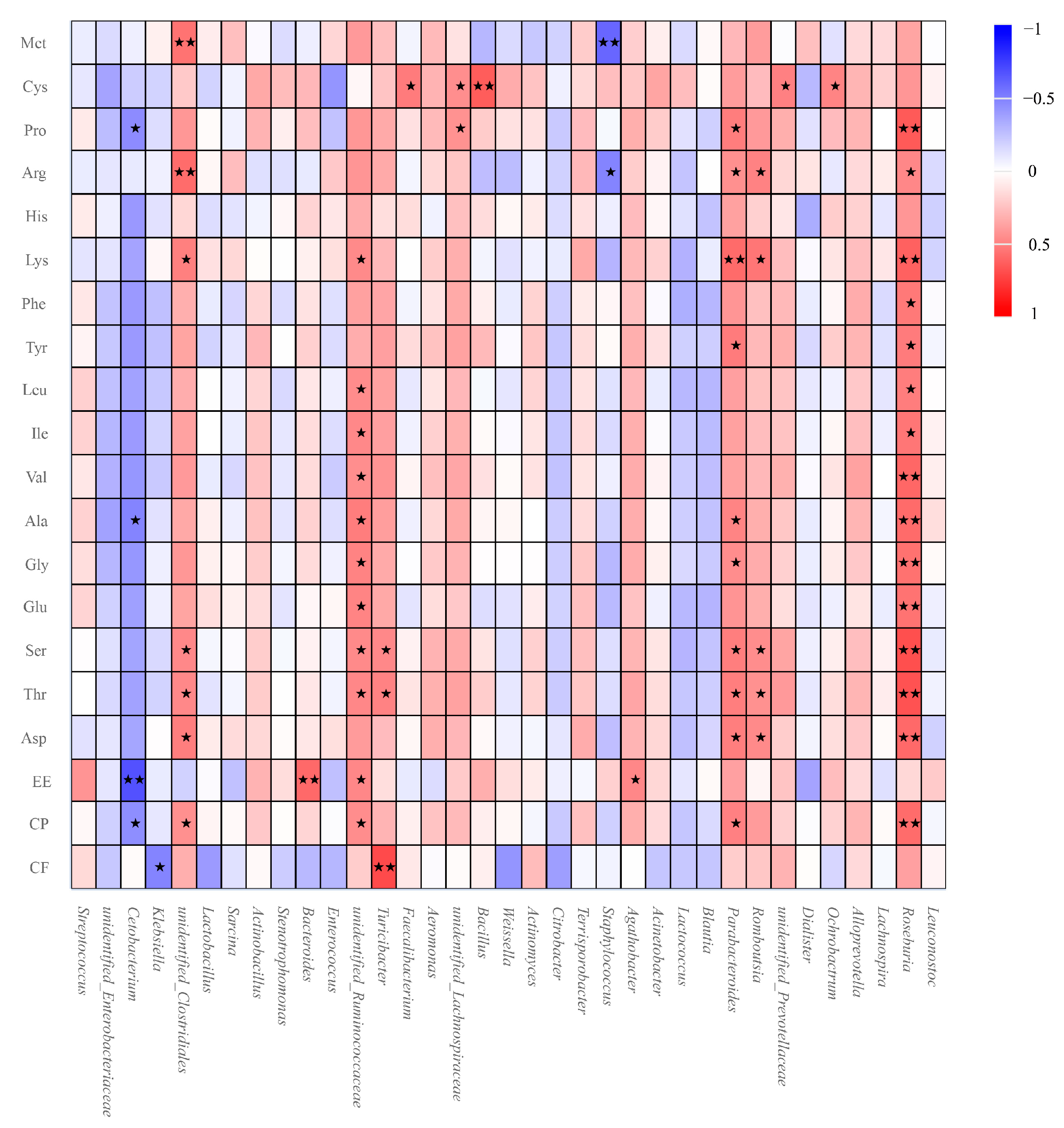
3.6. Relationship between Gut Microbiota and Nutrient Apparent Digestibility in GPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wei, F.; Wang, X.; Wu, Q. The giant panda gut microbiome. Trends Microbiol. 2015, 23, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Shi, W.; Wang, L.; Qu, Q.; Zuo, Z.; Wang, J.; Zhao, F.; Wei, F. PandaGUT provides new insights into bacterial diversity, function, and resistome landscapes with implications for conservation. Microbiome 2023, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhao, W.; Xiang, G.; Lv, R.; Dong, Y.; Yan, H.; Li, M. Bamboo Plant Part Preference Affects the Nutrients Digestibility and Intestinal Microbiota of Geriatric Giant Pandas. Animals 2023, 13, 844. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Michalak, M.; Agellon, L.B. Importance of Nutrients and Nutrient Metabolism on Human Health. Yale J. Biol. Med. 2018, 91, 95–103. [Google Scholar] [PubMed]
- Warne, R.W. The micro and macro of nutrients across biological scales. Integr. Comp. Biol. 2014, 54, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Seo, K.; Cho, H.-W.; Jeon, J.-H.; Kim, C.H.; Jung, J.; Chun, J.L. Age-related digestibility of nutrients depending on the moisture content in aged dogs. J. Anim. Sci. Technol. 2021, 63, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Tancharoenrat, P.; Ravindran, V.; Zaefarian, F.; Ravindran, G. Influence of age on the apparent metabolisable energy and total tract apparent fat digestibility of different fat sources for broiler chickens. Anim. Feed Sci. Technol. 2013, 186, 186–192. [Google Scholar] [CrossRef]
- Schauf, S.; Stockman, J.; Haydock, R.; Eyre, R.; Fortener, L.; Park, J.S.; Bakke, A.M.; Watson, P. Healthy Ageing Is Associated with Preserved or Enhanced Nutrient and Mineral Apparent Digestibility in Dogs and Cats Fed Commercially Relevant Extruded Diets. Animals 2021, 11, 2127. [Google Scholar] [CrossRef]
- Zeng, X.; Xing, X.; Gupta, M.; Keber, F.C.; Lopez, J.G.; Lee, Y.-C.J.; Roichman, A.; Wang, L.; Neinast, M.D.; Donia, M.S.; et al. Gut bacterial nutrient preferences quantified in vivo. Cell 2022, 185, 3441–3456 e3419. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Jin, L.; Wu, D.; Li, C.; Zhang, A.; Xiong, Y.; Wei, R.; Zhang, G.; Yang, S.; Deng, W.; Li, T.; et al. Bamboo nutrients and microbiome affect gut microbiome of giant panda. Symbiosis 2020, 80, 293–304. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, Q.; Yao, Y.; Ayala, J.; Hou, R.; Wang, H. Fecal Metabolomics Reveals the Foraging Strategies of Giant Pandas for Different Parts of Bamboo. Animals 2023, 13, 1278. [Google Scholar] [CrossRef]
- Deng, F.; Wang, C.; Li, D.; Peng, Y.; Deng, L.; Zhao, Y.; Zhang, Z.; Wei, M.; Wu, K.; Zhao, J.; et al. The unique gut microbiome of giant pandas involved in protein metabolism contributes to the host’s dietary adaption to bamboo. Microbiome 2023, 11, 180. [Google Scholar] [CrossRef]
- Jin, L.; Huang, Y.; Yang, S.; Wu, D.; Li, C.; Deng, W.; Zhao, K.; He, Y.; Li, B.; Zhang, G.; et al. Diet, habitat environment and lifestyle conversion affect the gut microbiomes of giant pandas. Sci. Total Environ. 2021, 770, 145316. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Ding, Y.; Hu, Y.; Nie, Y.; Wei, W.; Ma, S.; Yan, L.; Zhu, L.; Wei, F. Seasonal variation in nutrient utilization shapes gut microbiome structure and function in wild giant pandas. Proc. Biol. Sci. 2017, 284, 20170955. [Google Scholar] [CrossRef]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef]
- Yang, S.; Deng, W.; Li, G.; Jin, L.; Huang, Y.; He, Y.; Wu, D.; Li, D.; Zhang, A.; Liu, C.; et al. Reference gene catalog and metagenome-assembled genomes from the gut microbiome reveal the microbial composition, antibiotic resistome, and adaptability of a lignocellulose diet in the giant panda. Environ. Res. 2023, 245, 118090. [Google Scholar] [CrossRef]
- Liu, F.; Li, R.; Zhong, Y.; Liu, X.; Deng, W.; Huang, X.; Price, M.; Li, J. Age-related alterations in metabolome and microbiome provide insights in dietary transition in giant pandas. mSystems 2023, 8, e0025223. [Google Scholar] [CrossRef]
- AOAC.USA: Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; AOAC.USA: Arlington, VA, USA, 2000. [Google Scholar]
- AOAC.USA: Association of Official Analytical Chemists. Official Methods of Analysis, 18th ed.; AOAC.USA: Arlington, VA, USA, 2005. [Google Scholar]
- GB/T 18246-2019; Determination of Amino Acids in Feeds. State Administration for Market Regulation, Standardization Administration: Beijing, China, 2020.
- Edga, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Wei, M.; Zhu, Y.; Liu, W.; Li, D.; Wei, R.; Deng, L.; Wu, K.; Song, S.; Li, T.; Zeng, W.; et al. Factors influencing bamboo intake of captive giant pandas (Ailuropoda melanoleuca). Sci. Rep. 2023, 13, 6262. [Google Scholar] [CrossRef]
- Qiu, Q.; Gao, C.; Rahman, M.A.U.; Cao, B.; Su, H. Digestive Ability, Physiological Characteristics, and Rumen Bacterial Community of Holstein Finishing Steers in Response to Three Nutrient Density Diets as Fattening Phases Advanced. Microorganisms 2020, 8, 335. [Google Scholar] [CrossRef]
- Lewis, R.M.; Emmans, G.C. The relationship between feed intake and liveweight in domestic animals. J. Anim. Sci. 2020, 98, skaa087. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, H.; Hou, R.; Ayala, J.; Liu, G.; Yuan, S.; Yan, Z.; Zhang, W.; Liu, Y.; Cai, K.; et al. A Diet Diverse in Bamboo Parts is Important for Giant Panda (Ailuropoda melanoleuca) Metabolism and Health. Sci. Rep. 2017, 7, 3377. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Wang, D.; Ye, F.; He, Y.; Hu, Z.; Zhao, G. A systematic review on the composition, storage, processing of bamboo shoots: Focusing the nutritional and functional benefits. J. Funct. Foods 2020, 71, 104015. [Google Scholar] [CrossRef]
- Guo, W.; Mishra, S.; Zhao, J.; Tang, J.; Zeng, B.; Kong, F.; Ning, R.; Li, M.; Zhang, H.; Zeng, Y.; et al. Metagenomic Study Suggests That the Gut Microbiota of the Giant Panda (Ailuropoda melanoleuca) May Not Be Specialized for Fiber Fermentation. Front. Microbiol. 2018, 9, 229. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, W.; Wang, L.; Hou, R.; Zhang, M.; Fei, L.; Zhang, X.; Huang, H.; Bridgewater, L.C.; Jiang, Y.; et al. The bamboo-eating giant panda harbors a carnivore-like gut microbiota, with excessive seasonal variations. mBio 2015, 6, e00022-00015. [Google Scholar] [CrossRef]
- Yao, R.; Yang, Z.; Zhang, Z.; Hu, T.; Chen, H.; Huang, F.; Gu, X.; Yang, X.; Lu, G.; Zhu, L. Are the gut microbial systems of giant pandas unstable? Heliyon 2019, 5, e02480. [Google Scholar] [CrossRef]
- Hu, P.; Wang, L.; Hu, Z.; Jiang, L.; Hu, H.; Rao, Z.; Wu, L.; Tang, Z. Effects of Multi-Bacteria Solid-State Fermented Diets with Different Crude Fiber Levels on Growth Performance, Nutrient Digestibility, and Microbial Flora of Finishing Pigs. Animals 2021, 11, 3079. [Google Scholar] [CrossRef]
- Holeček, M. Aspartic Acid in Health and Disease. Nutrients 2023, 15, 4023. [Google Scholar] [CrossRef]
- Meynial-Denis, D. Glutamine metabolism in advanced age. Nutr. Rev. 2016, 74, 225–236. [Google Scholar] [CrossRef]
- Canfield, C.-A.; Bradshaw, P.C. Amino acids in the regulation of aging and aging-related diseases. Transl. Med. Aging 2019, 3, 70–89. [Google Scholar] [CrossRef]
- Tessari, P.; Lante, A.; Mosca, G. Essential amino acids: Master regulators of nutrition and environmental footprint? Sci. Rep. 2016, 6, 26074. [Google Scholar] [CrossRef]
- Zhang, L.; Li, F.; Guo, Q.; Duan, Y.; Wang, W.; Zhong, Y.; Yang, Y.; Yin, Y. Leucine Supplementation: A Novel Strategy for Modulating Lipid Metabolism and Energy Homeostasis. Nutrients 2020, 12, 1299. [Google Scholar] [CrossRef]
- Baum, J.I.; Kim, I.-Y.; Wolfe, R.R. Protein Consumption and the Elderly: What Is the Optimal Level of Intake? Nutrients 2016, 8, 359. [Google Scholar] [CrossRef]
- Pellanda, P.; Ghosh, T.S.; O’toole, P.W. Understanding the impact of age-related changes in the gut microbiome on chronic diseases and the prospect of elderly-specific dietary interventions. Curr. Opin. Biotechnol. 2021, 70, 48–55. [Google Scholar] [CrossRef]
- Peng, Z.; Zeng, D.; Wang, Q.; Niu, L.; Ni, X.; Zou, F.; Yang, M.; Sun, H.; Zhou, Y.; Liu, Q.; et al. Decreased microbial diversity and Lactobacillus group in the intestine of geriatric giant pandas (Ailuropoda melanoleuca). World J. Microbiol. Biotechnol. 2016, 32, 79. [Google Scholar] [CrossRef]
- Ragonnaud, E.; Biragyn, A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun. Ageing 2021, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liao, C.; Wu, L.; Tang, J.; Chen, J.; Lei, C.; Zheng, L.; Zhang, C.; Liu, Y.-Y.; Xavier, J.; et al. Ecological dynamics of the gut microbiome in response to dietary fiber. ISME J. 2022, 16, 2040–2055. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gao, X.; Meng, J.; Zhang, A.; Zhou, Y.; Long, M.; Li, B.; Deng, W.; Jin, L.; Zhao, S.; et al. Metagenomic Analysis of Bacteria, Fungi, Bacteriophages, and Helminths in the Gut of Giant Pandas. Front. Microbiol. 2018, 9, 1717. [Google Scholar] [CrossRef]
- Santamaría, R.I.; Martínez-Carrasco, A.; de la Nieta, R.S.; Torres-Vila, L.M.; Bonal, R.; Martín, J.; Tormo, R.; Reyes, F.; Genilloud, O.; Díaz, M. Characterization of Actinomycetes Strains Isolated from the Intestinal Tract and Feces of the Larvae of the Longhorn Beetle Cerambyx welensii. Microorganisms 2020, 8, 2013. [Google Scholar] [CrossRef]
- Adams, A.S.; Jordan, M.S.; Adams, S.M.; Suen, G.; A Goodwin, L.; Davenport, K.W.; Currie, C.R.; Raffa, K.F. Cellulose-degrading bacteria associated with the invasive woodwasp Sirex noctilio. ISME J. 2011, 5, 1323–1331. [Google Scholar] [CrossRef]
- Propst, R.; Denham, L.; Deisch, J.K.; Kalra, T.; Zaheer, S.; Silva, K.; Magaki, S. Sarcina Organisms in the Upper Gastrointestinal Tract: A Report of 3 Cases With Varying Presentations. Int. J. Surg. Pathol. 2020, 28, 206–209. [Google Scholar] [CrossRef]
- Wu, Y.; Wan, J.; Choe, U.; Pham, Q.; Schoene, N.W.; He, Q.; Li, B.; Yu, L.; Wang, T.T.Y. Interactions Between Food and Gut Microbiota: Impact on Human Health. Annu. Rev. Food Sci. Technol. 2019, 10, 389–408. [Google Scholar] [CrossRef]
- Dai, Z.-L.; Wu, G.; Zhu, W.-Y. Amino acid metabolism in intestinal bacteria: Links between gut ecology and host health. Front. Biosci. 2011, 16, 1768–1786. [Google Scholar] [CrossRef] [PubMed]
- Libao-Mercado, A.J.O.; Zhu, C.L.; Cant, J.P.; Lapierre, H.; Thibault, J.-N.; Sève, B.; Fuller, M.F.; de Lange, C.F.M. Dietary and endogenous amino acids are the main contributors to microbial protein in the upper gut of normally nourished pigs. J. Nutr. 2009, 139, 1088–1094. [Google Scholar] [CrossRef]
- Lin, R.; Liu, W.; Piao, M.; Zhu, H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar] [CrossRef]
- Liu, Y.-K.; Kuo, H.-C.; Lai, C.-H.; Chou, C.-C. Single amino acid utilization for bacterial categorization. Sci. Rep. 2020, 10, 12686. [Google Scholar] [CrossRef]
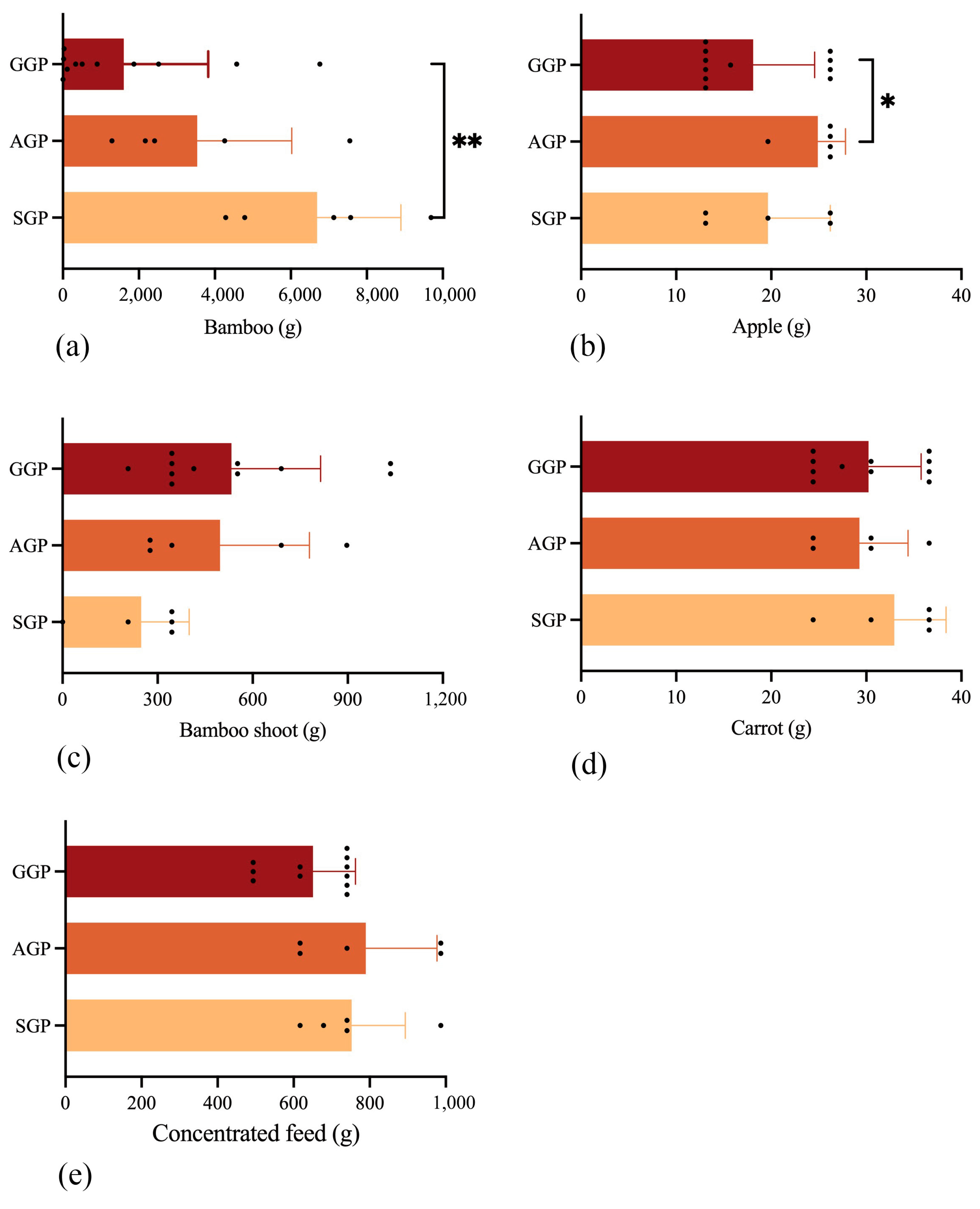
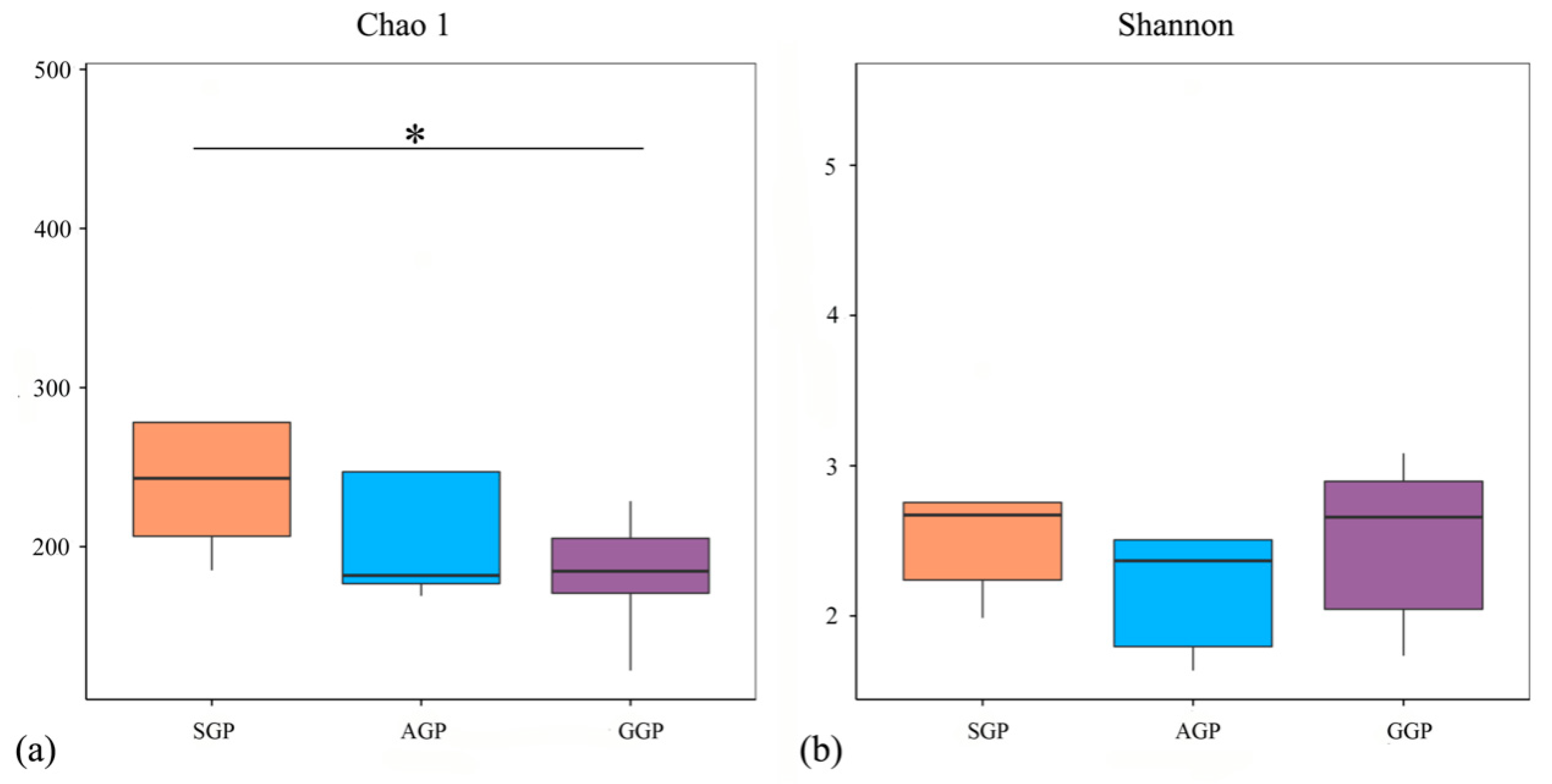

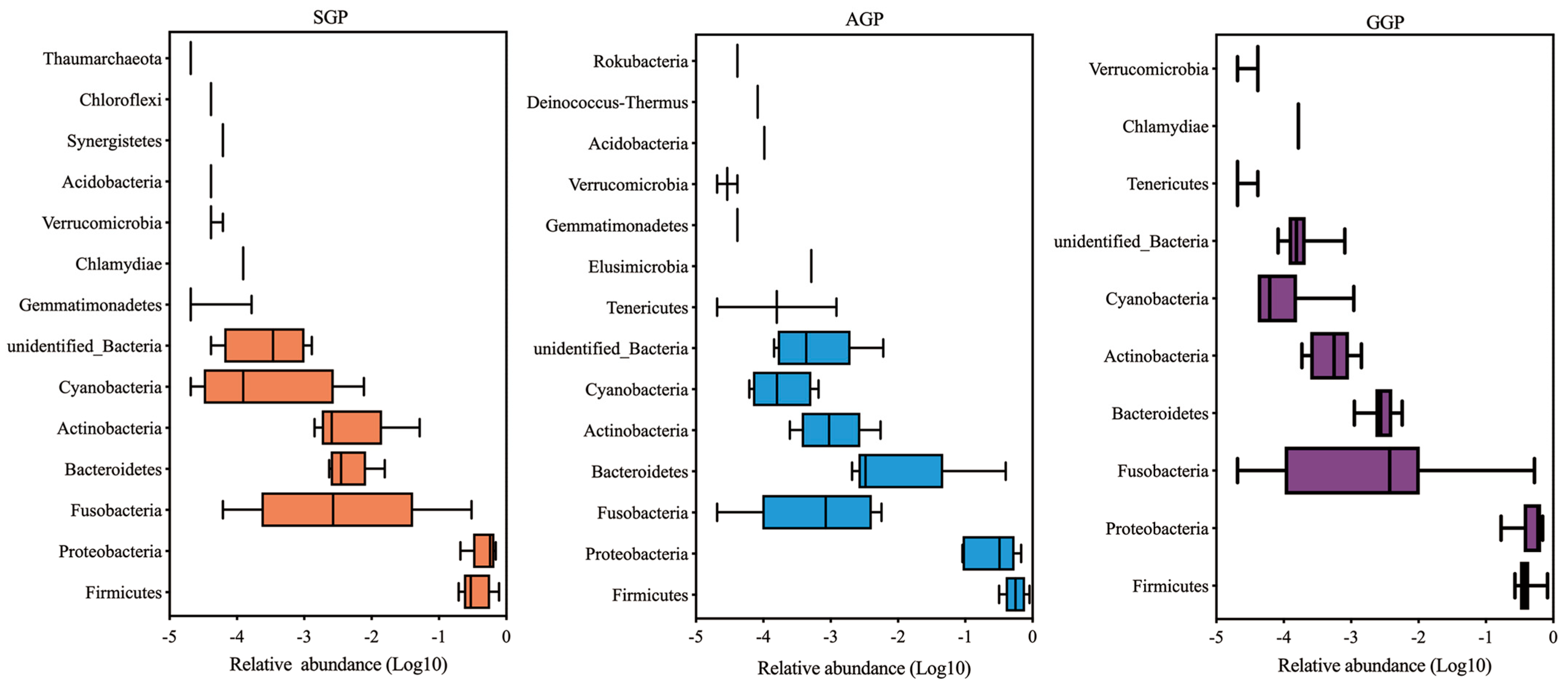
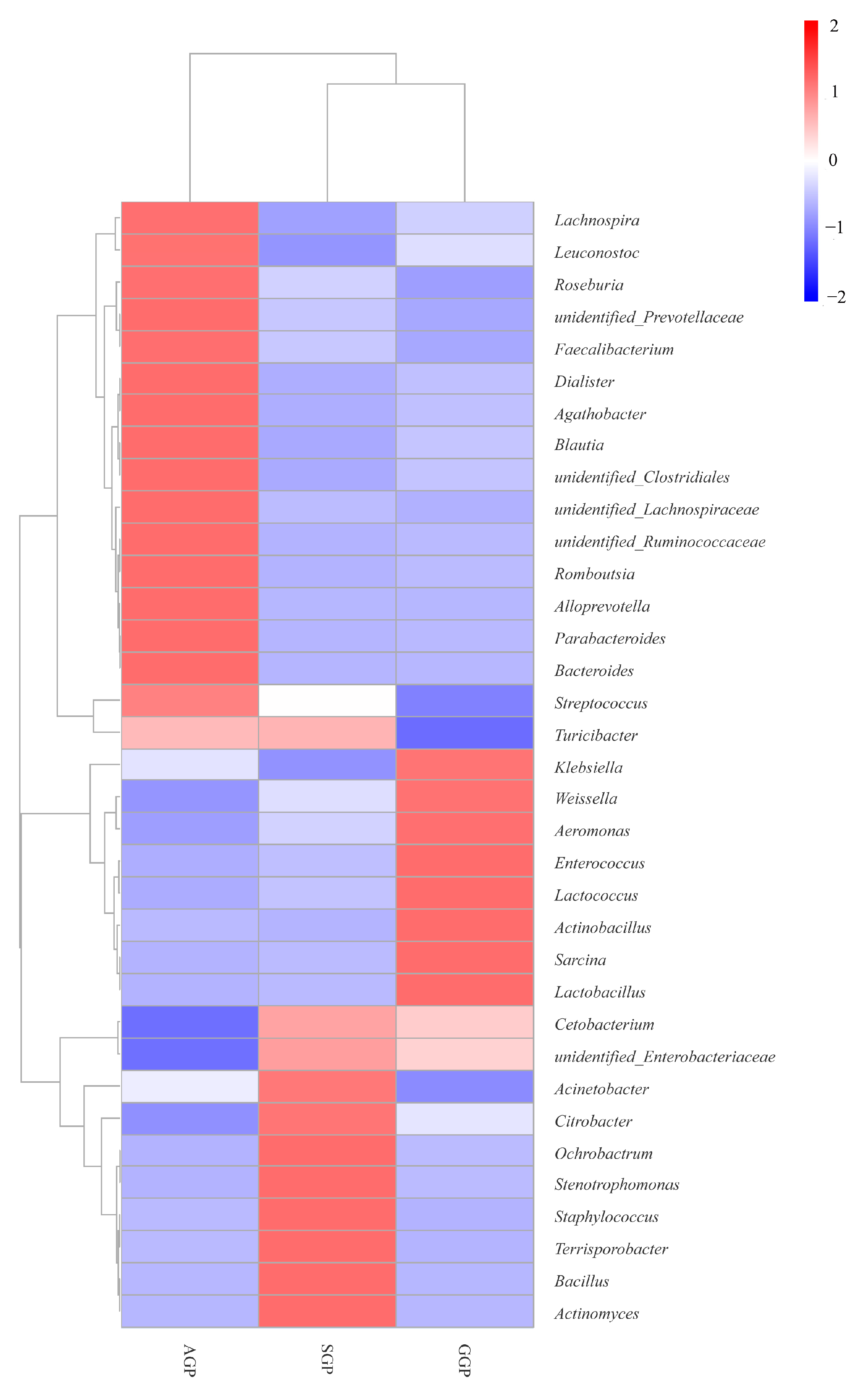
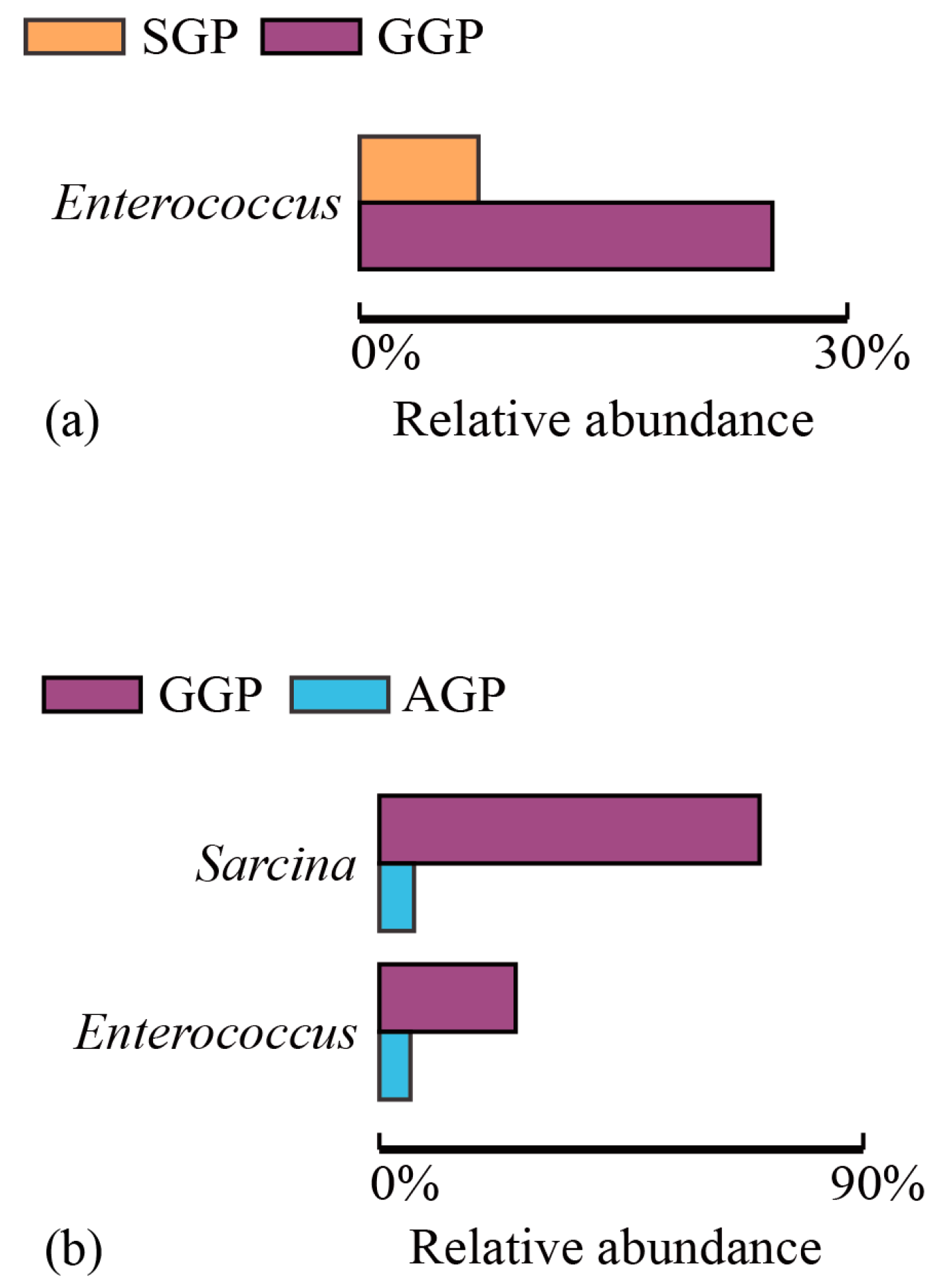
| Items | SGP | AGP | GGP |
|---|---|---|---|
| CF, g | 1955.88 ± 647.72 a | 1102.39 ± 720.35 | 557.06 ± 633.48 b |
| CP, g | 332.1 ± 99.44 | 322.14 ± 111.75 | 264.85 ± 91.30 |
| EE, g | 69.73 ± 17.48 | 69.12 ± 18.89 | 56.94 ± 14.36 |
| Thr, % | 12.07 ± 3.65 | 11.28 ± 4.00 | 9.11 ± 3.25 |
| Val, % | 16.05 ± 4.91 | 14.66 ± 5.34 | 11.67 ± 4.37 |
| Iso, % | 11.72 ± 3.35 | 11.08 ± 3.62 | 8.97 ± 2.90 |
| Leu, % | 23.03 ± 6.31 | 21.49 ± 6.63 | 17.20 ± 5.29 |
| Tyr, % | 10.54 ± 3.60 | 9.16 ± 4.01 | 7.13 ± 3.31 |
| Phe, % | 16.78 ± 4.93 a | 14.62 ± 5.14 | 11.27 ± 4.20 b |
| Lys, % | 15.03 ± 4.75 | 14.80 ± 5.50 | 12.32 ± 4.53 |
| His, % | 7.08 ± 2.08 | 6.74 ± 2.29 | 5.50 ± 1.83 |
| Met, % | 9.82 ± 5.38 | 18.26 ± 9.86 | 19.28 ± 9.69 |
| Asp, % | 42.14 ± 13.64 | 41.88 ± 15.98 | 35.06 ± 13.31 |
| Ser, % | 15.71 ± 4.70 | 14.60 ± 5.12 | 11.73 ± 4.17 |
| Glu, % | 37.41 ± 9.53 | 37.08 ± 10.35 | 30.57 ± 7.91 |
| Gly, % | 12.41 ± 3.66 | 12.06 ± 4.10 | 9.94 ± 3.31 |
| Ala, % | 15.47 ± 4.58 | 14.81 ± 5.07 | 12.09 ± 4.11 |
| Arg, % | 11.75 ± 3.04 | 12.59 ± 3.66 | 10.82 ± 2.78 |
| Pro, % | 18.11 ± 5.43 a | 15.46 ± 5.66 | 11.78 ± 4.66 b |
| Cys, % | 10.06 ± 3.33 a | 6.10 ± 3.64 | 3.43 ± 3.14 b |
| Items | SGP | AGP | GGP |
|---|---|---|---|
| CF | 12.41 ± 2.94 a | 11.77 ± 2.34 | 7.82 ± 4.07 b |
| CP | 69.58 ± 7.01 | 71.52 ± 7.44 | 70.70 ± 9.52 |
| EE | 63.96 ± 9.71 | 73.45 ± 10.17 | 56.86 ± 20.16 |
| Thr | 58.00 ± 8.10 | 57.38 ± 13.58 | 58.40 ± 11.96 |
| Val | 69.94 ± 7.42 | 70.02 ± 8.70 | 67.84 ± 9.71 |
| Iso | 69.52 ± 7.70 | 70.57 ± 7.40 | 68.18 ± 10.20 |
| Leu | 73.63 ± 7.26 | 74.60 ± 5.44 | 72.89 ± 9.80 |
| Tyr | 80.97 ± 4.38 | 78.57 ± 7.97 | 76.82 ± 7.14 |
| Phe | 77.47 ± 6.26 | 76.73 ± 4.97 | 74.43 ± 8.57 |
| Lys | 71.65 ± 7.83 | 75.14 ± 6.27 | 75.25 ± 9.21 |
| His | 68.52 ± 7.66 | 68.99 ± 9.04 | 69.23 ± 10.42 |
| Met | 81.36 ± 20.31 a | 93.98 ± 3.26 | 94.57 ± 4.33 b |
| Asp | 79.42 ± 4.73 | 80.65 ± 5.95 | 81.03 ± 6.85 |
| Ser | 71.05 ± 5.76 | 69.53 ± 9.19 | 70.47 ± 9.06 |
| Glu | 71.00 ± 6.89 | 72.46 ± 5.69 | 72.61 ± 8.16 |
| Gly | 61.04 ± 9.28 | 63.36 ± 8.70 | 61.77 ± 13.15 |
| Ala | 67.46 ± 8.61 | 69.98 ± 6.38 | 66.70 ± 11.82 |
| Arg | 66.54 ± 8.09 | 72.28 ± 6.35 | 74.68 ± 11.26 |
| Pro | 73.16 ± 7.13 | 72.12 ± 8.14 | 69.52 ± 8.67 |
| Cys | 83.70 ± 8.43 a | 67.93 ± 25.64 | 56.19 ± 21.57 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Deng, W.; Huang, Z.; Li, C.; Wei, R.; Zhu, Y.; Wu, K.; Li, C.; Deng, L.; Wei, M.; et al. Nutrient Utilization and Gut Microbiota Composition in Giant Pandas of Different Age Groups. Animals 2024, 14, 2324. https://doi.org/10.3390/ani14162324
Wang C, Deng W, Huang Z, Li C, Wei R, Zhu Y, Wu K, Li C, Deng L, Wei M, et al. Nutrient Utilization and Gut Microbiota Composition in Giant Pandas of Different Age Groups. Animals. 2024; 14(16):2324. https://doi.org/10.3390/ani14162324
Chicago/Turabian StyleWang, Chengdong, Wenwen Deng, Zhi Huang, Caiwu Li, Rongping Wei, Yan Zhu, Kai Wu, Chengyao Li, Linhua Deng, Ming Wei, and et al. 2024. "Nutrient Utilization and Gut Microbiota Composition in Giant Pandas of Different Age Groups" Animals 14, no. 16: 2324. https://doi.org/10.3390/ani14162324




