Effect of Agro-Industrial by Products Derived from Volatile Fatty Acids on Ruminant Feed In Vitro Digestibility
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of Feed Ingredients and the VFA Mixture
2.2. In Vitro Experiment Design
2.3. Analytical Method
2.4. Statistical Analysis
3. Results
3.1. Gas Production
3.1.1. Gas Production in the Concentrate Energy Replacement Experiment
3.1.2. Gas Production in the Silage Energy Replacement Experiment
3.2. Volatile Fatty Acid Production
3.2.1. VFA Production in the Concentrate Energy Replacement Experiment
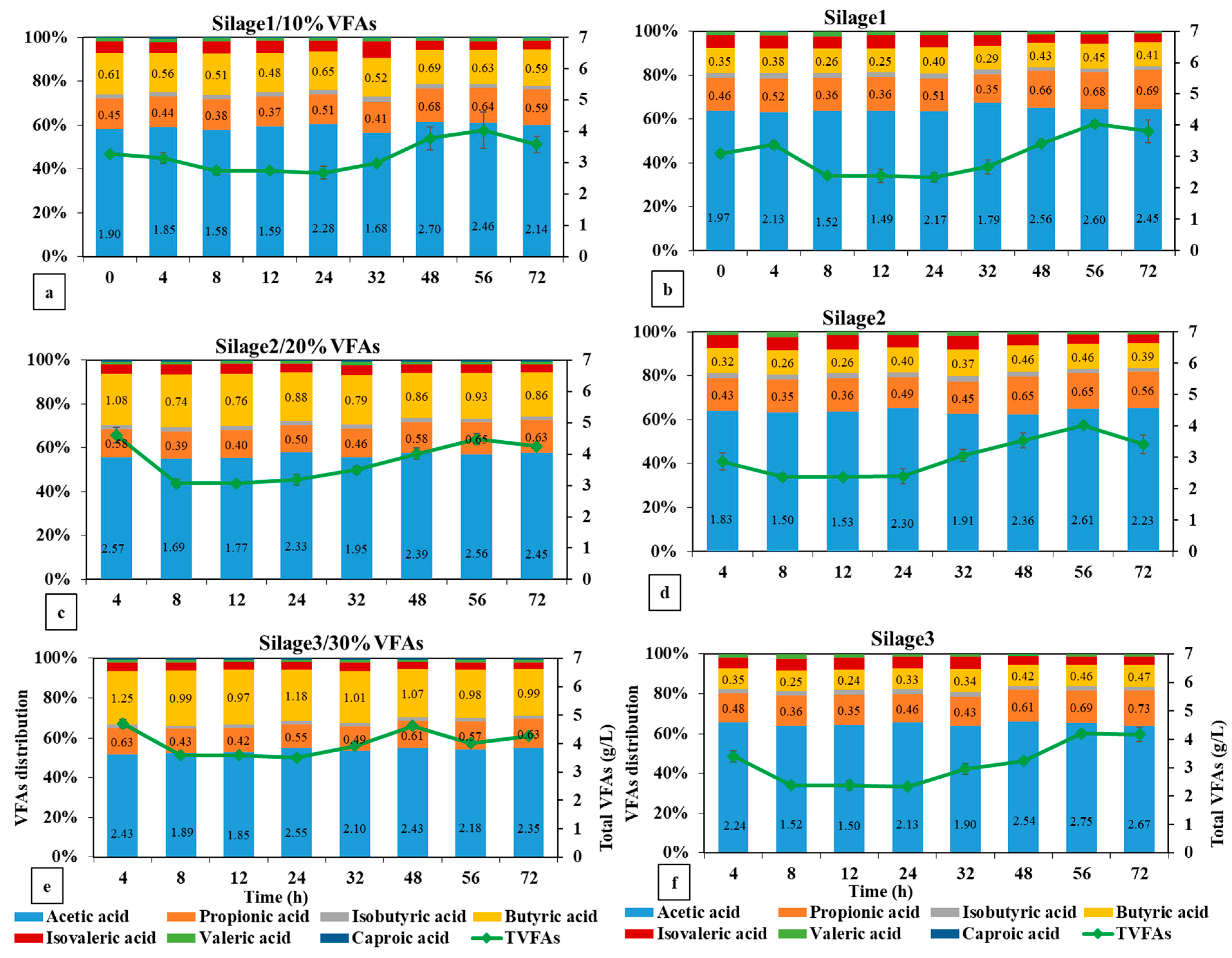
3.2.2. VFA Production in the Silage Energy Replacement Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ajita, T.; Roshna, K. Food Waste and Agro By-Products: A Step towards Food Sustainability. In Innovation in the Food Sector Through the Valorization of Food and Agro-Food By-Products; Ana Novo de, B., Irene, G., Eds.; IntechOpen: Rijeka, Croatia, 2021; Chapter 2. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste; FAO: Rome, Italy, 2011. [Google Scholar]
- Stancu, V.; Lähteenmäki, L. Consumer-related antecedents of food provisioning behaviors that promote food waste. Food Policy 2022, 108, 102236. [Google Scholar] [CrossRef]
- Georganas, A.; Giamouri, E.; Pappas, A.C.; Papadomichelakis, G.; Galliou, F.; Manios, T.; Tsiplakou, E.; Fegeros, K.; Zervas, G. Bioactive compounds in food waste: A review on the transformation of food waste to animal feed. Foods 2020, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Sarsaiya, S.; Jain, A.; Kumar Awasthi, S.; Duan, Y.; Kumar Awasthi, M.; Shi, J. Microbial dynamics for lignocellulosic waste bioconversion and its importance with modern circular economy, challenges and future perspectives. Bioresour. Technol. 2019, 291, 121905. [Google Scholar] [CrossRef] [PubMed]
- Ferronato, N.; Torretta, V. Waste mismanagement in developing countries: A review of global issues. Int. J. Environ. Res. Public Health 2019, 16, 1060. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Tan, X.; Show, P.L.; Rambabu, K.; Banat, F.; Veeramuthu, A.; Lau, B.F.; Ng, E.P.; Ling, T.C. Incorporating biowaste into circular bioeconomy: A critical review of current trend and scaling up feasibility. Environ. Technol. Innov. 2020, 19, 101034. [Google Scholar] [CrossRef]
- Hamadou, B.; Djomdi, D.; Falama, R.Z.; Gardarin, C.; Blavignac, C.; Audonnet, F.; Delattre, C.; Pierre, G.; Dubessay, P.; Darnan, R.D.; et al. Hydrogen and Fatty Acid Production by Dark Fermentation of Sweet Sorghum Stalks as an Efficient Pre-treatment for Energy Recovery Before Their Bioconversion into Methane. BioEnergy Res. 2024. [Google Scholar] [CrossRef]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current perspectives on acidogenic fermentation to produce volatile fatty acids from waste. Rev. Environ. Sci. Bio/Technol. 2021, 20, 439–478. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, S.; Tan, J.; Wang, Y.; Li, L.; Zhao, H.; Liu, M.; Jiang, L. Bioconversion of citrus waste by long-term DMSO-cryopreserved rumen fluid to volatile fatty acids and biogas is feasible: A microbiome perspective. J. Environ. Manag. 2024, 351, 119693. [Google Scholar] [CrossRef]
- Mahboubi, A.; Agnihotri, S.; Uwineza, C.; Jomnonkhaow, U.; Taherzadeh, M.J. Chapter 18—Waste-derived volatile fatty acids for sustainable ruminant feed supplementation. In Biomass, Biofuels, Biochemicals; Varjani, S., Pandey, A., Taherzadeh, M.J., Ngo, H.H., Tyagi, R.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 407–430. [Google Scholar] [CrossRef]
- Wolin, M.J. The rumen fermentation: A model for microbial interactions in anaerobic ecosystems. In Advances in Microbial Ecology: Volume 3; Springer: Berlin/Heidelberg, Germany, 1979; pp. 49–77. [Google Scholar]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef]
- Amanzougarene, Z.; Fondevila, M. Fitting of the In Vitro Gas Production Technique to the Study of High Concentrate Diets. Animals 2020, 10, 1935. [Google Scholar] [CrossRef]
- Parchami, M.; Uwineza, C.; Ibeabuchi, O.H.; Rustas, B.-O.; Taherzadeh, M.J.; Mahboubi, A. Membrane bioreactor assisted volatile fatty acids production from agro-industrial residues for ruminant feed application. Waste Manag. 2023, 170, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Kim, H.J.; Min, D.B. Tocopherol Stability and Prooxidant Mechanisms of Oxidized Tocopherols in Lipids. In Food Lipids; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Fritz, D.; Menke, K.H.; Raab, L.; Salewski, A.; Schneider, W.; Steingass, H. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Parchami, M.; Ferreira, J.; Taherzadeh, M.J.B. Brewing process development by integration of edible filamentous fungi to upgrade the quality of Brewer’s spent grain (BSG). BioResources 2021, 16, 1686. [Google Scholar] [CrossRef]
- Magomya, A.M.; Kubmarawa, D.; Ndahi, J.A.; Yebpella, G.G. Determination of plant proteins via the Kjeldahl method and amino acid analysis: A comparative study. Int. J. Sci. Technol. Res. 2014, 3, 68–72. [Google Scholar]
- Parchami, M.; Ferreira, J.A.; Taherzadeh, M.J. Starch and protein recovery from brewer’s spent grain using hydrothermal pretreatment and their conversion to edible filamentous fungi—A brewery biorefinery concept. Bioresour. Technol. 2021, 337, 125409. [Google Scholar] [CrossRef]
- Uwineza, C.; Bouzarjomehr, M.; Parchami, M.; Sar, T.; Taherzadeh, M.J.; Mahboubi, A. Evaluation of in vitro digestibility of Aspergillus oryzae fungal biomass grown on organic residue derived-VFAs as a promising ruminant feed supplement. J. Anim. Sci. Biotechnol. 2023, 14, 120. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1926; Volume 6. [Google Scholar]
- Vali, N.; Combres, A.; Hosseinian, A.; Pettersson, A. The Effect of the Elemental Composition of Municipal Sewage Sludge on the Phosphorus Recycling during Pyrolysis, with a Focus on the Char Chemistry—Modeling and Experiments. Separations 2023, 10, 31. [Google Scholar] [CrossRef]
- Parchami, M.; Rustas, B.-O.; Taherzadeh, M.J.; Mahboubi, A. An in vitro evaluation of partial energy replacement in a total mixed ration with volatile fatty acids derived from agro-industrial residues. Syst. Microbiol. Biomanuf. 2024. [Google Scholar] [CrossRef]
- Marvin-Sikkema, F.D.; Rees, E.; Kraak, M.N.; Gottschal, J.C.; Prins, R.A. Influence of metronidazole, CO, CO2, and methanogens on the fermentative metabolism of the anaerobic fungus Neocallimastix sp. strain L2. Appl. Environ. Microbiol. 1993, 59, 2678–2683. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Liang, G.; Guan, L.L. Regulation of rumen development in neonatal ruminants through microbial metagenomes and host transcriptomes. Genome Biol. 2019, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Wang, R.; Ma, Z.Y.; Zhang, X.M.; Jiao, J.Z.; Zhang, Z.G.; Ungerfeld, E.M.; Yi, K.L.; Zhang, B.Z.; Long, L.J.T.I.J. Dietary selection of metabolically distinct microorganisms drives hydrogen metabolism in ruminants. ISME J. 2022, 16, 2535–2546. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant; Cornell University Press: Ithaca, NY, USA, 2018. [Google Scholar]
- Ungerfeld, E.M. Metabolic Hydrogen Flows in Rumen Fermentation: Principles and Possibilities of Interventions. Front. Microbiol. 2020, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Ghimire, S.; Wenner, B.A.; Kohn, R.A.; Firkins, J.L.; Gill, B.; Hanigan, M.D. Effects of acetate, propionate, and pH on volatile fatty acid thermodynamics in continuous cultures of ruminal contents. J. Dairy Sci. 2022, 105, 8879–8897. [Google Scholar] [CrossRef] [PubMed]
- Joblin, K.N. Ruminal acetogens and their potential to lower ruminant methane emissions. Aust. J. Agric. Res. 1999, 50, 1307–1314. [Google Scholar] [CrossRef]
- Greening, C.; Geier, R.; Wang, C.; Woods, L.C.; Morales, S.E.; McDonald, M.J.; Rushton-Green, R.; Morgan, X.C.; Koike, S.; Leahy, S.C.; et al. Diverse hydrogen production and consumption pathways influence methane production in ruminants. ISME J. 2019, 13, 2617–2632. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, A. Rumen ciliates: Their metabolism and relationships with bacteria and their hosts. Anim. Feed. Sci. Technol. 1990, 30, 203–266. [Google Scholar] [CrossRef]
- Herrera-Saldana, R.; Gomez-Alarcon, R.; Torabi, M.; Huber, J.T. Influence of synchronizing protein and starch degradation in the rumen on nutrient utilization and microbial protein synthesis. J. Dairy Sci. 1990, 73, 142–148. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, S.C.; Kim, J.D.; Oh, Y.G.; Kim, B.K.; Kim, C.W.; Kim, K.J. Methane production potential of feed ingredients as measured by in vitro gas test. Asian-Australas. J. Anim. Sci. 2003, 16, 1143–1150. [Google Scholar] [CrossRef]
- Conrad, R. Contribution of hydrogen to methane production and control of hydrogen concentrations in methanogenic soils and sediments. FEMS Microbiol. Ecol. 1999, 28, 193–202. [Google Scholar] [CrossRef]
- Janssen, P.H. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed Sci. Technol. 2010, 160, 1–22. [Google Scholar] [CrossRef]
- Leahy, S.C.; Janssen, P.H.; Attwood, G.T.; Mackie, R.I.; McAllister, T.A.; Kelly, W.J. Electron flow: Key to mitigating ruminant methanogenesis. Trends Microbiol. 2022, 30, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Zicarelli, F.; Calabrò, S.; Cutrignelli, M.I.; Infascelli, F.; Tudisco, R.; Bovera, F.; Piccolo, V. In vitro fermentation characteristics of diets with different forage/concentrate ratios: Comparison of rumen and faecal inocula. J. Sci. Food Agric. 2011, 91, 1213–1221. [Google Scholar] [CrossRef] [PubMed]



| Characteristics of Feed | Ingredients | |
|---|---|---|
| Hay Silage | Concentrate | |
| Dry matter (DM) | 428.6 ± 0.3 g/kg | 884.9 ± 0.5 g/kg |
| Moisture | 571.4 ± 0.1 g/kg | 113.2 ± 0.8 g/kg |
| Acid detergent fiber | 502.4 ± 0.3 g/kg | 322.6 ± 0.1 g/kg |
| Neutral detergent fiber | 513.5 ± 0.15 g/kg | 398.6 ± 0.3 g/kg |
| Crude fiber | 471.7 ± 0.6 g/kg | 293.8 ± 0.4 g/kg |
| Ash | 82.1 ± 0.49 g/kg | 51.5 ± 0.4 g/kg |
| Organic matter | 917.9 ± 0.6 g/kg DM | 948.5 ± 0.4 g/kg DM |
| Gross energy content | 18.29 ± 0.2 MJ/kg DM | 20.17 ± 0.1 MJ/kg DM |
| Starch | ND | 250 ± 0.1 g/kg |
| Protein | 113.2± 0.15 g/kg | 360.6 ± 0.2 g/kg |
| Characteristics of VFA mixture | ||
| Total VFAs | 18.35 g/L | |
| Acetate | 7.28 g/L | |
| Propionate | 1.61 g/L | |
| Butyrate | 9.46 g/L | |
| Ammonium nitrogen (NH4+–N) | 1110 mg/L | |
| Total COD | 14 g/L | |
| Energy content | 378.01 J/mL |
| Number | Condition | Removed Feed Energy (J) | Replaced Energy with VFAs Mixture (mL) | Set pH |
|---|---|---|---|---|
| Silage experiment | ||||
| 1 | Silage1/10%VFAs | 731.6 | 1.9 | 7.63 |
| 2 | Silage 1 | - | - | 7.63 |
| 3 | Silage2 /20%VFAs | 1463.2 | 3.8 | 7.81 |
| 4 | Silage 2 | - | - | 7.81 |
| 5 | Silage3/30%VFAs | 2194.8 | 5.7 | 7.93 |
| 6 | Silage 3 | - | - | 7.93 |
| 7 | Blank | - | - | 7.52 |
| Concentrate experiment | ||||
| 1 | Con1/10%VFAs | 806.3 | 2.1 | 7.42 |
| 2 | Con1 | - | - | 7.42 |
| 3 | Con2 /20%VFAs | 1612.6 | 4.2 | 7.62 |
| 4 | Con2 | - | - | 7.62 |
| 5 | Con3 /30%VFAs | 2418.9 | 6.3 | 7.83 |
| 6 | Con3 | - | - | 7.83 |
| 7 | Blank | - | - | 7.58 |
| Response | Conditions | ||||||
|---|---|---|---|---|---|---|---|
| Con1/10%VFAs | Con1 | Con2/20%VFAs | Con2 | Con3/30% VFAs | Con3 | SEM | |
| H2 mL | 4.74 b | 5.85 a | 3.57 c | 5.21 b | 1.62 d | 1.99 d | 0.08 |
| CH4 mL | 22.44 a | 17.8 cd | 14.65 c | 18.26 b | 13.29 c | 14.77 c | 0.35 |
| CO2 mL | 25.52 a | 23.12 a | 19.49 de | 24.69 a | 18.01 f | 21.53 de | 0.42 |
| TVFAs g/L | 4.31 de | 4.35 de | 5.48 b | 4.28 de | 6.29 a | 5.15 bc | 0.13 |
| ΔTVFAs g/L | 1.56 c | 2.79 b | 2.85 ab | 2.49 b | 3.08 ab | 3.38 a | 0.18 |
| Acetic acid g/L | 2.39 d | 2.7 cd | 3.03 abc | 2.69 cd | 3.32 a | 3.14 ab | 0.04 |
| Propionic acid g/L | 0.83 bcd | 0.96 ab | 0.91 abc | 0.9 abc | 0.96 ab | 1.05 a | 0.05 |
| Butyric acid g/L | 0.68 d | 0.5 e | 1.12 b | 0.51 e | 1.49 a | 0.53 e | 0.04 |
| A:P ratio | 2.89 c | 2.8 c | 3.32 abc | 2.99 bc | 3.45 abc | 2.99 bc | 0.08 |
| Response | Conditions | ||||||
|---|---|---|---|---|---|---|---|
| Silage1/10%VFAs | Silage1 | Silage2/20%VFAs | Silage2 | Silage3/30%VFAs | Silage3 | SEM | |
| H2 mL | 0.79 e | 0.52 f | 0.62 f | 0.82 f | 0.78 f | 2.04 g | 0.08 |
| CH4 mL | 9.98 f | 11.92 de | 8.58 gh | 9.97 fg | 7.38 h | 7.37 h | 0.55 |
| CO2 mL | 21.85 bcd | 22.32 b | 17.76 ef | 23.89 bcd | 18.17 f | 21.17 de | 0.92 |
| TVFAs g/L | 4.66 cde | 4.26 de | 4.78 bcd | 4.03 e | 5.22 bc | 4.76 cd | 0.23 |
| ΔTVFAs g/L | 1.52 c | 0.78 cde | 0.18 e | 1.17 cd | 0.51 de | 1.36 c | 0.28 |
| Acetic acid g/L | 2.84 bc | 2.66 cd | 2.77 bcd | 2.63 cd | 2.86 bc | 3.05 abc | 0.14 |
| Propionic acid g/L | 0.77 cd | 0.75 cd | 0.72 d | 0.67 d | 0.78 dc | 0.83 bcd | 0.05 |
| Butyric acid g/L | 0.71 d | 0.46 e | 0.94 c | 0.45 e | 1.2 b | 0.53 e | 0.04 |
| A:P ratio | 3.69 ab | 3.52 abc | 3.83 a | 3.93 a | 3.67 ab | 3.68 ab | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parchami, M.; Rustas, B.-O.; Taherzadeh, M.J.; Mahboubi, A. Effect of Agro-Industrial by Products Derived from Volatile Fatty Acids on Ruminant Feed In Vitro Digestibility. Animals 2024, 14, 2330. https://doi.org/10.3390/ani14162330
Parchami M, Rustas B-O, Taherzadeh MJ, Mahboubi A. Effect of Agro-Industrial by Products Derived from Volatile Fatty Acids on Ruminant Feed In Vitro Digestibility. Animals. 2024; 14(16):2330. https://doi.org/10.3390/ani14162330
Chicago/Turabian StyleParchami, Milad, Bengt-Ove Rustas, Mohammad J. Taherzadeh, and Amir Mahboubi. 2024. "Effect of Agro-Industrial by Products Derived from Volatile Fatty Acids on Ruminant Feed In Vitro Digestibility" Animals 14, no. 16: 2330. https://doi.org/10.3390/ani14162330
APA StyleParchami, M., Rustas, B.-O., Taherzadeh, M. J., & Mahboubi, A. (2024). Effect of Agro-Industrial by Products Derived from Volatile Fatty Acids on Ruminant Feed In Vitro Digestibility. Animals, 14(16), 2330. https://doi.org/10.3390/ani14162330








