Whole-Genome Resequencing Reveals Signatures of Adaptive Evolution in Acanthopagrus latus and Rhabdosargus sarba
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Source of the Samples
2.3. Sequence Data and Variant Detection
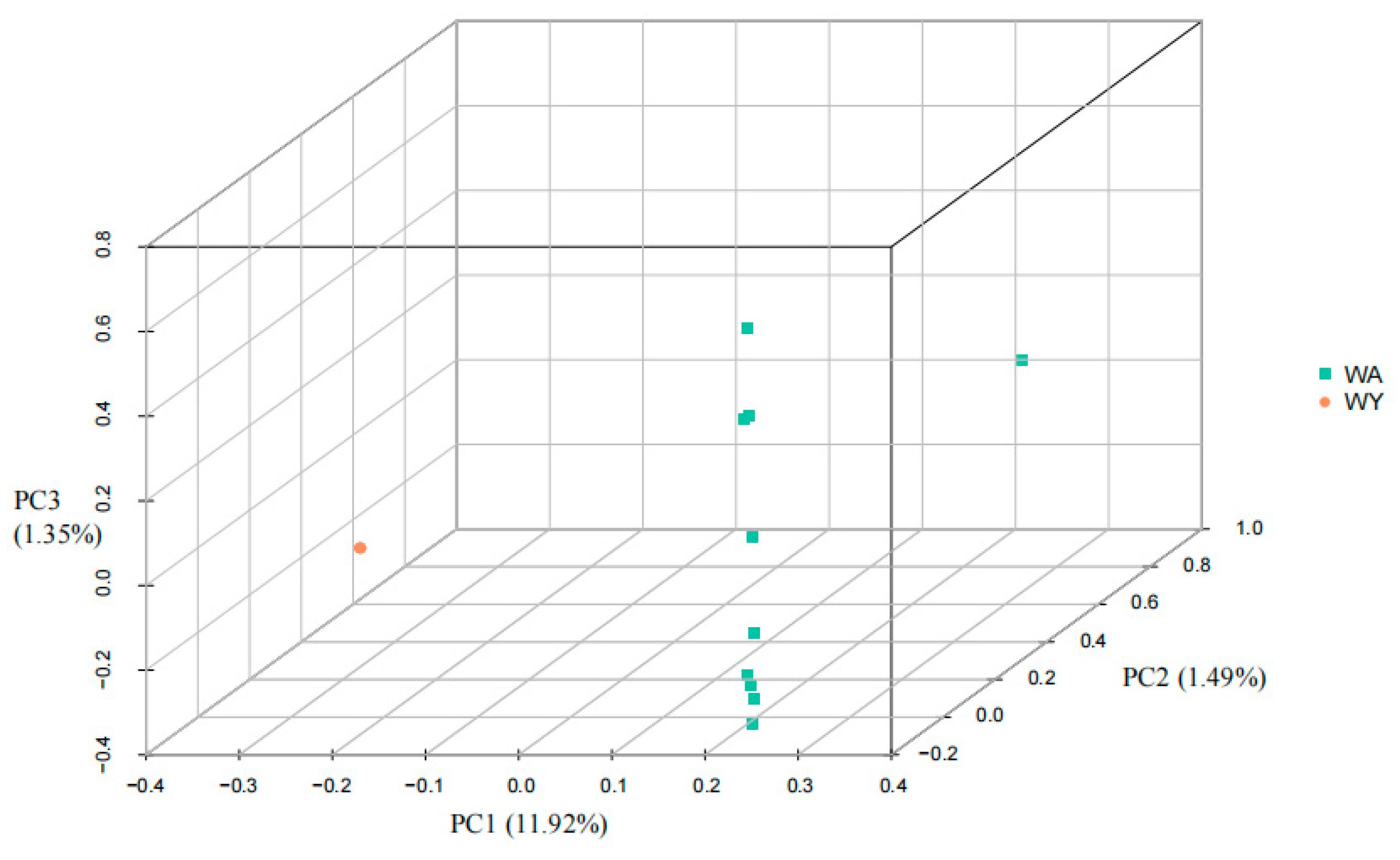
2.4. PCA Analysis
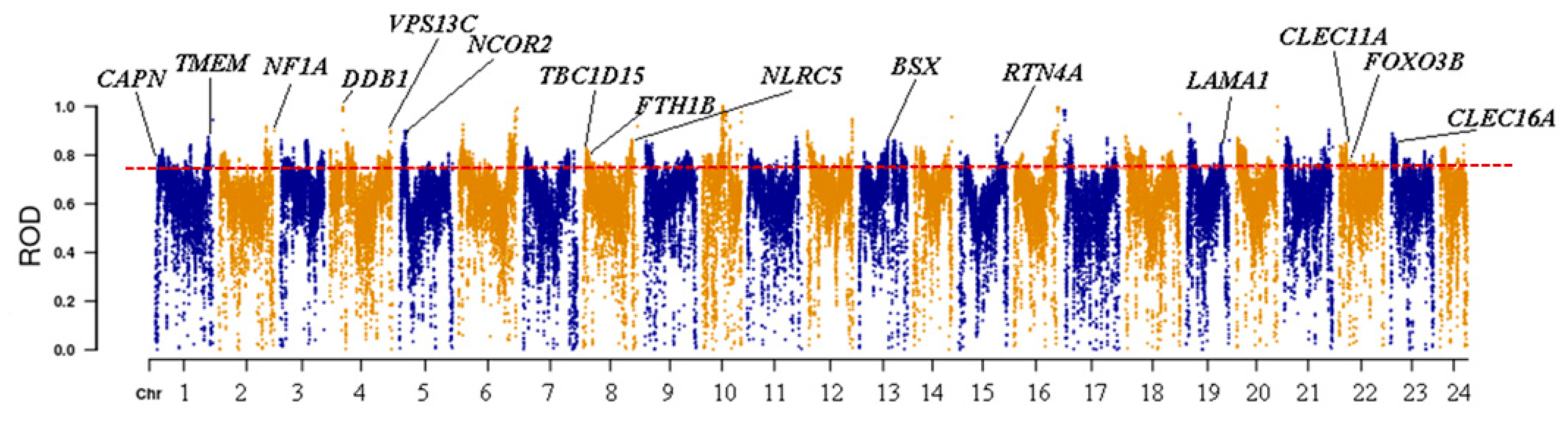
2.5. Genetic Diversity Analysis and Candidate Genes for Selection
2.6. Functional Annotation and Enrichment Analysis of Candidate Genes
3. Results
3.1. Whole-Genome Resequencing Data of Yellowfin-Seabream and Goldlined-Seabream Populations
3.2. Genetic Population Structure of the Yellowfin-Seabream and Goldline-Seabream Individuals
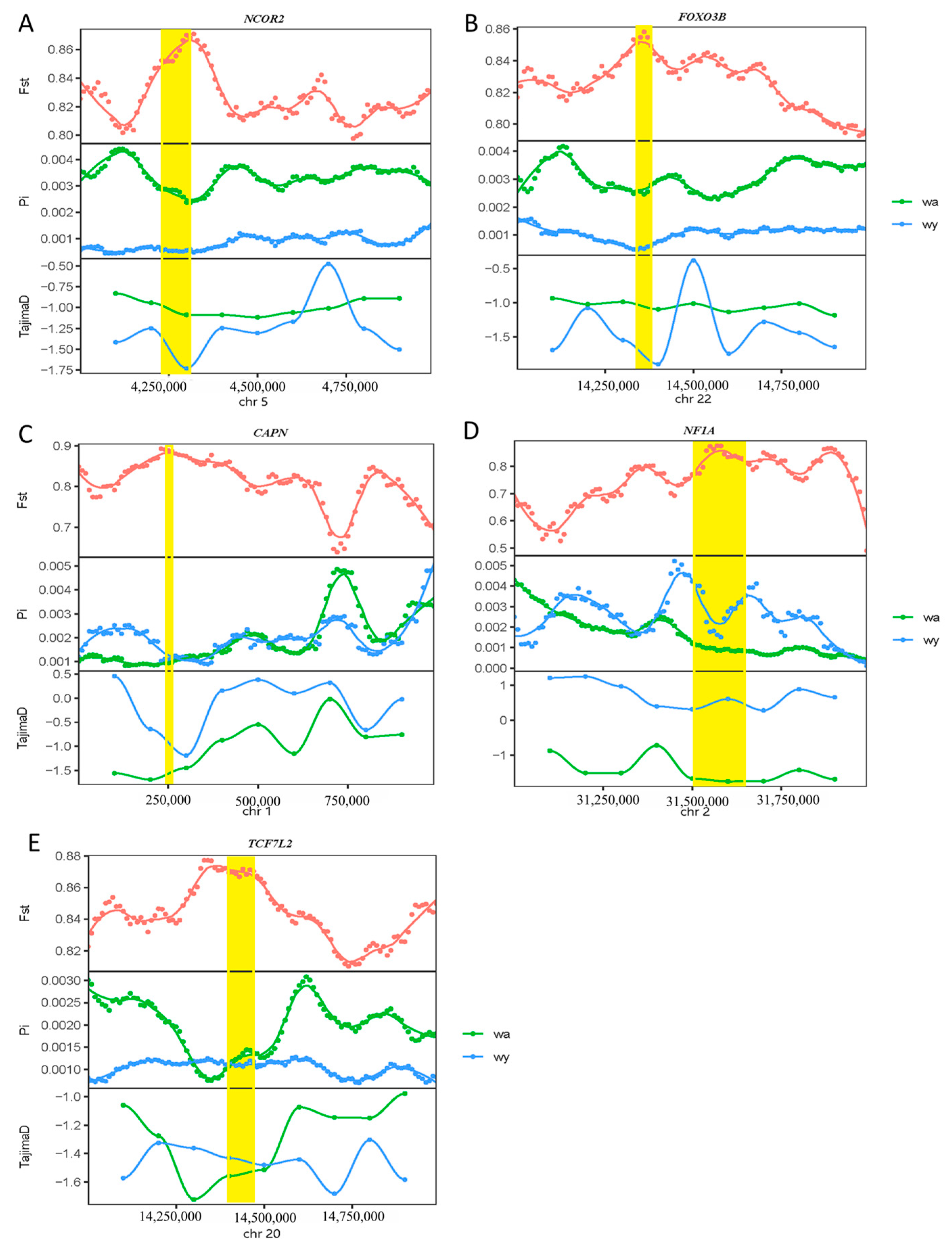
3.3. Detection of Genes Related to Adaptive Evolution in Yellowfin-Seabream and Goldlined-Seabream Populations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iwatsuki, Y. Review of the Acanthopagrus latus complex (Perciformes: Sparidae) with descriptions of three new species from the Indo-West Pacific Ocean. J. Fish Biol. 2013, 83, 64–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.B.; Hsu, T.L.; Joung, S.J. The reproductive biology of the hermaphroditic goldlined seabream Rhabdosargus sarba in the central waters of the Taiwan Strait. Fish. Sci. 2020, 86, 793–805. [Google Scholar] [CrossRef]
- Hesp, S.A. Biology of Two Species of Sparid on the West Coast of Australia. Ph.D. Thesis, Murdoch University, Perth, Australia, 2003. [Google Scholar]
- Zeng, J.; Chen, Q.; Huang, W.; Du, P.; Yang, H. Reform of the marine ecological protection system in China: From marine protected areas to marine ecological redline regions. Acta Ecol. Sin. 2016, 36, 1–10. [Google Scholar] [CrossRef]
- Hesp, S.A.; Potter, I.C. Reproductive biology of Rhabdosargus sarba (Sparidae) in Western Australian waters, in which it is a rudimentary hermaphrodite. J. Mar. Biol. Assoc. United Kingd. 2003, 83, 1333–1346. [Google Scholar] [CrossRef]
- Joung, S.-J.; Shyh, Y.-Y.; Liu, K.-M.; Wang, S.-B. Age, growth and mortality of the goldlined seabream Rhabdosargus sarba in waters off southwestern Taiwan. Pac. Sci. 2019, 73, 389–402. [Google Scholar] [CrossRef]
- Vahabnezhad, A.; Taghavimotlagh, S. Growth pattern and reproductive biology of Acanthopagrus latus from the Persian Gulf. J. Surv. Fish. Sci. 2017, 4, 18–28. [Google Scholar] [CrossRef]
- Grandcourt, E.; Al Abdessalaam, T.; Francis, F.; Al Shamsi, A. Demographic parameters and status assessments of Lutjanus ehrenbergii, Lethrinus lentjan, Plectorhinchus sordidus and Rhabdosargus sarba in the southern Arabian Gulf. J. Appl. Ichthyol. 2011, 27, 1203–1211. [Google Scholar] [CrossRef]
- Lin, G.; Zheng, M.; Li, S.; Xie, J.; Fang, W.; Gao, D.; Huang, J.; Lu, J. Response of gut microbiota and immune function to hypoosmotic stress in the yellowfin seabream (Acanthopagrus latus). Sci. Total Environ. 2020, 745, 140976. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.-H.; Huang, C.-W.; Lee, H.-T.; Kuo, Y.-H.; Liu, K.-M.; Lin, C.-H.; Gong, H.-Y. Population genetic analysis for stock enhancement of silver sea bream (Rhabdosargus sarba) in Taiwan. Fishes 2020, 5, 19. [Google Scholar] [CrossRef]
- Xia, J.H.; Huang, J.H.; Gong, J.B.; Jiang, S.G. Significant population genetic structure of yellowfin seabream Acanthopagrus latus in China. J. Fish Biol. 2008, 73, 1979–1992. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, J.; Liu, S.; Song, P.; Guan, Y.; Shan, B.; Li, Y.; Lin, L. Genetic diversity of the yellowfin seabream, Acanthopagrus latus (Actinopterygii: Perciformes: Sparidae)―An enhancement species in Dongshan Bay. Acta Ichthyol. Piscat. 2021, 51, 281–287. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Su, T.; Gong, S. Molecular phylogeny of mitochondrial cytochrome b gene sequences from four Sparicae fishes. J. Fish. Sci. China 2003, 10, 184–188. [Google Scholar] [CrossRef]
- Jr, B.; William, C. Uniparental inheritance of mitochondrial and chloroplast genes: Mechanisms and evolution. Proc. Natl. Acad. Sci. USA 1995, 92, 11331–11338. [Google Scholar] [CrossRef]
- Ankel-Simons, F.; Cummins, J.M. Misconceptions about mitochondria and mammalian fertilization: Implications for theories on human evolution. Proc. Natl. Acad. Sci. USA 1996, 93, 13859–13863. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Thudi, M.; Roorkiwal, M.; He, W.; Upadhyaya, H.D.; Yang, W.; Bajaj, P.; Cubry, P.; Rathore, A.; Jian, J.; et al. Resequencing of 429 chickpea accessions from 45 countries provides insights into genome diversity, domestication and agronomic traits. Nat. Genet. 2019, 51, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Liang, Z.; Yan, T.; Xu, Y.; Xuan, L.; Tang, J.; Zhou, G.; Lohwasser, U.; Hua, S.; Wang, H.; et al. Whole-Genome Resequencing of a Worldwide Collection of Rapeseed Accessions Reveals the Genetic Basis of Ecotype Divergence. Mol. Plant 2019, 12, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Pardo, A.P.; Ruzzante, D.E. Whole-genome sequencing approaches for conservation biology: Advantages, limitations and practical recommendations. Mol. Ecol. 2017, 26, 5369–5406. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, T.; Gao, T.; Song, N. Whole-genome resequencing reveals genetic diversity and selection signals in warm temperate and subtropical Sillago sinica populations. BMC Genom. 2023, 24, 547. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Qian, Y.; Zhang, Z.; Meng, M.; Deng, Y.; Wang, G.; He, S.; Yang, L. Chromosome-level genome assembly and whole-genome resequencing of topmouth culter (Culter alburnus) provide insights into the intraspecific variation of its semi-buoyant and adhesive eggs. Mol. Ecol. Resour. 2023, 23, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhuang, X.; Wu, L.; Lin, H.; Li, Y.; Wu, L.; Yao, J.; Liu, J.; Ding, S. Assessing the population genetic structure of yellow croaker in China: Insights into the ecological and genetic consequences of artificial breeding on natural populations. Aquaculture 2024, 590, 741026. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Abyzov, A.; Urban, A.E.; Snyder, M.; Gerstein, M. CNVnator: An approach to discover, genotype, and characterize typical and atypical CNVs from family and population genome sequencing. Genome Res. 2011, 21, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, N.; Liu, B.; Guo, L.; Guo, H.; Jiang, S.; Zhang, D. A chromosome-level genome assembly of the yellowfin seabream (Acanthopagrus latus) (Hottuyn, 1782) provides insights into its osmoregulation and sex reversal. bioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, Z.; Liu, F. Transcriptional and epigenetic control of hematopoietic stem cell fate decisions in vertebrates. Dev. Biol. 2021, 475, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Feng, Y.C.; Guo, S.T.; Wang, P.L.; Qi, T.F.; Yue, Y.M.; Wang, S.X.; Zhang, S.N.; Tang, C.X.; La, T.; et al. The pan-cancer lncRNA PLANE regulates an alternative splicing program to promote cancer pathogenesis. Nat. Commun. 2021, 12, 3734. [Google Scholar] [CrossRef]
- Webb, A.E.; Brunet, A. FOXO transcription factors: Key regulators of cellular quality control. Trends Biochem. Sci. 2014, 39, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.E.; Kundaje, A.; Brunet, A. Characterization of the direct targets of FOXO transcription factors throughout evolution. Aging Cell 2016, 15, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Shi, B.; Lu, H.; Zhang, L.; Zhang, W. Foxo3b but not Foxo3a activates cyp19a1a in Epinephelus coioides. J. Mol. Endocrinol. 2016, 56, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Min, Q.; Dong, R.; Zeng, S.; Zhang, X. Molecular Cloning and Expression Analyses of Foxo3a and Foxo3b Genes in Gobiocypris rarus. Fish. Sci. 2023, 42, 222–232. [Google Scholar] [CrossRef]
- Chen, X.; Ayala, I.; Shannon, C.; Fourcaudot, M.; Acharya, N.K.; Jenkinson, C.P.; Heikkinen, S.; Norton, L. The diabetes gene and Wnt pathway effector TCF7L2 regulates adipocyte development and function. Diabetes 2018, 67, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Fang, F.; Cang, J. The role of Notch signaling and its regulators in temporal specification of neural progenitor cells. Int. J. Anesthesiol. Resusc. 2018, 39, 6. [Google Scholar] [CrossRef]
- Zhou, J.R.; Li, M.; Luo, Z.; Zou, K.; Chen, Z.Z.; Yang, B.P.; Tang, Z.Y. Downregulation of Calpain 1 Causes Neurotoxicity and Apoptosis in Primary Neurons Induced by Aβ25-35. Nanosci. Nanotechnol. Lett. 2019, 11, 696–703. [Google Scholar] [CrossRef]
- Chen, W.X.; Ma, Y. Characterization and expression of the calpastatin gene in Cyprinus carpio. Genet. Mol. Res. 2015, 14, 7406–7416. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zheng, X.; Yang, D. Knockout fth1b affects early mineralization of zebrafish pharyngeal teeth. West China J. Stomatol. 2021, 39, 32–37. [Google Scholar] [CrossRef]
- Zoledziewska, M.; Costa, G.; Pitzalis, M.; Cocco, E.; Melis, C.; Moi, L.; Zavattari, P.; Murru, R.; Lampis, R.; Morelli, L.; et al. Variation within the CLEC16A gene shows consistent disease association with both multiple sclerosis and type 1 diabetes in Sardinia. Genes Immun. 2009, 10, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, J.; Wang, B.; Wang, S.; Qiao, Y.; Tian, F.; Li, F. Research Progress on the Immune Functions of TMEM Family Members. Chin. J. Immunol. 2016, 32, 127–130. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.-Y.; Hu, G.-S.; Tang, W.-S.; Weng, L.; Zhang, Y.; Guo, H.; Yao, S.-S.; Liu, S.-Y.; Zhang, G.-L.; et al. DDB1 binds histone reader BRWD3 to activate the transcriptional cascade in adipogenesis and promote onset of obesity. Cell Rep. 2021, 35, 109281. [Google Scholar] [CrossRef] [PubMed]
- Ruz, M.; Song, S.; Pan, J.; Yang, M.; Men, X.; Zhao, J.; Jiang, L.; Ma, Y. Identification of SNPs in VPS13C and EIF4G1 and their Association with Milk Production in Goat. J. Domest. Anim. Ecol. 2017, 38, 8. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Cheng, D.; Wang, T.; Lu, C.; Chen, J.; Nie, Z.; Zhang, W.; Lv, Z.; Wu, W.; et al. A New Member of the TBC1D15 Family from Chiloscyllium plagiosum: Rab GTPase-Activating Protein Based on Rab7 as a Substrate. Mar. Drugs 2015, 13, 2955–2966. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Cui, J.; Li, Q.; Zou, J.; Wang, H.Y.; Wang, R.-F. Enhanced TLR-induced NF-κB signaling and type I interferon responses in NLRC5 deficient mice. Cell Res. 2012, 22, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, M.B.; Hertz, H.; Bering, T.; Moller, M.; Rohde, K.; Klein, D.C.; Coon, S.L.; Rath, M.F. Circadian regulation and molecular role of the Bsx homeobox gene in the adult pineal gland. J. Pineal Res. 2020, 68, e12629. [Google Scholar] [CrossRef] [PubMed]
- Kulczynska-Przybik, A.; Dulewicz, M.; Slowik, A.; Borawska, R.; Kulakowska, A.; Kochanowicz, J.; Mroczko, B. The Clinical Significance of Cerebrospinal Fluid Reticulon 4 (RTN4) Levels in the Differential Diagnosis of Neurodegenerative Diseases. J. Clin. Med. 2021, 10, 5281. [Google Scholar] [CrossRef] [PubMed]
- Schiff, E.R.; Aychoua, N.; Nutan, S.; Davagnanam, I.; Moore, A.T.; Robson, A.G.; Patel, C.K.; Webster, A.R.; Arno, G. Variability of retinopathy consequent upon novel mutations in LAMA1. Ophthalmic Genet. 2022, 43, 671–678. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Cai, Z.; Fang, Y.; Shan, B.; Zhang, R.; Lin, L.; Li, Y.; Zhang, J. Whole-Genome Resequencing Reveals Signatures of Adaptive Evolution in Acanthopagrus latus and Rhabdosargus sarba. Animals 2024, 14, 2339. https://doi.org/10.3390/ani14162339
Yang J, Cai Z, Fang Y, Shan B, Zhang R, Lin L, Li Y, Zhang J. Whole-Genome Resequencing Reveals Signatures of Adaptive Evolution in Acanthopagrus latus and Rhabdosargus sarba. Animals. 2024; 14(16):2339. https://doi.org/10.3390/ani14162339
Chicago/Turabian StyleYang, Jingyu, Zizi Cai, Yan Fang, Binbin Shan, Ran Zhang, Longshan Lin, Yuan Li, and Jing Zhang. 2024. "Whole-Genome Resequencing Reveals Signatures of Adaptive Evolution in Acanthopagrus latus and Rhabdosargus sarba" Animals 14, no. 16: 2339. https://doi.org/10.3390/ani14162339



