Effects of Cryoprotectant Concentration and Exposure Time during Vitrification of Immature Pre-Pubertal Lamb Cumulus–Oocyte Complexes on Nuclear and Cytoplasmic Maturation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ovary Collection and COC Recovery
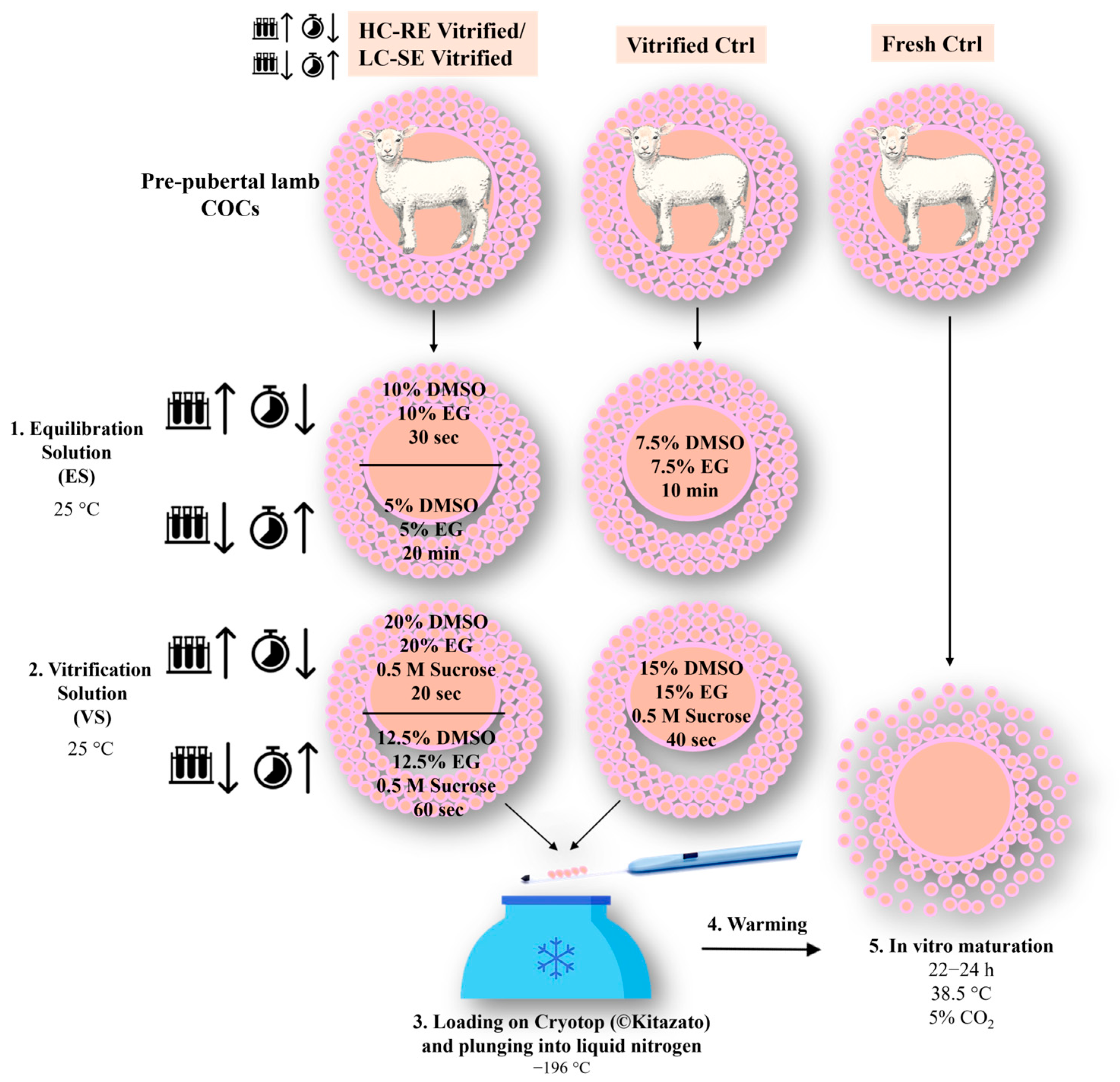
2.3. COC Vitrification and Warming
2.4. COC Morphology Assessment after Vitrification-Warming
2.5. In Vitro Maturation (IVM)
2.6. Cumulus Expansion Assessment and Oocyte Denuding
2.7. Oocyte Mitochondria and ROS Staining
2.8. Oocyte Nuclear Chromatin Evaluation
2.9. Mitochondrial Distribution Pattern and Intracellular ROS Localization Assessment
2.10. Quantification of Bioenergetic/Oxidative Parameters
2.11. Statistical Analysis
3. Results
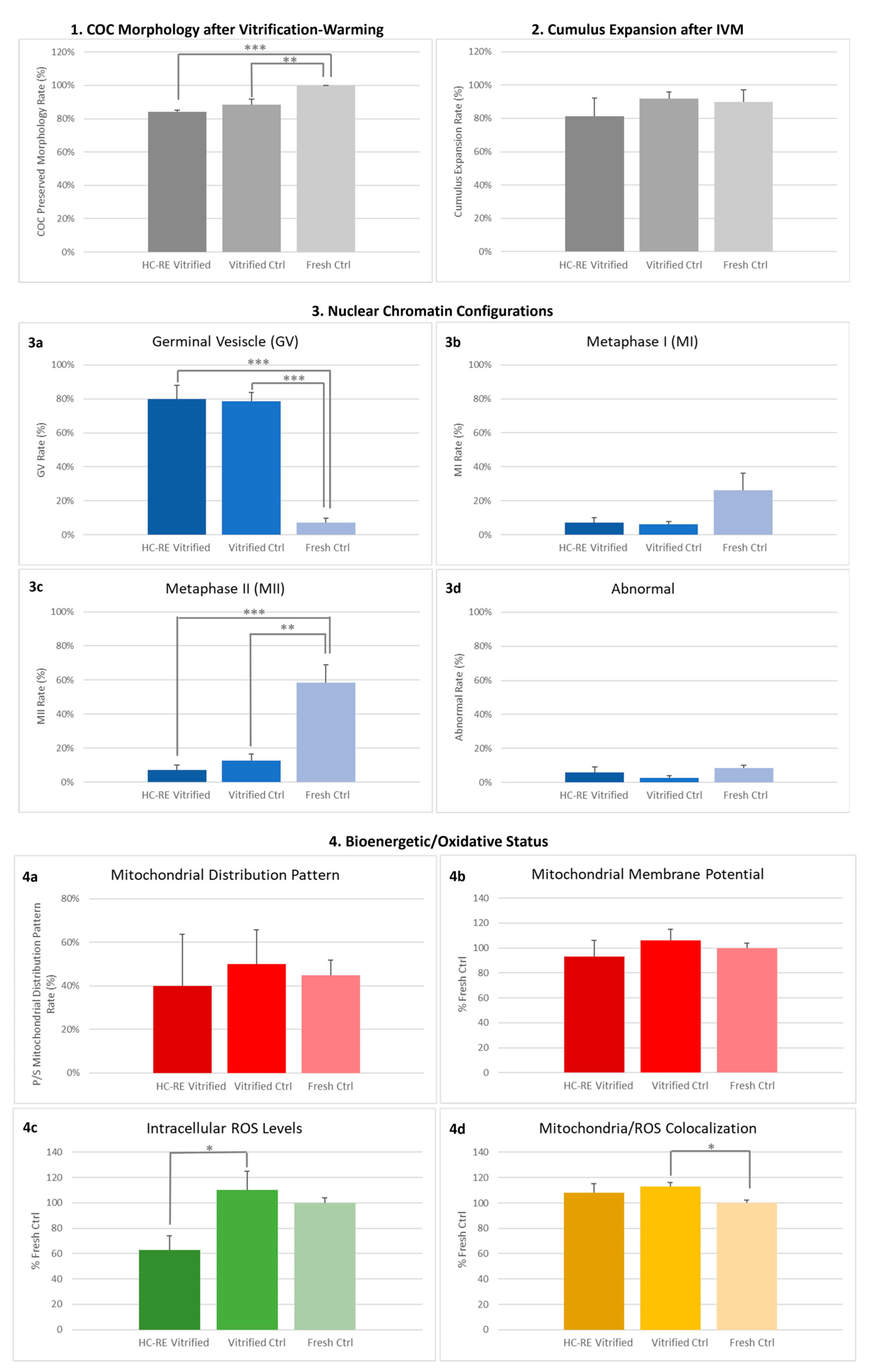
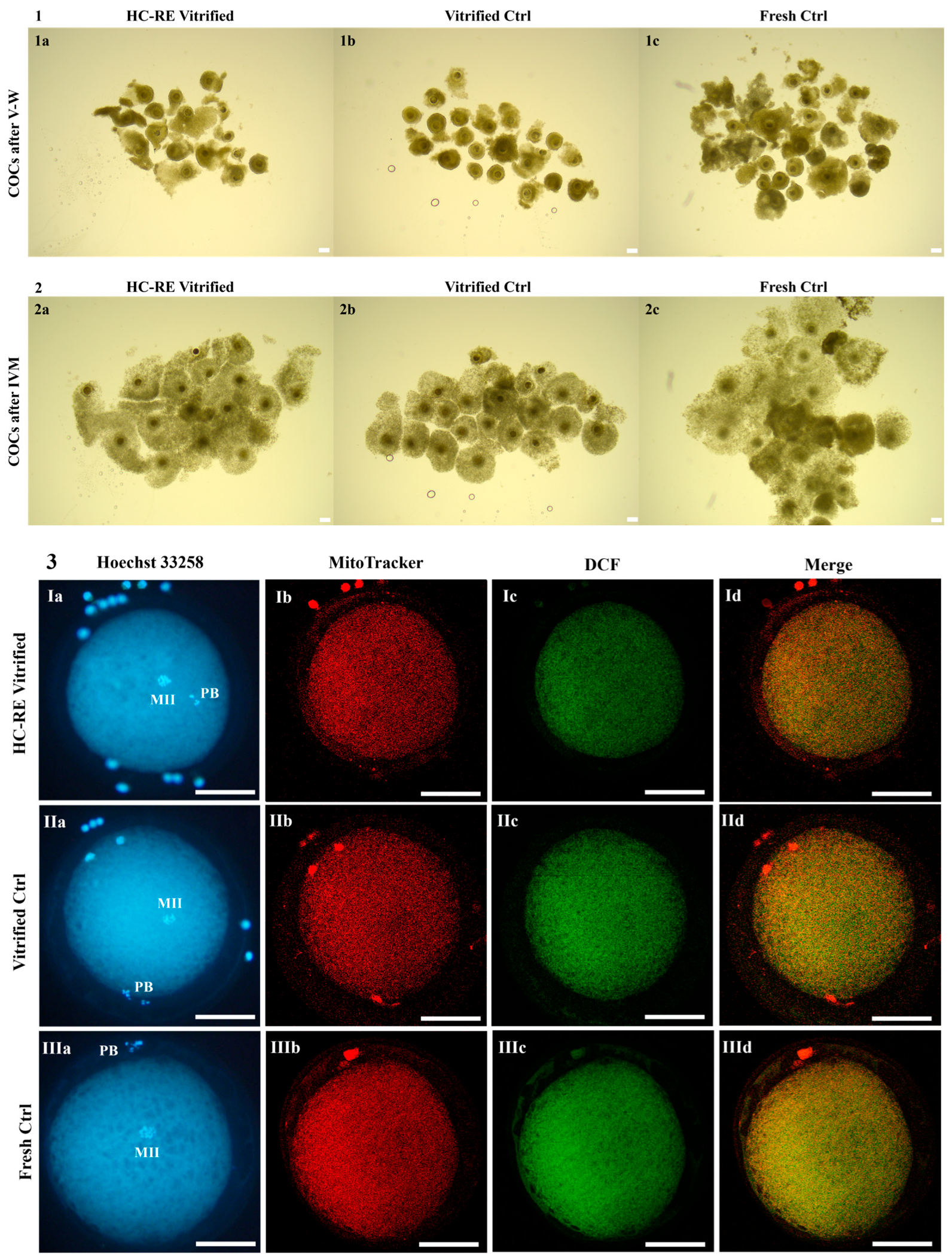
3.1. HC-RE Vitrification Protocol Preserved COC Morphology
3.2. HC-RE Vitrification Protocol Allowed Cumulus Expansion
3.3. HC-RE Vitrification Protocol Reduced Oocyte Meiotic Maturation
3.4. HC-RE Vitrification Protocol Did Not Affect Oocyte Mitochondria Distribution Pattern
3.5. HC-RE Vitrification Protocol Reduced Oocyte Intracellular ROS Levels
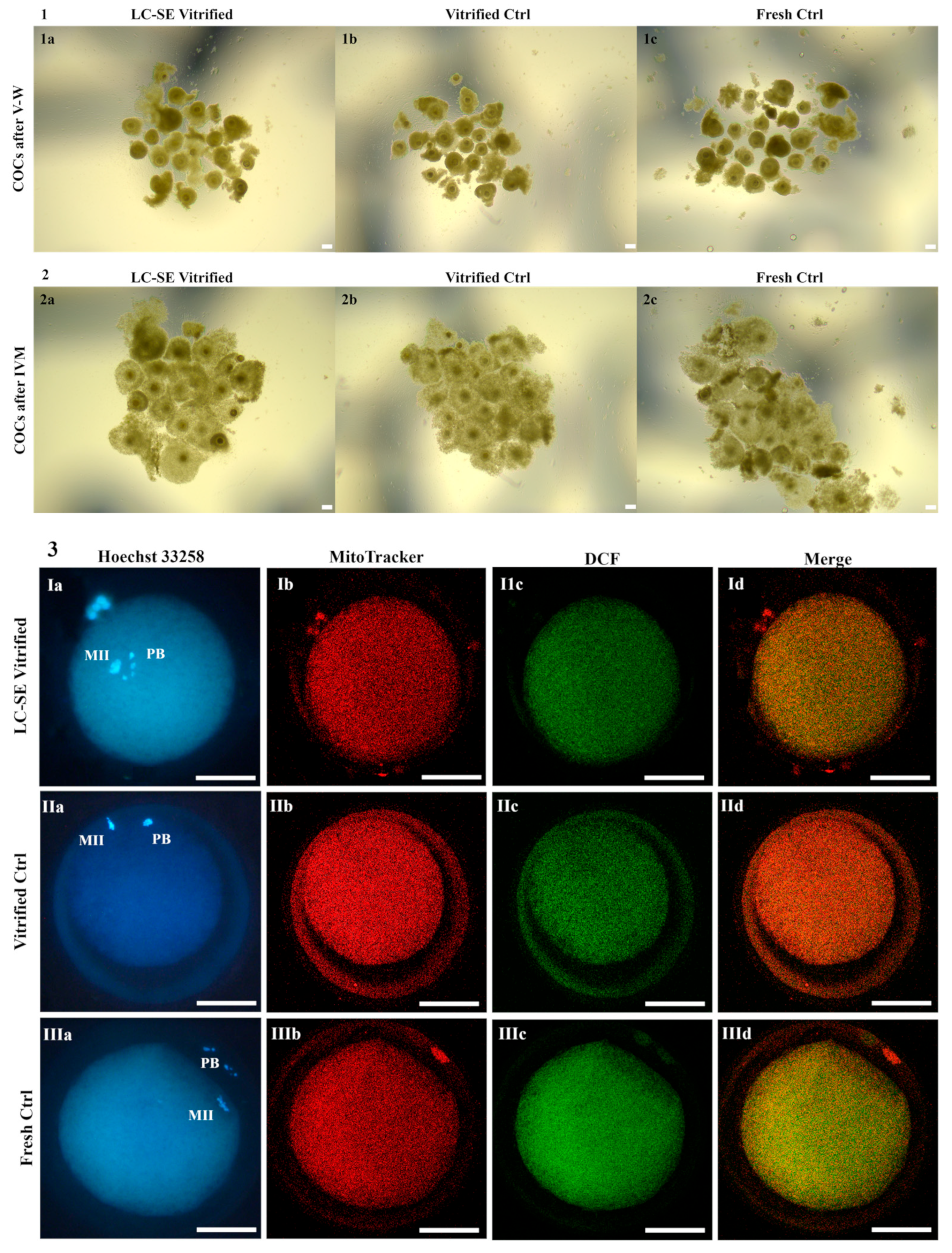
3.6. LC-SE Vitrification Protocol Preserved COC Morphology
3.7. LC-SE Vitrification Protocol Allowed Cumulus Expansion
3.8. LC-SE Vitrification Protocol Reduced Oocyte Meiotic Maturation
3.9. LC-SE Vitrification Protocol Did Not Affect Oocyte Mitochondria Distribution Pattern
3.10. LC-SE Vitrification Protocol Did Not Affect Quantitative Parameters of Bioenergetic-Oxidative Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Temerario, L.; Monaco, D.; Mastrorocco, A.; Martino, N.A.; Cseh, S.; Lacalandra, G.M.; Ciani, E.; Dell’Aquila, M.E. New Strategies for Conservation of Gentile di Puglia Sheep Breed, an Autochthonous Capital of Millennial Tradition in Southern Italy. Animals 2023, 13, 2371. [Google Scholar] [CrossRef] [PubMed]
- Sardaro, R.; La Sala, P. New Value to Wool: Innovative Garments for Preservation of Sheep Landraces in Italy. Animals 2021, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Sponenberg, D.P.; Beranger, J.; Martin, A.M.; Couch, C.R. Conservation of rare and local breeds of livestock. Rev. Sci. Tech. 2018, 37, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Woelders, H.; Windig, J.; Hiemstra, S.J. How developments in cryobiology, reproductive technologies and conservation genomics could shape gene banking strategies for (farm) animals. Reprod. Domest. Anim. 2012, 47 (Suppl. S4), 264–273. [Google Scholar] [CrossRef] [PubMed]
- Martino, N.A.; Picardi, E.; Ciani, E.; D’Erchia, A.M.; Bogliolo, L.; Ariu, F.; Mastrorocco, A.; Temerario, L.; Mansi, L.; Palumbo, V.; et al. Cumulus Cell Transcriptome after Cumulus-Oocyte Complex Exposure to Nanomolar Cadmium in an In Vitro Animal Model of Prepubertal and Adult Age. Biology 2023, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Succu, S.; Serra, E.; Gadau, S.; Varcasia, A.; Berlinguer, F. Vitrification of In Vitro Matured Oocytes Collected from Adult and Prepubertal Ovaries in Sheep. J. Vis. Exp. 2021, 173, e62272. [Google Scholar] [CrossRef]
- Reader, K.L.; Cox, N.R.; Stanton, J.A.; Juengel, J.L. Mitochondria and vesicles differ between adult and prepubertal sheep oocytes during IVM. Reprod. Fertil. Dev. 2015, 27, 513–522. [Google Scholar] [CrossRef]
- Leoni, G.G.; Palmerini, M.G.; Satta, V.; Succu, S.; Pasciu, V.; Zinellu, A.; Carru, C.; Macchiarelli, G.; Nottola, S.A.; Naitana, S.; et al. Differences in the Kinetic of the First Meiotic Division and in Active Mitochondrial Distribution between Prepubertal and Adult Oocytes Mirror Differences in their Developmental Competence in a Sheep Model. PLoS ONE 2015, 10, e0124911. [Google Scholar] [CrossRef]
- Bebbere, D.; Ariu, F.; Bogliolo, L.; Masala, L.; Murrone, O.; Fattorini, M.; Falchi, L.; Ledda, S. Expression of maternally derived KHDC3, NLRP5, OOEP and TLE6 is associated with oocyte developmental competence in the ovine species. BMC Dev. Biol. 2014, 14, 40. [Google Scholar] [CrossRef]
- Ledda, S.; Bogliolo, L.; Calvia, P.; Leoni, G.; Naitana, S. Meiotic progression and developmental competence of oocytes collected from juvenile and adult ewes. J. Reprod. Fertil. 1997, 109, 73–78. [Google Scholar] [CrossRef]
- O’Brien, J.K.; Catt, S.L.; Ireland, K.A.; Maxwell, W.M.; Evans, G. In vitro and in vivo developmental capacity of oocytes from prepubertal and adult sheep. Theriogenology 1997, 47, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Mastrorocco, A.; Cacopardo, L.; Lamanna, D.; Temerario, L.; Brunetti, G.; Carluccio, A.; Robbe, D.; Dell’Aquila, M.E. Bioengineering Approaches to Improve In Vitro Performance of Prepubertal Lamb Oocytes. Cells 2021, 10, 1458. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, D.; Catalá, M.G.; Paramio, M.T. Small ruminants: Prepubertal oocyte donors. Methods Mol. Biol. 2019, 2006, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Morton, K.M. Developmental capabilities of embryos produced in vitro from prepubertal lamb oocytes. Reprod. Domest. Anim. 2008, 43 (Suppl. S2), 137–143. [Google Scholar] [CrossRef] [PubMed]
- Souza-Fabjan, J.M.C.; Leal, G.R.; Monteiro, C.A.S.; Batista, R.I.T.P.; Barbosa, N.O.; Freitas, V.J.F. In vitro embryo production in small ruminants: What is still missing? Anim. Reprod. 2023, 20, e20230055. [Google Scholar] [CrossRef] [PubMed]
- Falchi, L.; Ledda, S.; Zedda, M.T. Embryo biotechnologies in sheep: Achievements and new improvements. Reprod. Domest. Anim. 2022, 57 (Suppl. S5), 22–33. [Google Scholar] [CrossRef] [PubMed]
- Paramio, M.T.; Izquierdo, D. Recent advances in in vitro embryo production in small ruminants. Theriogenology 2016, 86, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Alkali, I.M.; Luvoni, G.C. Microenvironment factors promoting the quality of vitrified cat oocytes. Theriogenology 2023, 196, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Tharasanit, T.; Thuwanut, P. Oocyte Cryopreservation in Domestic Animals and Humans: Principles, Techniques and Updated Outcomes. Animals 2021, 11, 2949. [Google Scholar] [CrossRef]
- Prentice, J.R.; Anzar, M. Cryopreservation of mammalian oocyte for conservation of animal genetics. Vet. Med. Int. 2010, 2011, 146405. [Google Scholar] [CrossRef]
- Pereira, R.M.; Marques, C.C. Animal oocyte and embryo cryopreservation. Cell Tissue Bank 2008, 9, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Boes, J.; Boettcher, P.; Honkatukia, M. Innovations in cryoconservation of animal genetic resources-Practical guide. In FAO Animal Production and Health Guidelines, No. 33; FAO: Rome, Italy, 2023; pp. 41–67. [Google Scholar]
- Brambillasca, F.; Guglielmo, M.C.; Coticchio, G.; Mignini Renzini, M.; Dal Canto, M.; Fadini, R. The current challenges to efficient immature oocyte cryopreservation. J. Assist. Reprod. Genet. 2013, 30, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Bogliolo, L.; Ariu, F.; Fois, S.; Rosati, I.; Zedda, M.T.; Leoni, G.; Succu, S.; Pau, S.; Ledda, S. Morphological and biochemical analysis of immature ovine oocytes vitrified with or without cumulus cells. Theriogenology 2007, 68, 1138–1149. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Carrageta, D.F.; Bernardino, R.L.; Alves, M.G.; Oliveira, P.F. Aquaporins and Animal Gamete Cryopreservation: Advances and Future Challenges. Animals 2022, 12, 359. [Google Scholar] [CrossRef] [PubMed]
- Iussig, B.; Maggiulli, R.; Fabozzi, G.; Bertelle, S.; Vaiarelli, A.; Cimadomo, D.; Ubaldi, F.M.; Rienzi, L. A brief history of oocyte cryopreservation: Arguments and facts. Acta Obstet. Gynecol. Scand. 2019, 98, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Ofosu, J.; Zhang, Y.; Liu, Y.; Sun, X.; Quan, G.; Alvarez Rodriguez, M.; Zhou, G. Editorial: Cryopreservation of mammalian gametes and embryos: Implications of oxidative and nitrosative stress and potential role of antioxidants. Front. Vet. Sci. 2023, 10, 1174756. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Qin, J.; Pan, B.; Qazi, I.H.; Ye, J.; Fang, Y.; Zhou, G. Oxidative Stress and Oocyte Cryopreservation: Recent Advances in Mitigation Strategies Involving Antioxidants. Cells 2022, 11, 3573. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Di Nardo, M.; Adiga, S.K.; Talevi, R. Mitochondrial Dysfunction and Oxidative Stress Caused by Cryopreservation in Reproductive Cells. Antioxidants 2021, 10, 337. [Google Scholar] [CrossRef]
- Mateo-Otero, Y.; Yeste, M.; Damato, A.; Giaretta, E. Cryopreservation and oxidative stress in porcine oocytes. Res. Vet. Sci. 2021, 135, 20–26. [Google Scholar] [CrossRef]
- Kuwayama, M.; Vajta, G.; Kato, O.; Leibo, S.P. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod. Biomed. Online 2005, 11, 300–308. [Google Scholar] [CrossRef]
- Liu, J.; Lee, G.Y.; Biggers, J.D.; Toth, T.L.; Toner, M. Low cryoprotectant concentration rapid vitrification of mouse oocytes and embryos. Cryobiology 2021, 98, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Whaley, D.; Damyar, K.; Witek, R.P.; Mendoza, A.; Alexander, M.; Lakey, J.R. Cryopreservation: An Overview of Principles and Cell-Specific Considerations. Cell Transplant. 2021, 30, 963689721999617. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, T.; Martínez-Rodero, I.; Roncero-Carol, J.; Yánez-Ortiz, I.; Higgins, A.Z.; Mogas, T. Impact of equilibration duration combined with temperature on the outcome of bovine oocyte vitrification. Theriogenology 2022, 184, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Canesin, H.S.; Brom-de-Luna, J.G.; Choi, Y.H.; Pereira, A.M.; Macedo, G.G.; Hinrichs, K. Vitrification of germinal-vesicle stage equine oocytes: Effect of cryoprotectant exposure time on in-vitro embryo production. Cryobiology 2018, 81, 185–191. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, T.; Mogas, T.; Mullen, S.F.; Martínez-Rodero, I.; Gulieva, R.E.; Higgins, A.Z. Effect of cryoprotectant concentration on bovine oocyte permeability and comparison of two membrane permeability modelling approaches. Sci. Rep. 2021, 11, 15387. [Google Scholar] [CrossRef] [PubMed]
- Ledda, S.; Bogliolo, L.; Leoni, G.; Naitana, S. Cell coupling and maturation-promoting factor activity in in vitro-matured prepubertal and adult sheep oocytes. Biol. Reprod. 2001, 65, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Agca, Y.; Liu, J.; Peter, A.T.; Critser, E.S.; Critser, J.K. Effect of developmental stage on bovine oocyte plasma membrane water and cryoprotectant permeability characteristics. Mol. Reprod. Dev. 1998, 49, 408–415. [Google Scholar] [CrossRef]
- Fabbri, R.; Porcu, E.; Marsella, T.; Primavera, M.R.; Rocchetta, G.; Ciotti, P.M.; Magrini, O.; Seracchioli, R.; Venturoli, S.; Flamigni, C. Technical aspects of oocyte cryopreservation. Mol. Cell Endocrinol. 2000, 169, 39–42. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Santos, M.V.; de Queiroz Neta, L.B.; Avezedo Borges, A.; Fernandes Pereira, A. Influence of commercially available follicle stimulating hormone on the in vitro maturation of bovine oocytes. Semin. Ciênc. Agrár. 2017, 38, 1393–1402. [Google Scholar] [CrossRef]
- Yang, H.W.; Hwang, K.J.; Kwon, H.C.; Kim, H.S.; Choi, K.W.; Oh, K.S. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum. Reprod. 1998, 13, 998–1002. [Google Scholar] [CrossRef]
- Manders, E.M.M.; Verbeek, F.J.; Aten, J.A. Measurement of co-localization of objects in dual-colour confocal images. J. Microsc. 1993, 169, 375–382. [Google Scholar] [CrossRef]
- Dos Santos-Neto, P.C.; Vilariño, M.; Cuadro, F.; Barrera, N.; Crispo, M.; Menchaca, A. Cumulus cells during in vitro fertilization and oocyte vitrification in sheep: Remove, maintain or add? Cryobiology 2020, 92, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, E.; Shirazi, A.; Shams-Esfandabadi, N.; Nazari, H. Antioxidants and glycine can improve the developmental competence of vitrified/warmed ovine immature oocytes. Reprod. Domest. Anim. 2019, 54, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Moawad, A.R.; Choi, I.; Zhu, J.; El-Wishy, A.B.A.; Amarnath, D.; Chen, W.; Campbell, K.H.S. Caffeine and oocyte vitrification: Sheep as an animal model. Int. J. Vet. Sci. Med. 2018, 6, S41–S48. [Google Scholar] [CrossRef] [PubMed]
- Aono, A.; Nagatomo, H.; Takuma, T.; Nonaka, R.; Ono, Y.; Wada, Y.; Abe, Y.; Takahashi, M.; Watanabe, T.; Kawahara, M. Dynamics of intracellular phospholipid membrane organization during oocyte maturation and successful vitrification of immature oocytes retrieved by ovum pick-up in cattle. Theriogenology 2013, 79, 1146–1152.e1. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.D.; Forell, F.; Feltrin, C.; Rodrigues, J.L. Calves born after direct transfer of vitrified bovine in vitro-produced blastocysts derived from vitrified immature oocytes. Reprod. Domest. Anim. 2008, 43, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Hara, K.; Matsumoto, H.; Kobayashi, J.; Sasada, H.; Ekwall, H.; Rodriguez-Martinez, H.; Sato, E. Feasibility of a nylon-mesh holder for vitrification of bovine germinal vesicle oocytes in subsequent production of viable blastocysts. Biol. Reprod. 2005, 72, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.D.; Mezzalira, A.; Barbieri, D.P.; Lehmkuhl, R.C.; Rubin, M.I.; Vajta, G. Calves born after open pulled straw vitrification of immature bovine oocytes. Cryobiology 2002, 45, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Somfai, T.; Yoshioka, K.; Tanihara, F.; Kaneko, H.; Noguchi, J.; Kashiwazaki, N.; Nagai, T.; Kikuchi, K. Generation of live piglets from cryopreserved oocytes for the first time using a defined system for in vitro embryo production. PLoS ONE 2014, 9, e97731. [Google Scholar] [CrossRef]
- Clérico, G.; Taminelli, G.; Veronesi, J.C.; Polola, J.; Pagura, N.; Pinto, C.; Sansinena, M. Mitochondrial function, blastocyst development and live foals born after ICSI of immature vitrified/warmed equine oocytes matured with or without melatonin. Theriogenology 2021, 160, 40–49. [Google Scholar] [CrossRef]
- Ortiz-Escribano, N.; Bogado Pascottini, O.; Woelders, H.; Vandenberghe, L.; De Schauwer, C.; Govaere, J.; Van den Abbeel, E.; Vullers, T.; Ververs, C.; Roels, K.; et al. An improved vitrification protocol for equine immature oocytes, resulting in a first live foal. Equine Vet. J. 2018, 50, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Rui, R.; Dai, J.; Zhang, C.; Ju, S.; Xie, B.; Lu, X.; Zheng, X. Effects of cryopreservation on the developmental competence, ultrastructure and cytoskeletal structure of porcine oocytes. Mol. Reprod. Dev. 2006, 73, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Luvoni, G.C. Minimum volume vitrification of immature feline oocytes. J. Vis. Exp. 2020, 160, e61523. [Google Scholar] [CrossRef]
- Moawad, A.R.; Zhu, J.; Choi, I.; Amarnath, D.; Chen, W.; Campbell, K.H. Production of good-quality blastocyst embryos following IVF of ovine oocytes vitrified at the germinal vesicle stage using a cryoloop. Reprod. Fertil. Dev. 2013, 25, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Moawad, A.R.; Fisher, P.; Zhu, J.; Choi, I.; Polgar, Z.; Dinnyes, A.; Campbell, K.H.S. In vitro fertilization of ovine oocytes vitrified by solid surface vitrification at germinal vesicle stage. Cryobiology 2012, 65, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Dilixiati, A.; Zhang, L.; Aihemaiti, A.; Song, Y.; Zhao, G.; Fu, X.; Wang, X.; Wusiman, A. Mito-TEMPO Improves the Meiosis Resumption and Mitochondrial Function of Vitrified Sheep Oocytes via the Recovery of Respiratory Chain Activity. Animals 2024, 14, 152. [Google Scholar] [CrossRef]
- Quan, G.B.; Wu, G.Q.; Wang, Y.J.; Ma, Y.; Lv, C.R.; Hong, Q.H. Meiotic maturation and developmental capability of ovine oocytes at germinal vesicle stage following vitrification using different cryodevices. Cryobiology 2016, 72, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Reyez, F.; Ducolomb, Y.; Romo, S.; Casas, E.; Salazar, Z.; Betancourt, M. Viability, maturation and embryo development in vitro of immature porcine and ovine oocytes vitrified in different devices. Cryobiology 2012, 64, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, H.; Zheng, L.; Luo, Y.; Zhou, G.; Li, J.; Hou, Y.; Fu, X. Vitrification of Mammalian Oocytes: Recent Studies on Mitochondrial Dysfunction. Biopreserv. Biobank 2024. ahead of print. [Google Scholar] [CrossRef]
- Somoskoi, B.; Martino, N.A.; Cardone, R.A.; Lacalandra, G.M.; Dell’Aquila, M.E.; Cseh, S. Different chromatin and energy/redox responses of mouse morulae and blastocysts to slow freezing and vitrification. Reprod. Biol. Endocrinol 2015, 13, 22. [Google Scholar] [CrossRef]
- Fabbri, R.; Vicenti, R.; Macciocca, M.; Martino, N.A.; Dell’Aquila, M.E.; Pasquinelli, G.; Morselli-Labate, A.M.; Seracchioli, R.; Paradisi, R. Morphological, ultrastructural and functional imaging of frozen/thawed and vitrified/warmed human ovarian tissue retrieved from oncological patients. Hum. Reprod. 2016, 31, 1838–1849. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temerario, L.; Martino, N.A.; Bennink, M.; de Wit, A.; Hiemstra, S.J.; Dell’Aquila, M.E.; Lamy, J. Effects of Cryoprotectant Concentration and Exposure Time during Vitrification of Immature Pre-Pubertal Lamb Cumulus–Oocyte Complexes on Nuclear and Cytoplasmic Maturation. Animals 2024, 14, 2351. https://doi.org/10.3390/ani14162351
Temerario L, Martino NA, Bennink M, de Wit A, Hiemstra SJ, Dell’Aquila ME, Lamy J. Effects of Cryoprotectant Concentration and Exposure Time during Vitrification of Immature Pre-Pubertal Lamb Cumulus–Oocyte Complexes on Nuclear and Cytoplasmic Maturation. Animals. 2024; 14(16):2351. https://doi.org/10.3390/ani14162351
Chicago/Turabian StyleTemerario, Letizia, Nicola Antonio Martino, Monika Bennink, Agnes de Wit, Sipke Joost Hiemstra, Maria Elena Dell’Aquila, and Julie Lamy. 2024. "Effects of Cryoprotectant Concentration and Exposure Time during Vitrification of Immature Pre-Pubertal Lamb Cumulus–Oocyte Complexes on Nuclear and Cytoplasmic Maturation" Animals 14, no. 16: 2351. https://doi.org/10.3390/ani14162351
APA StyleTemerario, L., Martino, N. A., Bennink, M., de Wit, A., Hiemstra, S. J., Dell’Aquila, M. E., & Lamy, J. (2024). Effects of Cryoprotectant Concentration and Exposure Time during Vitrification of Immature Pre-Pubertal Lamb Cumulus–Oocyte Complexes on Nuclear and Cytoplasmic Maturation. Animals, 14(16), 2351. https://doi.org/10.3390/ani14162351




