Dynamics of Gill Responses to a Natural Infection with Neoparamoeba perurans in Farmed Tasmanian Atlantic Salmon
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
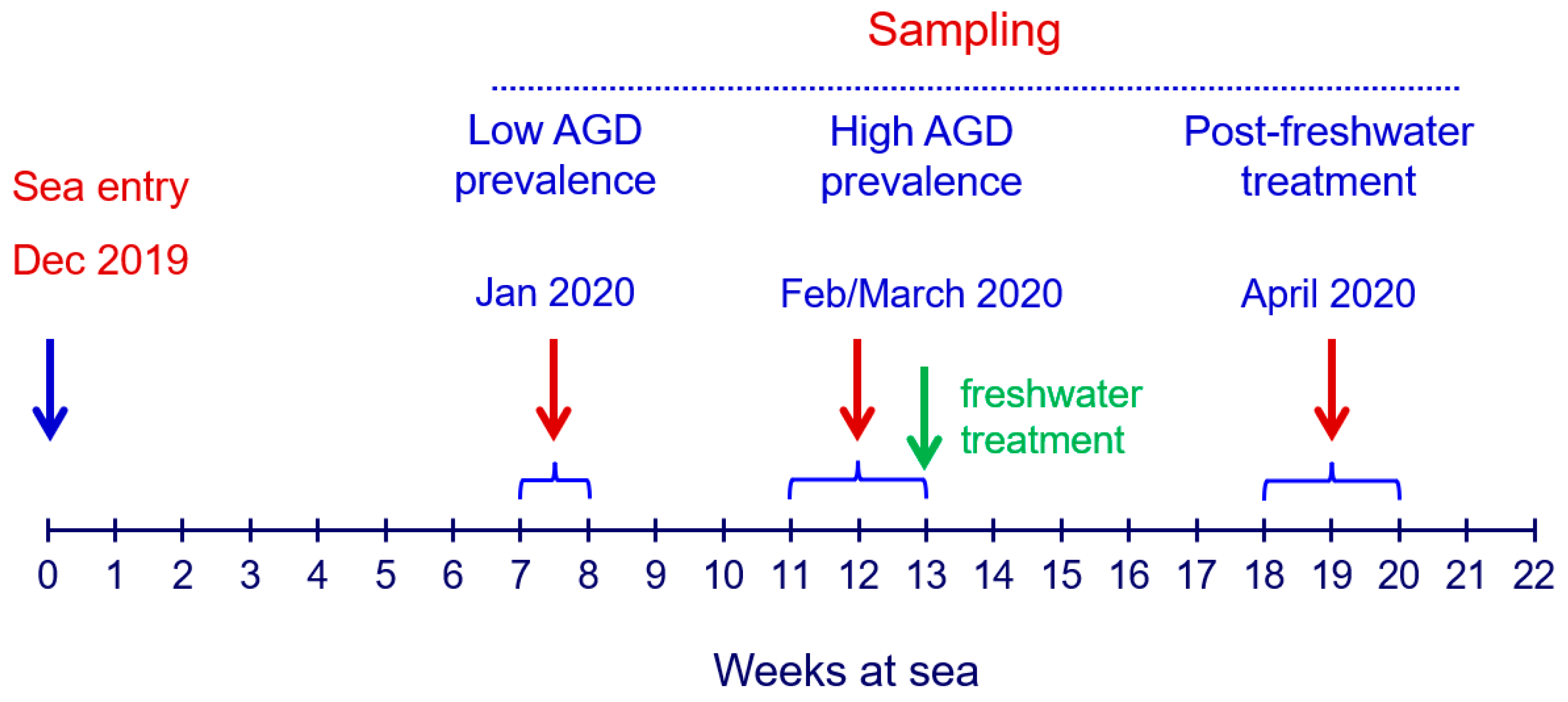
2.1. Fish Husbandry and Sampling Regime
2.2. Gill Histopathology
2.3. RNA Extraction and cDNA Synthesis
2.4. Detection of N. perurans and Expression of Host Genes in Gill Tissue
2.5. Statistical Analysis
3. Results
3.1. Gill Scores
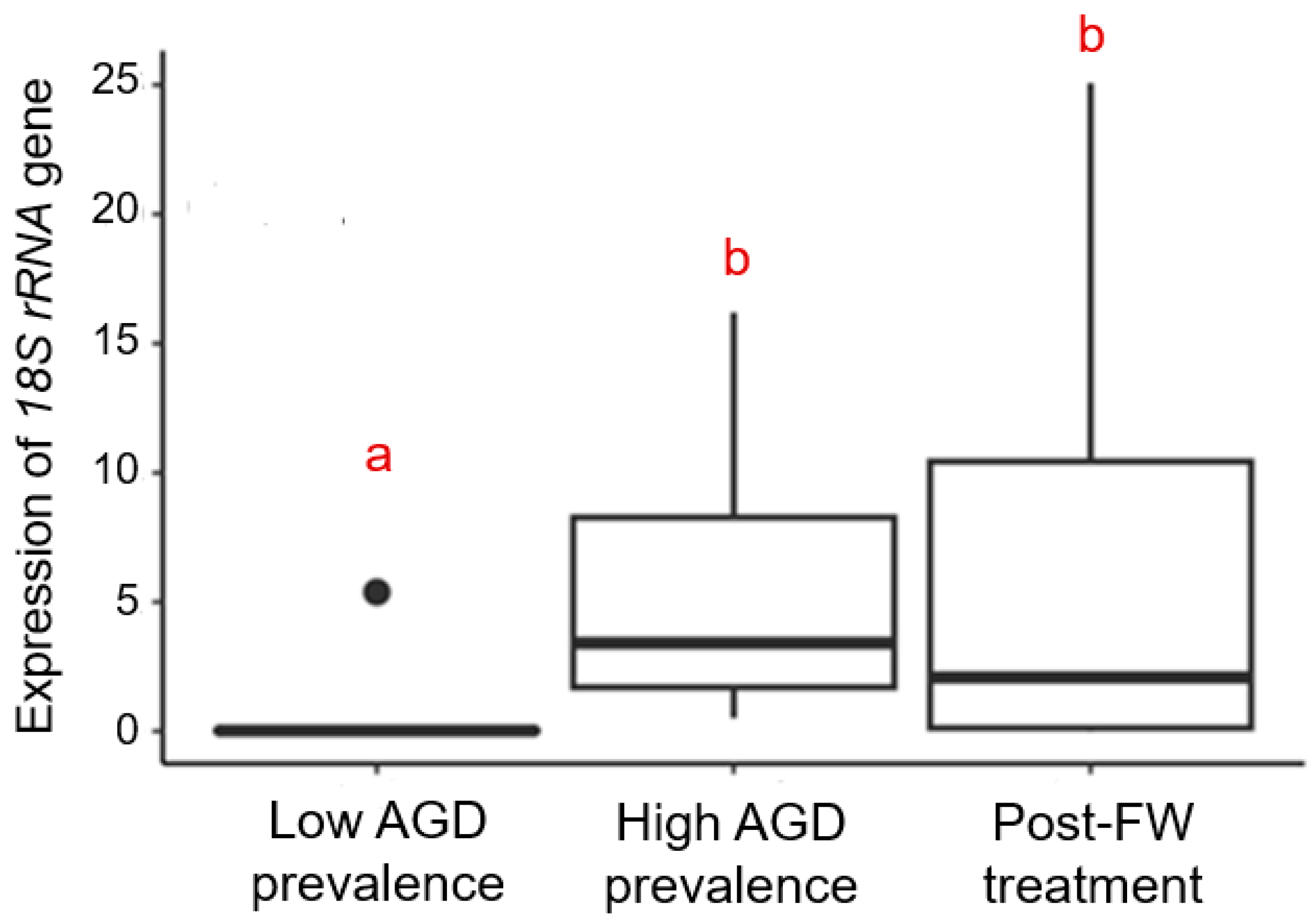
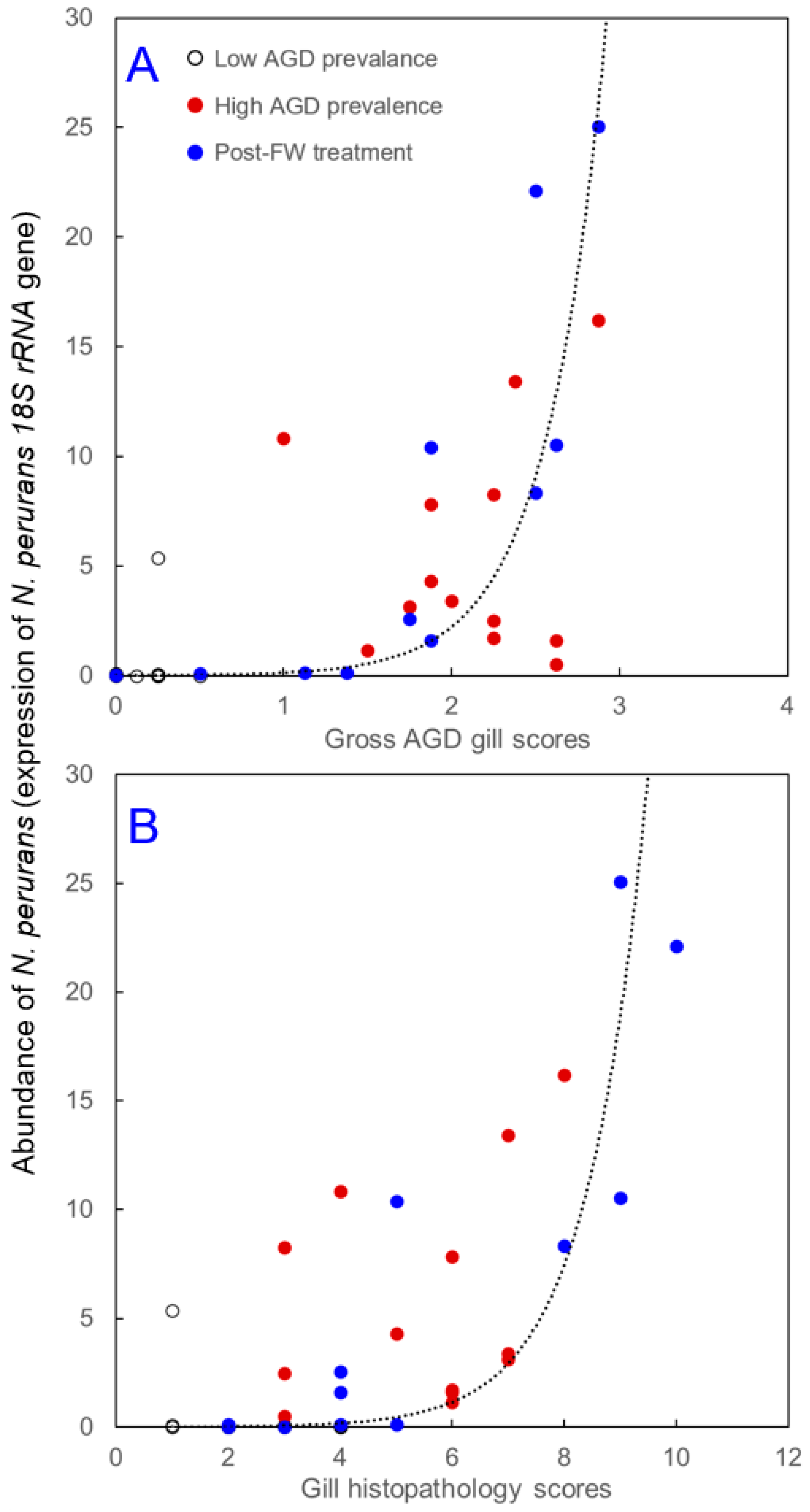
3.2. Abundance of N. perurans in Gill Tissue
3.3. Expression of Salmon Genes
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munday, B.L. Diseases of salmonids. In Proceedings of the Workshop on Diseases of Australian Fish and Shellfish; Humphrey, J.D., Langdon, J.A., Eds.; Victorian Development of Agriculture and Rural Affairs: Benalla, Australia, 1985; pp. 127–141. [Google Scholar]
- Young, N.D.; Crosbie, P.B.B.; Adams, M.B.; Nowak, B.F.; Morrison, R.N. Neoparamoeba perurans n. sp., an agent of amoebic gill disease of Atlantic salmon (Salmo salar). Int. J. Parasitol. 2007, 37, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Rodger, H.D. Gill disorders: An emerging problem for farmed Atlantic salmon (Salmo salar) in the marine environment? Fish Vet. J. 2007, 9, 38–48. [Google Scholar]
- Herrero, A.; Thompson, K.D.; Ashby, A.; Rodger, H.D.; Dagleish, M.P. Complex gill disease: An emerging syndrome in farmed Atlantic salmon (Salmo salar L.). J. Comp. Pathol. 2018, 163, 23–28. [Google Scholar] [CrossRef]
- Foyle, K.L.; Hess, S.; Powell, M.D.; Herbert, N.A. What is gill health and what is its role in marine finfish aquaculture in the face of a changing climate? Front. Mar. Sci. 2020, 7, 400. [Google Scholar] [CrossRef]
- Calado, R.; Mota, V.C.; Madeira, D.; Leal, M.C. Summer is coming! Tackling ocean warming in Atlantic salmon cage farming. Animals 2021, 11, 1800. [Google Scholar] [CrossRef]
- Sajid, Z.; Gamperl, A.K.; Parrish, C.C.; Colombo, S.M.; Santander, J.; Mather, C.; Neis, B.; Holmen, I.M.; Filgueira, R.; McKenzie, C.H.; et al. An aquaculture risk model to understand the causes and consequences of Atlantic salmon mass mortality events: A review. Rev. Aquac. 2024, 1–22. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Król, E.; Noguera, P.; Shaw, S.; Costelloe, E.; Gajardo, K.; Valdengro, V.; Bickerdinke, R.; Douglas, A.; Martin, S.A.M. Integration of transcriptome, gross morphology and histopathology in gill of sea farmed Atlantic salmon (Salmo salar): Lessons from multi-site sampling. Front. Genet. 2020, 11, 610. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.R.; Pinder, A.W. The respiratory development of Atlantic salmon: I. Morphometry of gills, yolk sac and body surface. J. Exp. Biol. 1996, 199, 2725–2736. [Google Scholar] [CrossRef]
- Koppang, E.O.; Fischer, U.; Moore, L.; Tranulis, M.A.; Dijkstra, J.M.; Köllner, B.; Aune, L.; Jirillo, E.; Hordvik, I. Salmonid T cells assemble in the thymus, spleen and in novel interbranchial lymphoid tissue. J. Anat. 2010, 217, 728–739. [Google Scholar] [CrossRef]
- Salinas, I. The mucosal immune system of teleost fish. Biology 2015, 4, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Rességuier, J.; Dalum, A.S.; Du Pasquier, L.; Zhang, Y.; Koppang, E.O.; Boudinot, P.; Wiegertjes, G.F. Lymphoid tissue in teleost gills: Variations on a theme. Biology 2020, 9, 127–214. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Takizawa, F.; Casadei, E.; Shibasaki, Y.; Ding, Y.; Sauters, T.J.C.; Yu, Y.; Salinas, I.; Sunyer, J.O. Specialization of mucosal immunoglobulins in pathogen control and microbiota homeostasis occurred early in vertebrate evolution. Sci. Immunol. 2020, 5, eaay3254. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.O.; Rodger, H.D. A review of infectious gill disease in marine salmonid fish. J. Fish Dis. 2011, 34, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.O.; Rodger, H.D.; Holland, Á.C.; Baxter, E.J. Development of a novel histopathological gill scoring protocol for assessment of gill health during a longitudinal study in marine-farmed Atlantic salmon (Salmo salar). Aquat. Int. 2012, 20, 813–825. [Google Scholar] [CrossRef]
- Gjessing, M.C.; Steinum, T.; Olsen, A.B.; Lie, K.I.; Tavornpanich, S.; Colquhoun, S.; Gjevre, A. Histopathological investigation of complex gill disease in sea farmed Atlantic salmon. PLoS ONE 2019, 14, e0222926. [Google Scholar] [CrossRef] [PubMed]
- Nowak, B.F.; Archibald, B.M. Opportunistic but lethal: The mystery of Paramoebae. Trends Parasitol. 2018, 34, 404–419. [Google Scholar] [CrossRef]
- Ajasa, A.A.; Boison, S.A.; Gjøen, H.M.; Lillehammer, M. Genome-assisted prediction of amoebic gill disease resistance in different populations of Atlantic salmon during field outbreak. Aquaculture 2024, 578, 740078. [Google Scholar] [CrossRef]
- Kent, M.L.; Sawyer, T.; Hedrick, R. Paramoeba pemaquidensis (Sarcomastigophora: Paramoebidae) infestation of the gills of coho salmon Oncorhynchus kisutch reared in sea water. Dis. Aquat. Org. 1988, 5, 163–169. [Google Scholar] [CrossRef]
- Steinum, T.; Kvellestad, A.; Rønneberg, L.B.; Nilsen, H.; Asheim, A.; Fiell, K.; Nygård, S.M.R.; Olsen, A.B.; Dale, O.B. First cases of amoebic gill disease (AGD) in Norwegian seawater farmed Atlantic salmon, Salmo salar L., and phylogeny of the causative amoeba using 18S cDNA sequences. J. Fish Dis. 2008, 31, 205–214. [Google Scholar] [CrossRef]
- Bustos, P.A.; Young, N.D.; Rozas, M.A.; Bohle, H.M.; Ildefonso, R.S.; Morrison, R.N.; Nowak, B.F. Amoebic gill disease (AGD) in Atlantic salmon (Salmo salar) farmed in Chile. Aquaculture 2011, 310, 281–288. [Google Scholar] [CrossRef]
- Powell, M.D.; Reynolds, P.; Kristensen, T. Freshwater treatment of amoebic gill disease and sea-lice in seawater salmon production: Considerations of water chemistry and fish welfare in Norway. Aquaculture 2015, 448, 18–22. [Google Scholar] [CrossRef]
- Oldham, T.; Rodger, H.; Nowak, B.F. Incidence and distribution of amoebic gill disease (AGD)—An epidemiological review. Aquaculture 2016, 457, 35–42. [Google Scholar] [CrossRef]
- Adams, M.B.; Nowak, B.F. Distribution and structure of lesions in the gills of Atlantic salmon, Salmo salar L., affected with amoebic gill disease. J. Fish Dis. 2001, 24, 535–542. [Google Scholar] [CrossRef]
- Adams, M.B.; Nowak, B.F. Amoebic gill disease: Sequential pathology in cultured Atlantic salmon, Salmo salar L. J. Fish Dis. 2003, 26, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.D.; Nowak, B.F.; Adams, M.B. Cardiac morphology in relation to amoebic gill disease history in Atlantic salmon, Salmo salar L. J. Fish Dis. 2002, 25, 209–215. [Google Scholar] [CrossRef]
- Kube, P.D.; Taylor, R.S.; Elliott, N.G. Genetic variation in parasite resistance of Atlantic salmon to amoebic gill disease over multiple infections. Aquaculture 2012, 364, 165–172. [Google Scholar] [CrossRef]
- Benedicenti, O.; Pottinger, T.G.; Collins, C.; Secombes, C.J. Effects of temperature on amoebic gill disease development: Does it play a role? J. Fish Dis. 2019, 42, 1241–1258. [Google Scholar] [CrossRef]
- Bowden, A.J.; Adams, M.B.; Andrewartha, S.J.; Elliott, N.G.; Frappell, P.B.; Clark, T.D. Amoebic gill disease increases energy requirements and decreases hypoxia tolerance in Atlantic salmon (Salmo salar) smolts. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2022, 265, 111128. [Google Scholar] [CrossRef]
- Taylor, R.S.; Muller, W.J.; Cook, M.T.; Kube, P.D.; Elliott, N.G. Gill observations in Atlantic salmon (Salmo salar L.) during repeated amoebic gill disease (AGD) field exposure and survival challenge. Aquaculture 2009, 290, 1–8. [Google Scholar] [CrossRef]
- Downes, J.K.; Rigby, M.L.; Taylor, R.S.; Maynard, B.T.; MacCarthy, E.; O’Connor, I.; Marcos-Lopez, M.; Rodger, H.D.; Collins, E.; Ruane, N.M.; et al. Evaluation of non-destructive molecular diagnostics for the detection of Neoparamoeba perurans. Front. Mar. Sci. 2017, 4, 61. [Google Scholar] [CrossRef]
- Noguera, P.; Olsen, A.B.; Hoare, J.; Lie, K.I.; Marcos-López, M.; Poppe, T.T.; Rodger, H. Complex gill disorder (CGD): A histopathology workshop report. Bull. Eur. Ass. Fish Pathol. 2019, 39, 172–176. [Google Scholar]
- Bridle, A.R.; Crosbie, P.B.B.; Cadoret, K.; Nowak, B.F. Rapid detection and quantification of Neoparamoeba perurans in the marine environment. Aquaculture 2010, 309, 56–61. [Google Scholar] [CrossRef]
- Fringuelli, E.; Gordon, A.W.; Rodger, H.; Welsh, M.D.; Graham, D.A. Detection of Neoparamoeba perurans by duplex quantitative Taqman real-time PCR in formalin-fixed, paraffin-embedded Atlantic salmonid gill tissues. J. Fish Dis. 2012, 35, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Downes, J.K.; Henshilwood, K.; Collins, E.M.; Ryan, A.; O’Connor, I.; Rodger, H.D.; MacCarthy, E.; Ruane, N.M. A longitudinal study of amoebic gill disease on a marine Atlantic salmon farm utilising a real-time PCR assay for the detection of Neoparamoeba perurans. Aquac. Environ. Interact. 2015, 7, 239–251. [Google Scholar] [CrossRef]
- Wynne, J.W.; Rigby, M.L.; Maynard, B.T.; Taylor, R.S. Improved environmental detection of Neoparamoeba perurans using sensitive RNA-based qPCR. J. Fish Dis. 2024, 47, e13879. [Google Scholar] [CrossRef]
- Parsons, H.; Nowak, B.; Fisk, D.; Powell, M. Effectiveness of commercial freshwater bathing as a treatment against amoebic gill disease in Atlantic salmon. Aquaculture 2001, 195, 205–210. [Google Scholar] [CrossRef]
- Rodger, H.D. Amoebic gill disease (AGD) in farmed salmon (Salmo salar) in Europe. Fish Vet. J. 2014, 14, 16–26. [Google Scholar]
- Munday, B.L.; Zilberg, D.; Findlay, V. Gill disease of marine fish caused by infection with Neoparamoeba pemaquidensis. J. Fish Dis. 2001, 24, 497–507. [Google Scholar] [CrossRef]
- Clark, G.; Powell, M.; Nowak, B. Effects of commercial fresh-water bathing on reinfection of Atlantic salmon, Salmo salar, with amoebic gill disease. Aquaculture 2003, 219, 135–142. [Google Scholar] [CrossRef]
- Adams, M.B.; Crosbie, P.B.B.; Nowak, B.F. Preliminary success using hydrogen peroxide to treat Atlantic salmon, Salmo salar L., affected with experimentally induced amoebic gill disease (AGD). J. Fish Dis. 2012, 35, 839–848. [Google Scholar] [CrossRef]
- Wynne, J.W.; Stratford, C.; Slinger, J.; Samsing, F.; Rigby, M.; McCulloch, R.; Quezada-Rodriguez, P.; Taylor, R.S. The interaction between temperature and dose on the efficacy and biochemical response of Atlantic salmon to hydrogen peroxide treatment for amoebic gill disease. J. Fish Dis. 2020, 43, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, P.; Yeager, T. Prospect of hydroxyl exposure during seawater bathing to treat amoebic gill disease in Atlantic salmon. Aquat. Res. 2023, 2023, 5074580. [Google Scholar] [CrossRef]
- Cadoret, K.; Bridle, A.R.; Leef, M.J.; Nowak, B.F. Evaluation of fixation methods for demonstration of Neoparamoeba perurans infection in Atlantic salmon, Salmo salar L., gills. J. Fish Dis. 2013, 36, 831–839. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 6th ed.; Churchill Livingstone Elsevier: London, UK, 2007; 744p. [Google Scholar]
- R Core Team, R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Clinton, M.; Król, E.; Sepúlveda, D.; Andersen, N.R.; Brierley, A.S.; Ferrier, D.E.K.; Hansen, P.J.; Lorenzen, N.; Martin, S.A.M. Gill transcriptomic responses to toxin-producing alga Prymnesium parvum in rainbow trout. Front. Immunol. 2021, 12, 794593. [Google Scholar] [CrossRef]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Hoem, K.S.; Tveten, A.K. Sea transfer and net pen cleaning induce changes in stress-related gene expression in commercial Atlantic salmon (Salmo salar) gill tissue. Aquacult. Int. 2023, 31, 2245–2262. [Google Scholar] [CrossRef]
- English, C.J.; Tyml, T.; Botwright, N.A.; Barnes, A.C.; Wynne, J.W.; Lima, P.C.; Cook, M.T. A diversity of amoebae colonise the gills of farmed Atlantic salmon (Salmo salar) with amoebic gill disease (AGD). Eur. J. Protistol. 2019, 67, 27–45. [Google Scholar] [CrossRef]
- Steinum, T.; Kvellestad, A.; Colquhoun, D.J.; Heum, M.; Mohammad, S.; Grøntvedt, R.N.; Falk, K. Microbial and pathological findings in farmed Atlantic salmon Salmo salar with proliferative gill inflammation. Dis. Aquat. Org. 2010, 91, 201–211. [Google Scholar] [CrossRef]
- Robledo, D.; Hamilton, A.; Gutiérrez, A.P.; Bron, J.E.; Houston, R.D. Characterising the mechanisms underlying genetic resistance to amoebic gill disease in Atlantic salmon using RNA sequencing. BMC Genom. 2020, 21, 271. [Google Scholar] [CrossRef]
- Oldham, T.; Dempster, T.; Crosbie, P.; Adams, M.; Nowak, B. Cyclic hypoxia exposure accelerates the progression of amoebic gill disease. Pathogens 2020, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Okon, E.M.; Okocha, R.C.; Taiwo, A.B.; Falana, B.M.; Adeniran, M.B. Dynamics of co-infection in fish: A review of pathogen-host interaction and clinical outcome. Fish Shellfish Immunol. Rep. 2023, 4, 100096. [Google Scholar] [CrossRef] [PubMed]
- Emam, M.; Caballero-Solares, A.; Xue, X.; Umasuthan, N.; Milligan, B.; Taylor, R.G.; Balder, R.; Rise, M.L. Gill and liver transcript expression changes associated with gill damage in Atlantic salmon (Salmo salar). Front. Immunol. 2022, 13, 806484. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, D.; Feng, L.; Zhang, C.; Xi, D.; Liu, H.; Yan, C.; Xu, Z.; Zhang, Y.; Li, Y.; et al. Transcriptome analysis reveals the high temperature induced damage is a significant factor affecting the osmotic function of gill tissue in Siberian sturgeon (Acipenser baerii). BMC Genom. 2023, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- Valdenegro-Vega, V.A.; Crosbie, P.; Bridle, A.; Leef, M.; Wilson, R.; Nowak, B.F. Differentially expressed proteins in gill and skin mucus of Atlantic salmon (Salmo salar) affected by amoebic gill disease. Fish Shellfish Immunol. 2014, 40, 69–77. [Google Scholar] [CrossRef]
- Pizzo, E.; Varcamonti, M.; Maro, A.D.; Zanfardino, A.; Giancola, C.; D’Alessio, G. Ribonucleases with angiogenic and bactericidal activities from the Atlantic salmon. FEBS J. 2008, 275, 1283–1295. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, W.; Yin, L.; Chilian, W.M.; Krieger, J.; Zhang, P. Vascular precursor cells in tissue injury repair. Transl. Res. 2017, 184, 77–100. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Zhang, Q.; Zhao, L.; Yang, J.; Zou, Z.; Zhao, Y.; Li, C.; Sun, X.; Lin, X.; Jin, M. African swine fever virus infection activates inflammatory responses through downregulation of the anti-inflammatory molecule C1QTNF3. Front. Immunol. 2022, 13, 1002616. [Google Scholar] [CrossRef]
- Campo, A.; Nitzan, T.; Slosman, T.; Doron-Faigenboim, A.; D’Cotta, H.; Baroiller, J.-F.; Cnaani, A. Different transcriptomic architecture of the gill epithelia in Nile and Mozambique tilapia after salinity challenge. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 41, 100927. [Google Scholar] [CrossRef] [PubMed]
- Umasuthan, N.; Xue, X.; Caballero-Solares, A.; Kumar, S.; Westcott, J.D.; Chen, Z.; Fast, M.D.; Skugor, S.; Nowak, B.F.; Taylor, R.G.; et al. Transcriptomic profiling in fins of atlantic salmon parasitized with sea lice: Evidence for an early imbalance between chalimus-induced immunomodulation and the host’s defense response. Int. J. Mol. Sci. 2020, 21, 2417. [Google Scholar] [CrossRef]



| Gene Symbol 1 | Gene Name | Species | NCBI Accession Number | Ensembl Accession Number | Primers 5′-3′ (Forward and Reverse) 2 |
|---|---|---|---|---|---|
| 18S rRNA gene | 18S ribosomal RNA gene | N. perurans | EF216905 | n/a | F:GTTCTTTCGGGAGCT R:GAACTATCGCCGGCA |
| ANG1 | Angiogenin-1 | S. salar | XM_014199172 | ENSSSAT00000023457 | F:ACTGTGGCAGATATTTGGGGAAGA R:GTCACCCTGGACACCTGTGG |
| LL | Ladderlectin | S. salar | XM_014125761 | ENSSSAT00000132935 | F:GATCTACGTGCCGCAAAGGC R:TTTGGTCCAACCTCCGGGAC |
| IL8 | Interleukin 8 | S. salar | NM_001140710 | ENSSSAT00000013831 | F:GAATGTCAGCCAGCCTTGT R:TCCAGACAAATCTCCTGACCG |
| GPX6 | Glutathione peroxidase 6-like | S. salar | XM_014155258 | ENSSSAT00000067910 | F:TAGCATGCAGGGTTACACAATGG R:GAGCACCTTGCCCCTGTAGT |
| C1QTNF3 | Complement C1q tumour necrosis factor-related protein 3-like | S. salar | XM_014134640 | ENSSSAT00000142506 | F:AGACGATGCTTCCTCTCCAGAT R:ACACCCACAGAGTTGCGTGA |
| EF1A | Elongation factor 1-alpha | S. salar | XM_014177562 | ENSSSAT00000157959 | F:CAAGGATATCCGTCGTGGCA R:ACAGCGAAACGACCAAGAGG |
| RPS13 | Ribosomal protein S13 | S. salar | BT059859 | ENSSSAT00000078374 | F:CCCTCTCAGATCGGTGTGATCC R:TCCTGTCCTTTCTGTTCCTCTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallarino, M.C.; Dagen, S.L.; Costelloe, E.; Oyenekan, S.I.; Tinsley, J.; Valdenegro, V.; Król, E.; Noguera, P.; Martin, S.A.M. Dynamics of Gill Responses to a Natural Infection with Neoparamoeba perurans in Farmed Tasmanian Atlantic Salmon. Animals 2024, 14, 2356. https://doi.org/10.3390/ani14162356
Vallarino MC, Dagen SL, Costelloe E, Oyenekan SI, Tinsley J, Valdenegro V, Król E, Noguera P, Martin SAM. Dynamics of Gill Responses to a Natural Infection with Neoparamoeba perurans in Farmed Tasmanian Atlantic Salmon. Animals. 2024; 14(16):2356. https://doi.org/10.3390/ani14162356
Chicago/Turabian StyleVallarino, Max Charles, Sarah L. Dagen, Eoin Costelloe, Shalom Inioluwa Oyenekan, John Tinsley, Victoria Valdenegro, Elżbieta Król, Patricia Noguera, and Samuel A. M. Martin. 2024. "Dynamics of Gill Responses to a Natural Infection with Neoparamoeba perurans in Farmed Tasmanian Atlantic Salmon" Animals 14, no. 16: 2356. https://doi.org/10.3390/ani14162356







