Effect of Postbiotic Supplementation on Nutrient Digestibility and Milk Yield during the Transition Period in Dairy Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Diets
2.3. Experimental Design and Measurements
2.4. Chemical Analyses
2.5. Calculations and Statistical Analysis
3. Results
| CT | PC | PR | rsd | p | |
|---|---|---|---|---|---|
| Milk (kg/d) | 29.61 b | 33.73 a | 30.06 b | 6.908 | 0.044 |
| Fat (%) | 4.03 | 4.19 | 3.80 | 0.668 | 0.068 |
| Protein (%) | 3.10 | 3.22 | 3.27 | 0.307 | 0.083 |
| Solids-not-fat (%) | 8.73 b | 8.68 b | 8.99 a | 0.413 | 0.007 |
| Lactose (%) | 4.83 | 4.79 | 5.08 | 0.648 | 0.148 |
| Urea (mg/kg) | 223 | 210 | 229 | 48.3 | 0.296 |
| Milk performance | |||||
| FPCM (kg/d) | 29.26 b | 34.30 a | 29.12 b | 7.046 | 0.004 |
| kg fat/d | 1.16 b | 1.42 a | 1.13 b | 0.336 | 0.002 |
| kg protein/d | 0.92 b | 1.08 a | 0.98 b | 0.211 | 0.014 |
| Nitrogen efficiency (%) | 34.16 | 39.93 | 36.68 | 9.546 | 0.078 |
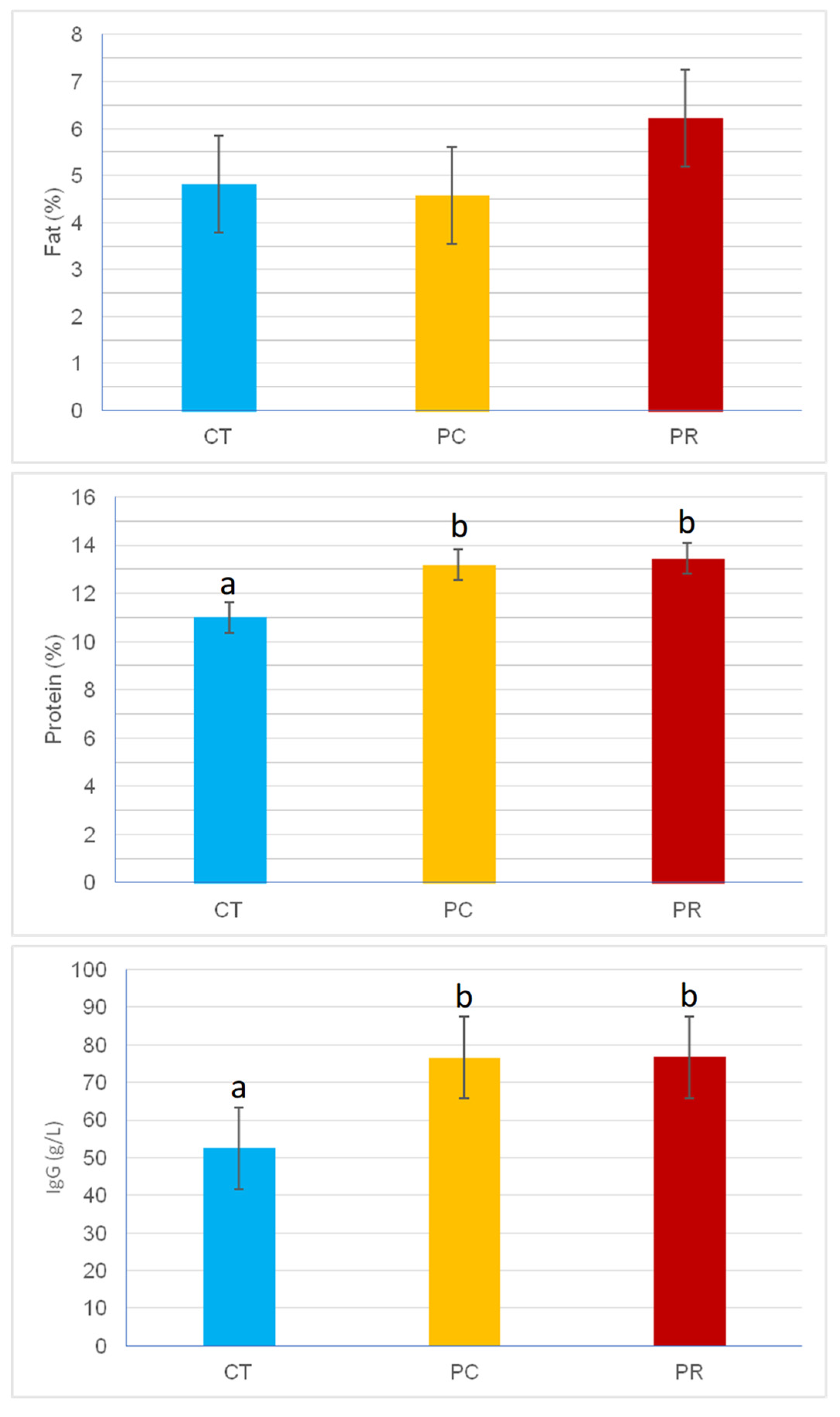
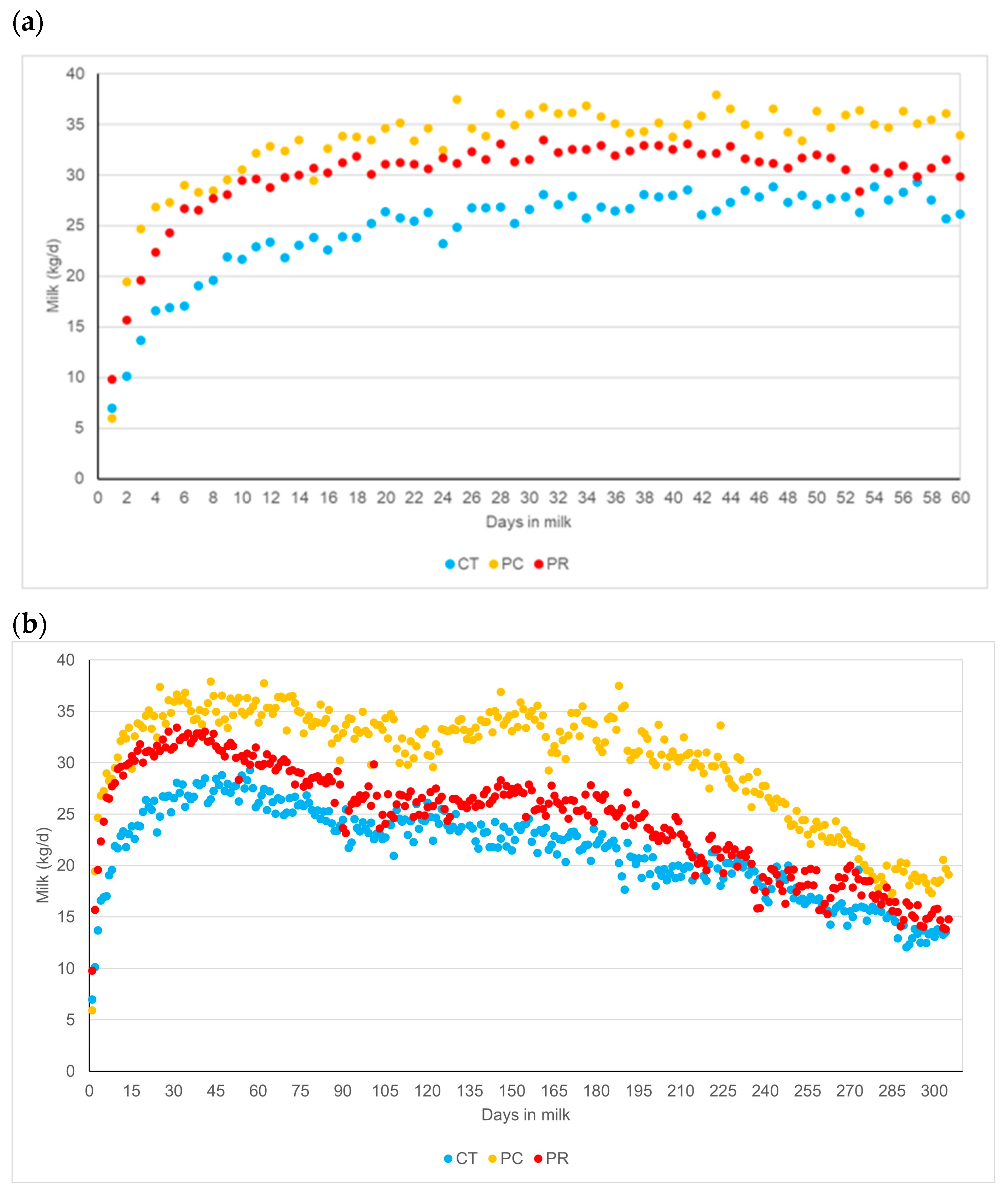
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mottet, A.; de Haan, C.; Falcucci, A.; Tempio, G.; Opio, C.; Gerber, P. Livestock: On our plates or eating at our table? A new analysis of the feed/food debate. Glob. Food Sec. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Zebeli, Q.; Metzler-Zebeli, B.U. Interplay between rumen digestive disorders and diet-induced inflammation in dairy cattle. Res. Vet. Sci. 2012, 93, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Kim, S.; Kim, M.; Upadhaya, S.; Kam, D.; Ha, J. Direct-fed microbials for ruminant animals. Asian-Aust. J. Anim. Sci. 2010, 23, 1657–1667. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 5, 4745–4767. [Google Scholar] [CrossRef]
- Li, T.; Teng, D.; Mao, R.; Hao, Y.; Wang, X.; Wang, J. A critical review of antibiotic resistance in probiotic bacteria. Food Res. Int. 2020, 136, 109571. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, E.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Thanh, N.T.; Loh, T.C.; Foo, H.L.; Hair-bejo, M.; Azhar, B.K. Effects of feeding metabolite combinations produced by Lactobacillus plantarum on growth performance, faecal microbial population, small intestine villus height and faecal volatile fatty acids in broilers. Br. Poult. Sci. 2009, 50, 298–306. [Google Scholar] [CrossRef]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; García-Varela, R.; García, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Izuddin, W.I.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Humam, A.M. Postbiotic L. plantarum RG14 improves ruminal epithelium growth, immune status and upregulates the intestinal barrier function in post-weaning lambs. Sci. Rep. 2019, 9, 9938. [Google Scholar] [CrossRef]
- Kareem, K.Y.; Ling, F.H.; Chwen, L.T.; Foong, O.M.; Asmara, S.A. Inhibitory activity of postbiotic produced by strains of Lactobacillus plantarum using reconstituted media supplemented with inulin. Gut Pathog. 2014, 6, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Rovai, M.; Guifarro, L.; Salama, A.A. Effects of long-term postbiotic supplementation on dairy heifer calves: Health status and wound healing after dehorning. J. Dairy Sci. 2019, 102 (Suppl. 1), 221. [Google Scholar]
- Izuddin, W.I.; Loh, T.C.; Samsudin, A.A.; Foo, H.L. In vitro study of postbiotics from Lactobacillus plantarum RG14 on rumen fermentation and microbial population. Rev. Bras. Zootecn. 2018, 47, e20170255. [Google Scholar] [CrossRef]
- Fernández, C.; Romero, T.; Badiola, I.; Díaz-Cano, J.; Sanzol, G.; Loor, J.J. Postbiotic yeast fermentation product supplementation to lactating goats increases the efficiency of milk production by enhancing fiber digestibility and ruminal propionate, and reduces energy losses in methane. J. Anim Sci. 2023, 101, skac370. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods of dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- ISO 23318:2022; Milk, Dried Milk Products and Cream. Determination of Fat Content—Gravimetric Method, Edition 1, 2022. International Organization for Standardization: Geneva, Switzerland, 2022.
- ISO 8968-1:2014; IDF 20-1:2014 Milk and Milk Products—Determination of Nitrogen Content Part 1: Kjeldahl Principle and Crude Protein Calculation, Edition 2, 2014. International Organization for Standardization: Geneva, Switzerland, 2014.
- MAFF. Energy Allowances and Feeding Systems for Ruminants. Reference Book 433; Ministry of Agriculture, Fisheries and Food: Reading, UK, 1984.
- ADAS. Compound Feed Evaluation Fir the Ruminants. Technical Bulletin 85/21; Ministry of Agriculture, Fisheries and Food: Reading, UK, 1985.
- Quigley, J.D.; Lago, A.; Chapman, C.; Erickson, P.; Polo, J. Evaluation of the Brix refractometer to estimate immunoglobulin G concentration in bovine colostrum. J. Dairy Sci. 2013, 96, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- IDF. A Common Carbon Footprint Approach for Dairy. The IDF Guide to Standard Lifecycle Assessment Methodology for the Dairy Sector; Bulletin IDF No. 479/2010; International Dairy Federation: Brussels, Belgium, 2015. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Olagaray, K.E.; Sivinski, S.E.; Saylor, B.A.; Mamedova, L.K.; Sauls-Hiesterman, J.A.; Yoon, I.; Bradford, B.J. Effect of Saccharomyces cerevisiae fermentation product on feed intake parameters, lactation performance, and metabolism of transition dairy cattle. J. Dairy Sci. 2019, 102, 8092–8107. [Google Scholar] [CrossRef] [PubMed]
- Dann, H.M.; Drackley, J.K.; McCoy, G.C.; Hutjens, M.F.; Garrett, J.E. Effects of yeast culture (Saccharomyces cerevisiae) on prepartum intake and postpartum intake and milk production of Jersey cows. J. Dairy Sci. 2000, 83, 123–127. [Google Scholar] [CrossRef]
- Shi, W.; Knoblock, C.E.; Murphy, K.V.; Bruinjé, T.C.; Yoon, I.; Ambrose, D.J.; Oba, M. Effects of supplementing a Saccharomyces cerevisiae fermentation product during the periparturient period on performance of dairy cows fed fresh diets differing in starch content. J. Dairy Sci. 2019, 102, 3082–3096. [Google Scholar] [CrossRef]
- Gross, J.J.; Wellnitz, O.; Bruckmaier, R.M. Cortisol secretion in response to metabolic and inflammatory challenges in dairy cows. J. Anim Sci. 2015, 93, 3395–3401. [Google Scholar] [CrossRef]
- Zaworski, E.M.; Shriver-Munsch, C.M.; Fadden, N.A.; Sanchez, W.K.; Yoon, I.; Bobe, G. Effects of feeding various dosages of Saccharomyces cerevisiae fermentation product in transition dairy cows. J. Dairy Sci. 2014, 97, 3081–3098. [Google Scholar] [CrossRef]
- Arambel, M.J.; Kent, B.A. Effect of yeast culture on nutrient digestibility and milk yield response in early- to midlactation dairy cows. J. Dairy Sci. 1990, 73, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Khalouei, H.; Seranatne, V.; Fehr, K.; Guo, J.; Yoon, I.; Khafipour, E.; Plaizier, J.C. Effects of Saccharomyces cerevisiae fermentation products and subacute ruminal acidosis on feed intake, fermentation, and nutrient digestibilities in lactating dairy cows. Can. J. Anim Sci. 2021, 101, 143–157. [Google Scholar] [CrossRef]
- Rovai, M.; Guifarro, L.; Anderson, J.; Salama, A.A.K. Effects of long-term postbiotic supplementation on dairy heifer calves: Performance and metabolic indicators. In Proceedings of the ADSA Annual Meeting, Cincinnati, OH, USA, 23–26 June 2019. [Google Scholar]
- Shi, W.; Knoblock, C.E.; Yoon, I.; Oba, M. Effects of supplementing a Saccharomyces cerevisiae fermentation product during the transition period on rumen fermentation of dairy cows fed fresh diets differing in starch content. J. Dairy Sci. 2019, 102, 9943–9955. [Google Scholar] [CrossRef]
- Izuddin, W.I.; Loh, T.C.; Samsudin, A.A.; Foo, H.L.; Humam, A.M.; Shazali, N. Effect of postbiotic supplementation on growth performance, ruminal fermentation and microbial profile, blood metabolite and GHR, IGF-1 and MCT-1 gene expression in post-weaning lambs. BMC Vet. Res. 2019, 15, 315. [Google Scholar] [CrossRef] [PubMed]
- Poppy, G.D.; Rabiee, A.R.; Lean, I.J.; Sanchez, W.K.; Dorton, K.L.; Morley, P.S. A meta-analysis of the effects of feeding yeast culture produced by anaerobic fermentation of Saccharomyces cerevisiae on milk production of lactating dairy cows. J. Dairy Sci. 2012, 95, 6027–6041. [Google Scholar] [CrossRef]
- Latham, M.J.; Sutton, J.D.; Sharpe, M.E. Fermentation and microorganisms in the rumen and the content of fat in the milk of cows given low roughage rations. J. Dairy Sci. 1974, 57, 803–810. [Google Scholar] [CrossRef]
- Díaz, A.; Ranilla, M.J.; Saro, C.; Tejido, M.L.; Pérez-Quintana, M.; Carro, M.D. Influence of increasing doses of a yeast hydrolyzate obtained from sugarcane processing on in vitro rumen fermentation of two different diets and bacterial diversity in batch cultures and Rusitec fermenters. Anim. Feed Sci. Technol. 2017, 232, 129–138. [Google Scholar] [CrossRef]
- Williams, P.E.V.; Tait, C.A.G.; Innes, G.M.; Newbold, C.J. Effects of the inclusion of yeast culture (Saccharomyces cerevisiae plus growth medium) in the diet of dairy cows on milk yield and forage degradation and fermentation patterns in the rumen of steers. J. Anim Sci. 1991, 69, 3016–3026. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E.S.; Martin, S.A. Effects of a Saccharomyces cerevisiae culture on ruminal bacteria that utilize lactate and digest cellulose. J. Dairy Sci. 1997, 80, 2035–2044. [Google Scholar] [CrossRef]
- Wiedmeier, R.D.; Arambel, M.J.; Walters, J.L. Effects of yeast culture and Aspergillus oryzae fermentation extract on ruminal characteristics and nutrient digestibility. J. Dairy Sci. 1987, 70, 2063–2068. [Google Scholar] [CrossRef] [PubMed]
- Quigley, J.D., III; Martin, K.R.; Dowlen, H.H.; Wallis, L.B.; Lamar, K. Immunoglobulin concentration, specific gravity, and nitrogen fractions of colostrum from Jersey cattle. J. Dairy Sci. 1994, 77, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Sneddon, N.W.; Goi, A.; Visentin, G.; Mammi, L.M.E.; Savarino, E.V.; Zingone, F.; Formigoni, A.; Penasa, M.; De Marchi, M. Bovine colostrum, a promising ingredient for humans and animals—Properties, processing technologies, and uses. J. Dairy Sci. 2023, 106, 5197–5217. [Google Scholar] [CrossRef]
- Galvao, K.N.; Santos, J.E.; Coscioni, A.; Villasenor, M.; Sischo, W.M.; Berge, A.C. Effect of feeding live yeast products to calves with failure of passive transfer on performance and patterns of antibiotic resistance in fecal Escherichia coli. Reprod. Nutr. Dev. 2005, 45, 427–440. [Google Scholar] [CrossRef]
- Salmon, H. Immunophysiology of the mammary gland and transmission of immunity to the young. Reprod. Nutr. Dev. 2003, 43, 471–475. [Google Scholar] [CrossRef]
- Agazzi, A. The beneficial role of probiotics in monogastric animal nutrition and health. Dairy Vet. Anim. Res. 2015, 2, 116–132. [Google Scholar] [CrossRef][Green Version]
- Zanello, G.; Meurens, F.; Serreau, D.; Chevaleyre, C.; Melo, S.; Berri, M.; D’Inca, R.; Auclair, E.; Salmon, H. Effects of dietary yeast strains on immunoglobulin in colostrum and milk of sows. Vet. Immunol. Immunopathol. 2013, 152, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xu, C.; Wang, J.; Hu, C.; Ji, F.; Xie, J.; Yang, Y.; Yu, X.; Diao, X.; Lv, R. Effects of dietary probiotics and acidifiers on the production performance, colostrum components, serum antioxidant activity and hormone levels, and gene expression in mammary tissue of lactating sows. Animals 2023, 13, 1536. [Google Scholar] [CrossRef]
- Alonge, S.; Aiudi, G.G.; Lacalandra, G.M.; Leoci, R.; Melandri, M. Pre and probiotics to increase the immune power of colostrum in dogs. Front. Vet. Sci. 2020, 7, 570414. [Google Scholar] [CrossRef]
- Ramsing, E.M.; Davidson, J.A.; French, P.D.; Yoon, I.; Keller, M.; Peters-Fleckenstein, H. Effects of yeast culture on peripartum intake and milk production of primiparous and multiparous Holstein cows. Prof. Anim. Sci. 2009, 25, 487–495. [Google Scholar] [CrossRef]
- Stella, A.V.; Paratte, R.; Valnegri, L.; Cigalino, G.; Soncini, G.; Chevaux, E.; Dell’Orto, V.; Savoini, G. Effect of administration of live Saccharomyces cerevisiae on milk production, milk composition, blood metabolites, and faecal flora in early lactating dairy goats. Small Rumin. Res. 2007, 67, 7–13. [Google Scholar] [CrossRef]
- Wohlt, J.E.; Corcione, T.T.; Zajac, P.K. Effects of yeast on feed intake and performance of cows fed diets based on corn silage during early lactation. J. Dairy Sci. 1998, 81, 1345–1352. [Google Scholar] [CrossRef]
- Acharya, S.; Pretz, J.P.; Yoon, I.; Scott, M.F.; Casper, D.P. Effects of Saccharomyces cerevisiae fermentation products on the lactational performance of mid-lactation dairy cows. Transl. Anim. Sci. 2017, 1, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.L.G.; Freitas, J.A.; Micai, B.; Azevedo, R.A.; Greco, L.F.; Santos, J.E.P. Effects of supplementing yeast culture to diets differing in starch content on performance and feeding behavior of dairy cows. J. Dairy Sci. 2018, 101, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Liang, T.; Muckey, M.B.; Mendonça, L.G.D.; Hulbert, L.E.; Elrod, C.C.; Bradford, B.J. Yeast product supplementation modulated feeding behavior and metabolism in transition dairy cows. J. Dairy Sci. 2015, 98, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Piva, G.; Belladonna, S.; Fusconi, G.; Sicbaldi, F. Effects of yeast on dairy cow performance, ruminal fermentation, blood components, and milk manufacturing properties. J. Dairy Sci. 1993, 76, 2717–2722. [Google Scholar] [CrossRef] [PubMed]
- White, R.A.; Harrison, J.H.; Yoon, I.; Sanchez, W.K.; Nicholson, N. Effect of yeast culture on efficiency of nutrient utilization for milk production and impact on fiber digestibility and fecal particle size. Prof. Anim. Sci. 2008, 24, 114–119. [Google Scholar] [CrossRef]
- O’Regan, J.; Mulvihill, D. Preparation, characterization and selected functional properties of sodium caseinate–maltodextrin conjugates. Food Chem. 2009, 115, 1257–1267. [Google Scholar] [CrossRef]
- Kleefisch, M.T.; Zebeli, Q.; Humer, E.; Gruber, L.; Klevenhusen, F. Effects of feeding high-quality hay with graded amounts of concentrate on feed intake, performance and blood metabolites of cows in early lactation. Arch. Anim. Nutr. 2018, 72, 290–307. [Google Scholar] [CrossRef]
| Prepartum | Postpartum | Additional Concentrate 1 | |
|---|---|---|---|
| Diet composition (% on dry matter basis) | |||
| Grass silage | 41.16 | 46.81 | |
| Cereal straw | 12.14 | 6.12 | |
| Concentrate 1 | 46.70 | 47.07 | |
| Chemical composition (% on DM basis) | |||
| Dry matter (DM) | 46.98 | 43.01 | 88.71 |
| Organic matter (OM) | 90.39 | 90.26 | 91.36 |
| Crude protein (CP) | 13.10 | 13.96 | 22.82 |
| Crude fibre (CF) | 25.92 | 21.29 | 4.96 |
| Ether extract (EE) | 3.60 | 4.08 | 3.54 |
| Nitrogen-free extract (NFE) | 47.76 | 50.94 | 60.04 |
| Starch | 13.89 | 17.40 | 37.52 |
| Neutral detergent fibre (NDF) | 48.80 | 43.19 | 20.17 |
| Acid detergent fibre (ADF) | 30.63 | 27.19 | 8.56 |
| Net energy for lactation (Mcal/kg DM) | 1.44 | 1.52 | 1.89 |
| CT | PC | PR | rsd | p | |
|---|---|---|---|---|---|
| Intake (kg/day) | |||||
| Dry matter | 10.26 b | 10.46 ab | 10.97 a | 0.498 | 0.027 |
| Organic matter | 9.28 b | 9.50 ab | 9.94 a | 0.451 | 0.025 |
| Crude protein | 1.44 b | 1.60 a | 1.57 a | 0.068 | 0.001 |
| Neutral detergent fibre | 4.37 b | 4.71 a | 4.72 a | 0.233 | 0.009 |
| Digestibility (%) | |||||
| Dry matter | 62.00 | 66.91 | 64.92 | 4.030 | 0.091 |
| Organic matter | 62.87 | 67.59 | 66.03 | 3.889 | 0.088 |
| Crude protein | 60.16 b | 68.20 a | 63.01 ab | 4.059 | 0.005 |
| Neutral detergent fibre | 50.38 | 52.30 | 52.12 | 5.532 | 0.773 |
| Nitrogen balance (%) | 45.21 | 54.64 | 48.77 | 7.253 | 0.344 |
| CT | PC | PR | rsd | p | |
|---|---|---|---|---|---|
| Intake (kg/day) | |||||
| Dry matter | 18.37 b | 18.06 b | 19.88 a | 1.118 | 0.001 |
| Organic matter | 16.53 b | 16.49 b | 17.95 a | 1.014 | 0.015 |
| Crude protein | 3.35 a | 3.05 b | 3.24 a | 0.167 | 0.008 |
| Neutral detergent fibre | 5.90 b | 6.29 b | 7.30 a | 0.435 | 0.001 |
| Digestibility (%) | |||||
| Dry matter | 64.00 b | 64.76 b | 73.44 a | 7.406 | 0.036 |
| Organic matter | 65.90 b | 66.36 b | 74.68 a | 6.952 | 0.035 |
| Crude protein | 55.02 | 55.56 | 63.56 | 8.921 | 0.131 |
| Neutral detergent fibre | 57.67 b | 55.49 b | 71.65 a | 8.686 | 0.003 |
| Nitrogen balance (%) | 41.28 | 44.85 | 50.32 | 11.040 | 0.307 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente, F.; Campo-Celada, M.; Menéndez-Miranda, M.; García-Rodríguez, J.; Martínez-Fernández, A. Effect of Postbiotic Supplementation on Nutrient Digestibility and Milk Yield during the Transition Period in Dairy Cows. Animals 2024, 14, 2359. https://doi.org/10.3390/ani14162359
Vicente F, Campo-Celada M, Menéndez-Miranda M, García-Rodríguez J, Martínez-Fernández A. Effect of Postbiotic Supplementation on Nutrient Digestibility and Milk Yield during the Transition Period in Dairy Cows. Animals. 2024; 14(16):2359. https://doi.org/10.3390/ani14162359
Chicago/Turabian StyleVicente, Fernando, María Campo-Celada, Mario Menéndez-Miranda, Jairo García-Rodríguez, and Adela Martínez-Fernández. 2024. "Effect of Postbiotic Supplementation on Nutrient Digestibility and Milk Yield during the Transition Period in Dairy Cows" Animals 14, no. 16: 2359. https://doi.org/10.3390/ani14162359
APA StyleVicente, F., Campo-Celada, M., Menéndez-Miranda, M., García-Rodríguez, J., & Martínez-Fernández, A. (2024). Effect of Postbiotic Supplementation on Nutrient Digestibility and Milk Yield during the Transition Period in Dairy Cows. Animals, 14(16), 2359. https://doi.org/10.3390/ani14162359





