The Genetic Identification of Numerous Apicomplexan Sarcocystis Species in Intestines of Common Buzzard (Buteo buteo)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Common Buzzard Samples
2.2. Preparation of Intestines and Microscopical Characterisation of Sarcocystis spp.
2.3. Molecular Analysis of Sarcocystis spp.
2.4. Phylogenetic Analysis
3. Results
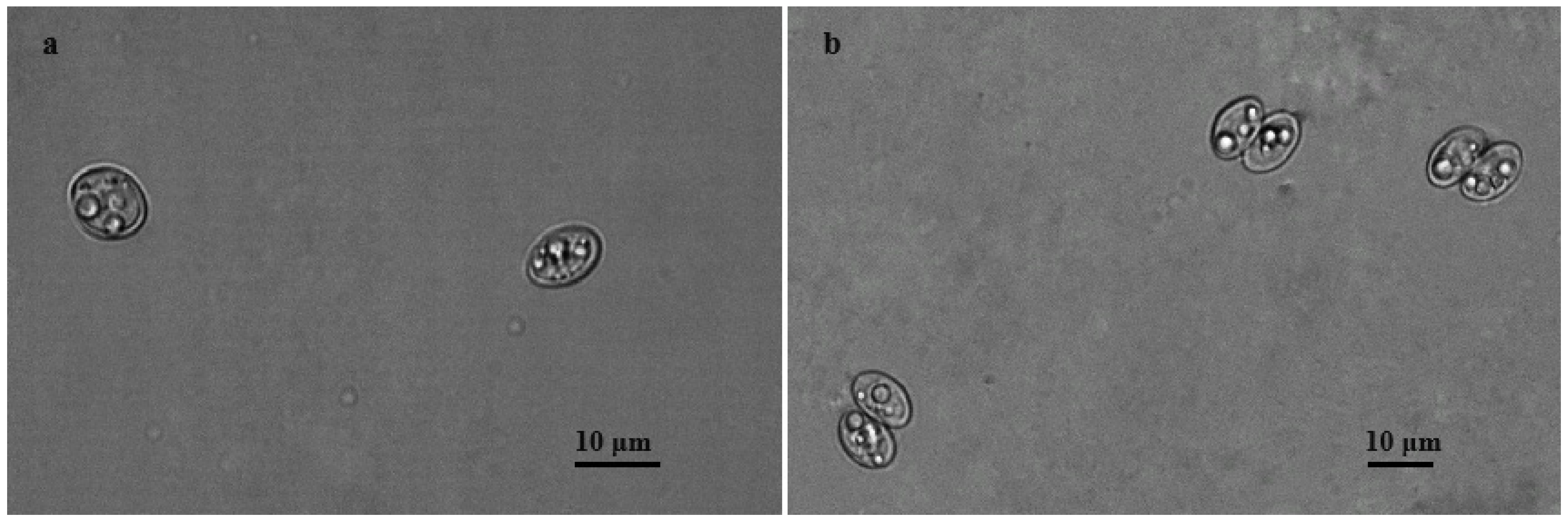
3.1. Microscopical Detection of Sarcocystis spp. Oocysts/Sporocyst
3.2. Molecular Identification of Sarcocystis spp. Using Birds as Their Intermediate Hosts and Common Buzzards as Definitive Hosts
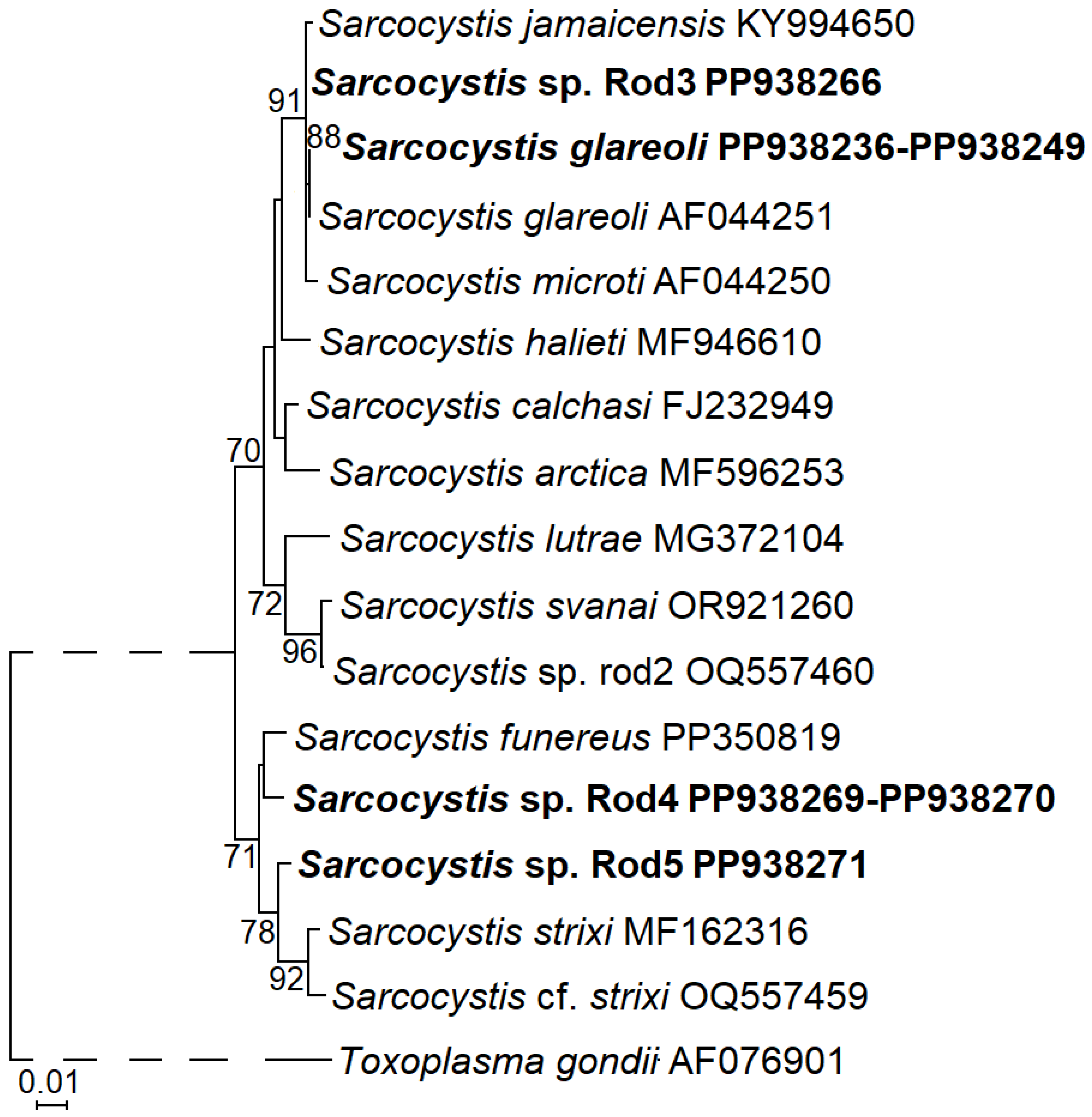
3.3. Molecular Characterisation of S. glareoli and Three Potentially New Sarcocystis Species Detected in Intestines of Common Buzzards
3.4. Phylogeny of Sarcocystis spp. Identified in Common Buzzards Using Rodents as Their Intermediate Hosts
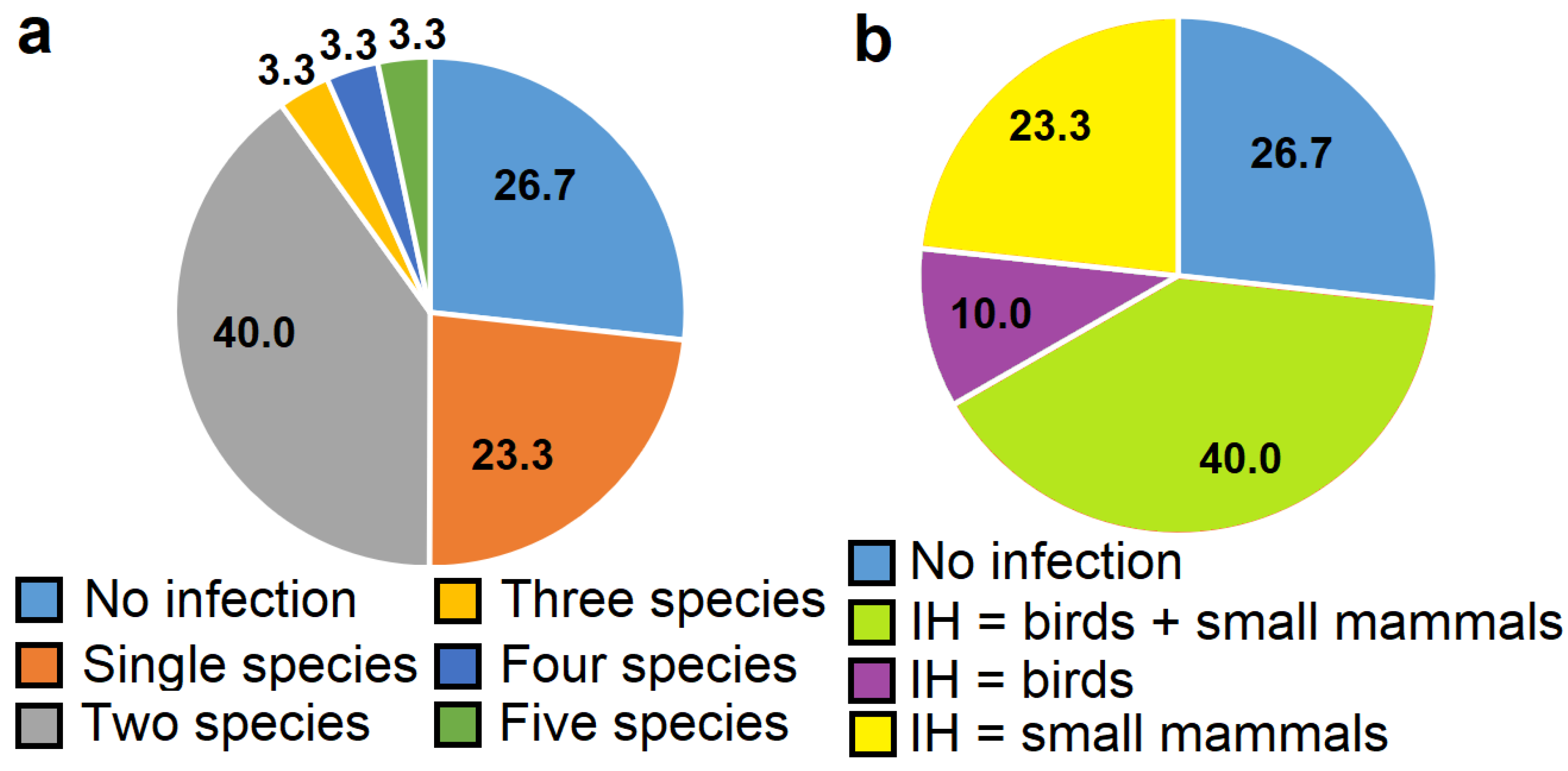
3.5. The Prevalence of Sarcocystis spp. Identified in the Common Buzzard from Lithuania
4. Discussion
4.1. The Role of Common Buzzards in the Transmission of Sarcocystis spp. Using Rodents as Their IH
4.2. The Role of Common Buzzards in the Transmission of Sarcocystis spp. Using Birds as Their IH
4.3. Common Buzzards as Definitive Hosts of Potentially New Sarcocystis Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walls, S.; Kenward, R. The Common Buzzard; T&AD Poyser: London, UK, 2020. [Google Scholar]
- The IUCN Red List of Threatened Species 2022. Available online: https://www.iucnredlist.org/species/181049859/181050999 (accessed on 19 June 2024).
- Jusys, V.; Karalius, S.; Raudonikis, L. The Guide to Birds of Lithuania; Lithuanian Ornithological Society: Vilnius, Lithuania, 2020. (In Lithuanian) [Google Scholar]
- Kurlavičius, P. (Ed.) Lithuanian Breeding Bird Atlas; Lututė: Kaunas, Lithuania, 2006. [Google Scholar]
- Snow, D.; Perrins, C. The Birds of the Western Palearctic; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Kontrimavičius, V.L.; Kazlauskas, R.; Logminas, V. Fauna of Lithuania: Birds; Mokslas: Vilnius, Lithuania, 1990. [Google Scholar]
- Hagemeijer, E.J.M.; Blair, M.J. (Eds.) The EBCC Atlas of European Breeding Birds: Their Distribution and Abundance; T & AD Poyser: London, UK, 1997. [Google Scholar]
- Drobelis, E. Birds of Prey in Lithuania. Acta Ornithol. Lithu. 1993, 7–8, 117–121. [Google Scholar]
- Mehlhorn, H.; Heydorn, A.O. The Sarcosporidia (Protozoa, Sporozoa): Life Cycle and Fine Structure. Adv. Parasitol. 1978, 16, 43–91. [Google Scholar] [CrossRef]
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Šukytė, T.; Butkauskas, D.; Juozaitytė-Ngugu, E.; Švažas, S.; Prakas, P. Molecular Confirmation of Accipiter Birds of Prey as Definitive Hosts of Numerous Sarcocystis Species, including Sarcocystis sp., Closely Related to Pathogenic S. calchasi. Pathogens 2023, 12, 752. [Google Scholar] [CrossRef] [PubMed]
- Prakas, P.; Jasiulionis, M.; Šukytė, T.; Juozaitytė-Ngugu, E.; Stirkė, V.; Balčiauskas, L.; Butkauskas, D. First Observations of Buzzards (Buteo) as Definitive Hosts of Sarcocystis Parasites Forming Cysts in the Brain Tissues of Rodents in Lithuania. Biology 2024, 13, 264. [Google Scholar] [CrossRef]
- Castro-Forero, P.S.; Bulla-Castañeda, M.D.; López Buitrago, A.H.; Díaz Anaya, M.A.; Madeira de Carvalho, M.L.; Pulido-Medellín, O.M. Sarcocystis spp., a Parasite with Zoonotic Potential. Bulg. J. Vet. Med. 2022, 25, 175–186. [Google Scholar] [CrossRef]
- Henry, D.P. Isopora buteonis sp. nov. from the Hawk and Owl, and Notes on Isopora lacazii (Labbé) in Birds. Univ. Calif. Publ. Zool. 1932, 37, 291–300. [Google Scholar]
- Krampitz, H.E.; Rommel, M.; Geisel, O.; Kaiser, E. Beiträge zum Lebenszyklus der Frenkelien II. Die ungeschlechtliche Entwicklung von Frenkelia clethrionomyobuteonis in der Rötelmaus. Z. Parasitenkd. 1976, 51, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Rommel, M.; Krampitz, H.E. Weitere Untersuchungen über das Zwischenwirtsspektrum und de Entwicklungszklus von Frenkelia microti aus der Erdmaus. Zbl. Vet. Med. B 1978, 25, 273–281. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Ambrus, S.I.; Blagburn, B.L. Frenkelia sp.-Like Infection in the Small Intestine of a Red-Tailed Hawk. J. Wildl. Dis. 1987, 23, 677–679. [Google Scholar] [CrossRef]
- Upton, S.J.; McKown, R.D. The Red-Tailed Hawk, Buteo jamaicensis, a Native Definitive Host of Frenkelia microti (Apicomplexa) in North America. J. Wildl. Dis. 1992, 28, 85–90. [Google Scholar] [CrossRef]
- Vorísek, P.; Votýpka, J.; Zvára, K.; Svobodová, M. Heteroxenous Coccidia Increase the Predation Risk of Parasitized Rodents. Parasitology 1998, 117, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Mugridge, N.B.; Morrison, D.A.; Johnson, A.M.; Luton, K.; Dubey, J.P.; Votýpka, J.; Tenter, A.M. Phylogenetic Relationships of the Genus Frenkelia: A Review of its History and New Knowledge Gained from Comparison of Large Subunit Ribosomal Ribonucleic Acid Gene Sequences. Int. J. Parasitol. 1999, 29, 957–972. [Google Scholar] [CrossRef]
- Mugridge, N.B.; Morrison, D.A.; Jäkel, T.; Heckeroth, A.R.; Tenter, A.M.; Johnson, A.M. Effects of Sequence Alignment and Structural Domains of Ribosomal DNA on Phylogeny Reconstruction for the Protozoan Family Sarcocystidae. Mol. Biol. Evol. 2000, 17, 1842–1853. [Google Scholar] [CrossRef] [PubMed]
- Modrý, D.; Votýpka, J.; Svobodová, M. Note on the Taxonomy of Frenkelia microti (Findlay & Middleton, 1934) (Apicomplexa: Sarcocystidae). Syst. Parasitol. 2004, 58, 185–187. [Google Scholar] [CrossRef]
- Odening, K. The Present State of Species-Systematics in Sarcocystis Lankester, 1882 (Protista, Sporozoa, Coccidia). Syst. Parasitol. 1998, 41, 209–233. [Google Scholar] [CrossRef]
- Laakkonen, J.; Henttonen, H. Ultrastructure of Frenkelia sp. from a Norwegian Lemming in Finland. J. Wildl. Dis. 2000, 36, 362–366. [Google Scholar] [CrossRef]
- Karstad, L. Toxoplasma microti (The M-Organism) in the Muskrat (Ondatra zibethica). Can. Vet. J. 1963, 4, 249–251. [Google Scholar] [PubMed]
- Kennedy, M.J.; Frelier, P.F. Frenkelia sp. from the Brain of a Porcupine (Erethizon Dorsatum) from Alberta, Canada. J. Wildl. Dis. 1986, 22, 112–114. [Google Scholar] [CrossRef]
- Fichet-Calvet, E.; Kia, E.B.; Giraudoux, P.; Quéré, J.P.; Delattre, P.; Ashford, R.W. Frenkelia Parasites in a Small Mammal Community. Dynamics of Infection and Effect on the Host. Parasite 2004, 11, 301–310. [Google Scholar] [CrossRef]
- Krücken, J.; Blümke, J.; Maaz, D.; Demeler, J.; Ramünke, S.; Antolová, D.; Schaper, R.; Von Samson-Himmelstjerna, G. Small Rodents as Paratenic or Intermediate Hosts of Carnivore Parasites in Berlin, Germany. PLoS ONE 2017, 12, e0172829. [Google Scholar] [CrossRef]
- Grikienienė, J.; Mažeikytė, R. Investigation of Sarcosporidians (Sarcocystis) of Small Mammals in Kamasta Landscape Reserve and Its Surroundings. Acta Zool. Litu. 2000, 10, 55–68. [Google Scholar] [CrossRef]
- Grikienienė, J.; Malakauskas, M.; Mažeikytė, R.; Balčiauskas, L.; Senutaitė, J. Muscle Parasites (Sarcocystis, Trichinella, Alaria) of Wild Mammals in Lithuania. Theriol. Litu. 2001, 1, 29–46. [Google Scholar]
- Grikienienė, J.; Mažeikytė, R.; Balčiauskas, L. The First Data on Brain Parasites of the Genus Frenkelia (Protista: Coccidia) in Some Small Rodent Species in Lithuania. Acta Zool. Litu. 2003, 13, 21–27. [Google Scholar] [CrossRef]
- Grikienienė, J. Investigations into Endoparasites of Small Mammals in the Environs of Lake Drūkšiai. Acta Zool. Litu. 2005, 15, 109–114. [Google Scholar] [CrossRef]
- Svobodová, M.; Vorisek, P.; Votýpka, J.; Weidinger, K. Heteroxenous Coccidia (Apicomplexa: Sarcocystidae) in the Populations of Their Final and Intermediate Hosts: European Buzzard and Small Mammals. Acta Protozool. 2004, 43, 251–260. [Google Scholar]
- Deter, J.; Bryja, J.; Chaval, Y.; Galan, M.; Henttonen, H.; Laakkonen, J.; Voutilainen, L.; Vapalahti, O.; Vaheri, A.; Salvador, A.R.; et al. Association between the DQA MHC Class II Gene and Puumala Virus Infection in Myodes glareolus, the Bank Vole. Infect. Genet. Evol. 2008, 8, 450–458. [Google Scholar] [CrossRef]
- Waindok, P.; Özbakış-Beceriklisoy, G.; Janecek-Erfurth, E.; Springer, A.; Pfeffer, M.; Leschnik, M.; Strube, C. Parasites in Brains of Wild Rodents (Arvicolinae and Murinae) in the City of Leipzig, Germany. Int. J. Parasitol. Parasites Wildl. 2019, 10, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Prakas, P.; Stirkė, V.; Šneideris, D.; Rakauskaitė, P.; Butkauskas, D.; Balčiauskas, L. Protozoan Parasites of Sarcocystis spp. in Rodents from Commercial Orchards. Animals 2023, 13, 2087. [Google Scholar] [CrossRef]
- Prakas, P.; Gudiškis, N.; Kitrytė, N.; Bagdonaitė, D.L.; Baltrūnaitė, L. Detection of Three Sarcocystis Species (Apicomplexa) in Blood Samples of the Bank Vole and Yellow-Necked Mouse from Lithuania. Life 2024, 14, 365. [Google Scholar] [CrossRef] [PubMed]
- Toyé, P.; Tappin, N. Frenkelia glareoli: A Record from Devon. Trans R. Soc. Trop. Med. Hyg. 1972, 66, 529. [Google Scholar]
- Fujita, O.; Oku, Y.; Ohbayashi, M. Frenkelia sp. from The Red-Backed Vole, Clethrionomys rufocanus bedfordiae, in Hokkaido, Japan. Jpn. J. Vet. Res. 1988, 36, 69–71. [Google Scholar] [PubMed]
- Reif, V.; Tornberg, R.; Jungell, S.; Korpimäki, E. Diet Variation of Common Buzzards in Finland Supports the Alternative Prey Hypothesis. Ecography 2001, 24, 267–274. [Google Scholar] [CrossRef]
- Selås, V. Predation on Reptiles and Birds by the Common Buzzard, Buteo buteo, in Relation to Changes in its Main Prey, Voles. Can. J. Zool. 2001, 79, 2086–2093. [Google Scholar] [CrossRef]
- Selås, V.; Tveiten, R.; Aanonsen, O. Diet of Common Buzzards (Buteo buteo) in Southern Norway Determined from Prey Remains and Video Recordings. Ornis Fennica 2007, 84, 97–104. [Google Scholar]
- Barauskas, R.; Karlonas, M. Birds of Lithuania. Breeding and Annually Occurring Species; Lututė: Kaunas, Lithuania, 2023. [Google Scholar]
- Olias, P.; Gruber, A.D.; Hafez, H.M.; Heydorn, A.O.; Mehlhorn, H.; Lierz, M. Sarcocystis calchasi sp. nov. of the Domestic Pigeon (Columba livia f. domestica) and the Northern Goshawk (Accipiter gentilis): Light and Electron Microscopical Characteristics. Parasitol. Res. 2010, 106, 577–585. [Google Scholar] [CrossRef]
- Wünschmann, A.; Rejmanek, D.; Conrad, P.A.; Hall, N.; Cruz-Martinez, L.; Vaughn, S.B.; Barr, B.C. Natural Fatal Sarcocystis falcatula Infections in Free-Ranging Eagles in North America. J. Vet. Diagn. Investig. 2010, 22, 282–289. [Google Scholar] [CrossRef]
- Maier-Sam, K.; Kaiponen, T.; Schmitz, A.; Schulze, C.; Bock, S.; Hlinak, A.; Olias, P. Encephalitis Associated with Sarcocystis halieti Infection in a Free-Ranging Little Owl (Athene noctua). J. Wildl. Dis. 2021, 57, 712–714. [Google Scholar] [CrossRef]
- Olias, P.; Olias, L.; Krücken, J.; Lierz, M.; Gruber, A.D. High Prevalence of Sarcocystis calchasi Sporocysts in European Accipiter Hawks. Vet. Parasitol. 2011, 175, 230–236. [Google Scholar] [CrossRef]
- Mayr, S.L.; Maier, K.; Müller, J.; Enderlein, D.; Gruber, A.D.; Lierz, M. Accipiter Hawks (Accipitridae) Confirmed as Definitive Hosts of Sarcocystis turdusi, Sarcocystis cornixi and Sarcocystis sp. ex Phalacrocorax carbo. Parasitol. Res. 2016, 115, 3041–3047. [Google Scholar] [CrossRef]
- Gjerde, B.; Vikøren, T.; Hamnes, I.S. Molecular identification of Sarcocystis halieti n. sp., Sarcocystis lari and Sarcocystis truncata in the intestine of a white-tailed sea eagle (Haliaeetus albicilla) in Norway. Int. J. Parasitol. Parasites Wildl. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Rogers, K.H.; Arranz-Solís, D.; Saeij, J.P.J.; Lewis, S.; Mete, A. Sarcocystis calchasi and Other Sarcocystidae Detected in Predatory Birds in California, USA. Int. J. Parasitol. Parasites Wildl. 2021, 17, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Juozaitytė-Ngugu, E.; Švažas, S.; Šneideris, D.; Rudaitytė-Lukošienė, E.; Butkauskas, D.; Prakas, P. The Role of Birds of the Family Corvidae in Transmitting Sarcocystis Protozoan Parasites. Animals 2021, 11, 3258. [Google Scholar] [CrossRef]
- Prakas, P.; Rudaitytė-Lukošienė, E.; Šneideris, D.; Butkauskas, D. Invasive American Mink (Neovison vison) as Potential Definitive Host of Sarcocystis elongata, S. entzerothi, S. japonica, S. truncata and S. silva using Different Cervid Species as Intermediate Hosts. Parasitol. Res. 2021, 120, 2243–2250. [Google Scholar] [CrossRef]
- Šneideris, D.; Moskaliova, D.; Butkauskas, D.; Prakas, P. The Distribution of Sarcocsytis Species Described by Ungulates-Canids Life Cycle in Intestines of Small Predators of the Family Mustelidae. Acta Parasitol. 2024, 69, 747–758. [Google Scholar] [CrossRef]
- Duszynski, D.W.; Box, E.D. The Opossum (Didelphis virginiana) as a Host for Sarcocystis debonei from Cowbirds (Molothrus ater) and Grackles (Cassidix mexicanus, Quiscalus quiscula). J. Parasitol. 1978, 64, 326–329. [Google Scholar] [CrossRef]
- Singh, L.A.L.; Raisinghani, P.M.; Kumar, D.; Swarankar, C.P. Clinical and Haematobiochemical Changes in Dogs Experimentally Infected with Sarcocystis capracanis. Indian J. Anim. Sci. 1993, 63, 1055–1057. [Google Scholar]
- Porter, R.A.; Ginn, P.E.; Dame, J.B.; Greiner, E.C. Evaluation of the Shedding of Sarcocystis falcatula Sporocysts in Experimentally Infected Virginia Opossums (Didelphis virginiana). Vet. Parasitol. 2001, 95, 313–319. [Google Scholar] [CrossRef]
- Máca, O.; Gudiškis, N.; Butkauskas, D.; González-Solís, D.; Prakas, P. Red Foxes (Vulpes vulpes) and Raccoon Dogs (Nyctereutes procyonoides) as Potential Spreaders of Sarcocystis Species. Front. Vet. Sci. 2024, 11, 1392618. [Google Scholar] [CrossRef] [PubMed]
- Prakas, P.; Balčiauskas, L.; Juozaitytė-Ngugu, E.; Butkauskas, D. The Role of Mustelids in the Transmission of Sarcocystis spp. Using Cattle as Intermediate Hosts. Animals 2021, 11, 822. [Google Scholar] [CrossRef]
- Prakas, P.; Kutkienė, L.; Butkauskas, D.; Sruoga, A.; Žalakevičius, M. Description of Sarcocystis lari sp. n. (Apicomplexa: Sarcocystidae) from the Great Black-Backed Gull, Larus marinus (Charadriiformes: Laridae), on the Basis of Cyst Morphology and Molecular Data. Folia Parasitol. 2014, 61, 11–17. [Google Scholar] [CrossRef]
- Prakas, P.; Butkauskas, D.; Švažas, S.; Juozaitytė-Ngugu, E.; Stanevičius, V. Morphologic and Genetic Identification of Sarcocystis fulicae n. sp. (Apicomplexa: Sarcocystidae) from the Eurasian coot (Fulica atra). J. Wildl. Dis. 2018, 54, 765–771. [Google Scholar] [CrossRef]
- Prakas, P.; Butkauskas, D.; Švažas, S.; Stanevičius, V. Morphological and Genetic Characterisation of Sarcocystis halieti from the Great Cormorant (Phalacrocorax carbo). Parasitol. Res. 2018, 117, 3663–3667. [Google Scholar] [CrossRef]
- Prakas, P.; Butkauskas, D.; Juozaitytė-Ngugu, E. Molecular and Morphological Description of Sarcocystis kutkienae sp. nov. from the Common Raven (Corvus corax). Parasitol. Res. 2020, 119, 4205–4210. [Google Scholar] [CrossRef]
- Gjerde, B. Phylogenetic Relationships among Sarcocystis Species in Cervids, Cattle and Sheep Inferred from the Mitochondrial Cytochrome C Oxidase Subunit I Gene. Int. J. Parasitol. 2013, 43, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, B. Molecular Characterisation of Sarcocystis bovifelis, Sarcocystis bovini n. sp., Sarcocystis hirsuta and Sarcocystis cruzi from Cattle (Bos taurus) and Sarcocystis sinensis from Water Buffaloes (Bubalus bubalis). Parasitol. Res. 2016, 115, 1473–1492. [Google Scholar] [CrossRef]
- Prakas, P.; Oksanen, A.; Butkauskas, D.; Sruoga, A.; Kutkienė, L.; Švažas, S.; Isomursu, M.; Liaugaudaitė, S. Identification and Intraspecific Genetic Diversity of Sarcocystis rileyi from Ducks, Anas spp., in Lithuania and Finland. J. Parasitol. 2014, 100, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Lindsay, D.S.; Grigg, M.E.; Dubey, J.P. Isolation, Culture and Cryopreservation of Sarcocystis Species. Curr. Protoc. Microbiol. 2017, 45, 20D.1.1–20D.1.27. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Dubey, J.P.; Cerqueira-Cézar, C.K.; Murata, F.H.A.; Mowery, J.D.; Scott, D.; von Dohlen, A.R.; Lindsay, D.S. Confirmation of Sarcocystis jamaicensis Sarcocysts in IFN-γ Gene Knockout Mice Orally Inoculated with Sporocysts from a Red-Tailed Hawk (Buteo jamaicensis). J. Parasitol. 2019, 105, 143–145. [Google Scholar] [CrossRef]
- Verma, S.K.; Von Dohlen, A.R.; Mowery, J.D.; Scott, D.; Cerqueira-Cézar, C.K.; Rosenthal, B.M.; Dubey, J.P.; Lindsay, D.S. Sarcocystis strixi n. sp. from a Barred Owl (Strix varia) Definitive Host and Interferon Gamma Gene Knockout Mice as Experimental Intermediate Host. J. Parasitol. 2017, 103, 768–777. [Google Scholar] [CrossRef]
- Máca, O.; Kouba, M.; Korpimäki, E.; González-Solís, D. Molecular Identification of Sarcocystis sp. (Apicomplexa, Sarcocystidae) in Offspring of Tengmalm’s Owls, Aegolius funereus (Aves, Strigidae). Front. Vet. Sci. 2021, 8, 804096. [Google Scholar] [CrossRef]
- Máca, O.; Kouba, M.; Langrová, I.; Panská, L.; Korpimäki, E.; González-Solís, D. The Tengmalm’s owl Aegolius funereus (Aves, Strigidae) as the definitive host of Sarcocystis funereus sp. nov. (Apicomplexa). Front. Vet. Sci. 2024, 11, 1356549. [Google Scholar] [CrossRef]
- Verma, S.K.; Von Dohlen, A.R.; Mowery, J.D.; Scott, D.; Rosenthal, B.M.; Dubey, J.P.; Lindsay, D.S. Sarcocystis jamaicensis n. sp., from Red-Tailed Hawks (Buteo jamaicensis) Definitive Host and IFN-γ Gene Knockout Mice as Experimental Intermediate Host. J. Parasitol. 2017, 103, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Kutkienė, L.; Prakas, P.; Sruoga, A.; Butkauskas, D. Sarcocystis in the Birds Family Corvidae with Description of Sarcocystis cornixi sp. nov. from the Hooded Crow (Corvus cornix). Parasitol. Res. 2009, 104, 329–336. [Google Scholar] [CrossRef]
- Juozaitytė-Ngugu, E.; Butkauskas, D.; Švažas, S.; Prakas, P. Investigations on Sarcocystis Species in the Leg Muscles of the Bird Family Corvidae in Lithuania. Parasitol. Res. 2022, 121, 703–711. [Google Scholar] [CrossRef]
- Kutkienė, L.; Prakas, P.; Butkauskas, D.; Sruoga, A. Description of Sarcocystis turdusi sp. nov. from the Common Blackbird (Turdus merula). Parasitology 2012, 139, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Kutkienė, L.; Prakas, P.; Sruoga, A.; Butkauskas, D. The Mallard Duck (Anas platyrhynchos) as Intermediate Host for Sarcocystis wobeseri sp. nov. from the Barnacle Goose (Branta leucopsis). Parasitol. Res. 2010, 107, 879–888. [Google Scholar] [CrossRef]
- Prakas, P.; Butkauskas, D.; Juozaitytė-Ngugu, E. Molecular Identification of four Sarcocystis Species in the Herring Gull, Larus argentatus, from Lithuania. Parasites Vectors 2020, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Prakas, P.; Bea, A.; Juozaitytė-Ngugu, E.; Olano, I.; Villanúa, D.; Švažas, S.; Butkauskas, D. Molecular Identification of Sarcocystis halieti in the Muscles of two Species of Birds of Prey from Spain. Parasites Vectors 2021, 14, 414. [Google Scholar] [CrossRef]
- Shadbolt, T.; Pocknell, A.; Sainsbury, A.W.; Egerton-Read, S.; Blake, D.P. Molecular Identification of Sarcocystis wobeseri-like Parasites in a New Intermediate Host Species, the White-Tailed Sea Eagle (Haliaeetus albicilla). Parasitol. Res. 2021, 120, 1845–1850. [Google Scholar] [CrossRef]
- Máca, O.; González-Solís, D. Role of three Bird Species in the Life Cycle of two Sarcocystis spp. (Apicomplexa, Sarcocystidae) in the Czech Republic. Int. J. Parasitol. Parasites Wildl. 2022, 17, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.P.; da Silva, T.C.E.; de Pontes, T.P.; Sanches, A.W.D.; Prakas, P.; Locatelli-Dittrich, R. Molecular Characterization of Sarcocystis spp. in Seabirds from Southern Brazil. Parasitol. Int. 2022, 90, 102595. [Google Scholar] [CrossRef] [PubMed]
- Juozaitytė-Ngugu, E.; Prakas, P. The Richness of Sarcocystis Species in the Common Gull (Larus canus) and Black-Headed Gull (Larus ridibundus) from Lithuania. Parasitologia 2023, 3, 172–180. [Google Scholar] [CrossRef]
- Prakas, P.; Estruch, J.; Velarde, R.; Ilgūnas, M.; Šneideris, D.; Nicolás-Francisco, O.; Marco, I.; Calero-Bernal, R. First Report of Sarcocystis halieti (Apicomplexa) in Bearded Vulture (Gypaetus barbatus). Vet. Res. Commun. 2024, 48, 541–546. [Google Scholar] [CrossRef]
- Prakas, P.; Butkauskas, D.; Sruoga, A.; Švažas, S.; Kutkienė, L. Identification of Sarcocystis columbae in Wood Pigeons (Columba palumbus) in Lithuania. Vet. Zootech. 2011, 55, 33–39. [Google Scholar]
- Lau, Y.L.; Chang, P.Y.; Subramaniam, V.; Ng, Y.H.; Mahmud, R.; Ahmad, A.F.; Fong, M.Y. Genetic Assemblage of Sarcocystis spp. in Malaysian Snakes. Parasites Vectors 2013, 6, 257. [Google Scholar] [CrossRef] [PubMed]
- Moré, G.; Maksimov, A.; Conraths, F.J.; Schares, G. Molecular Identification of Sarcocystis spp. in Foxes (Vulpes vulpes) and Raccoon Dogs (Nyctereutes procyonoides) from Germany. Vet. Parasitol. 2016, 220, 9–14. [Google Scholar] [CrossRef]
- Lesniak, I.; Franz, M.; Heckmann, I.; Greenwood, A.D.; Hofer, H.; Krone, O. Surrogate Hosts: Hunting Dogs and Recolonizing Grey Wolves share their Endoparasites. Int. J. Parasitol. Parasites Wildl. 2017, 6, 278–286. [Google Scholar] [CrossRef]
- Basso, W.; Alvarez Rojas, C.A.; Buob, D.; Ruetten, M.; Deplazes, P. Sarcocystis Infection in Red Deer (Cervus elaphus) with Eosinophilic Myositis/Fasciitis in Switzerland and Involvement of Red Foxes (Vulpes vulpes) and Hunting Dogs in the Transmission. Int. J. Parasitol. Parasites Wildl. 2020, 13, 130–141. [Google Scholar] [CrossRef]

| Primer Name | Orientation | Primer Sequence (5′-3′) | Locus | Length (bp) | Target Species | Ref. |
|---|---|---|---|---|---|---|
| SU1F | Forward | GATTGAGTGTTCCGGTGAATTATT | ITS1 region * | ~1000 | Sarcocystis spp. | [49] |
| 5.8SR2 | Reverse | AAGGTGCCATTTGCGTTCAGAA | ||||
| GsScalF2 | Forward | CCTTTTGTAAGGTTGGGGACATA | ITS1 | 584 | S. calchasi | [11] |
| GsScalR2 | Reverse | GCCTCCCTCCCTCTTTTTG | ||||
| GsScolF | Forward | ATATGTTCATCCTTTCGTAGCGTTG | ITS1 | 579 | S. columbae | [51] |
| GsScolR | Reverse | GCCATCCCTTTTTCTAAGAGAAGTC | ||||
| GsScornF2 | Forward | AGTTGTTGACGTTCGTGAGGTC | ITS1 | 483 | S. cornixi | [51] |
| GsScornR2 | Reverse | ACACACTACTCATTATCTCCTACTCCT | ||||
| GsScovF | Forward | TATTCATTCTTTCGGTAGTGTTGAG | ITS1 | 524 | S. corvusi | [51] |
| GsScovR | Reverse | TTACTCTTTTAACAGCTTCGCTGAG | ||||
| GsSfulF | Forward | CAAAGATGAAGAAGGTATATACGTGAA | ITS1 | 449 | S. fulicae | [51] |
| GsSfulR | Reverse | CTTTACTCTTGAAGAACGACGTTGA | ||||
| GsShalF | Forward | GATAATTGACTTTACGCGCCATTAC | ITS1 | 644 | S. halieti | [51] |
| GsShalR2 | Reverse | CCATCCCTTTTTCTAAAGGAGGTC | ||||
| GsSkutkF2 | Forward | ACACACGGTCGAGTTGATATGAC | ITS1 | 625 | S. kutkienae | [51] |
| GsSkutkR2 | Reverse | TCTTTACCCTTAAACAATTTCGTTG | ||||
| GsSlarF | Forward | TTCGTGAGGTTATTATCATTGTGCT | ITS1 | 545 | S. lari | [51] |
| GsSlarR | Reverse | GGCGATAGAAATCAAAGCAGTAGTA | ||||
| GsSturF | Forward | GATTTTTGATGTCCGTTGAAGTTAT | ITS1 | 561 | S. turdusi | [51] |
| GsSturR | Reverse | CATTCAAATATGCTCTCTTCCTTCT | ||||
| GsSwobF | Forward | ATGAACTGCTTTTTCTTCCATCTTT | ITS1 | 532 | S. wobeseri | [51] |
| GsSwobR2 | Reverse | CTCCTCTTGAAGGTGGTCGTGT | ||||
| GsSglajamF1 | Forward | TTTCGTAGCGCTGAGGAGATT | ITS1 | ~560 | S. glareoli/S. jamaicensis | PS |
| GsSglajamR1 | Reverse | TGCTTTTCTTCCTTTACTTTTGAATG | PS | |||
| Sgrau281 | Forward | GCGGAGGAAAAGAAAATAACAAT | 28S rRNA | ~900 | Sarcocystis spp. from rodents | [36] |
| Sgrau282 | Reverse | CTATCGCTTAGGACCGGCTA | ||||
| GsSglaF1 | Forward | GCAAAATGTGTGGTAAGTTTCACAT | 28S rRNA | 565 | S. glareoli | [12] |
| GsSglaR1 | Reverse | CCCTCTAAAAAGATGTTACCCTTCT | ||||
| GsSmicF1 | Forward | TGTGGTAAGTTTCACATAAGGCTAA | 28S rRNA | 553 | S. microti | [12] |
| GsSmicR1 | Reverse | CTTTCTAAAAAGATGTACCTTCTCCT | ||||
| SgraupaukF | Forward | CGTATTTGCCCTGTGTCCTT | 28S rRNA | ~660 | Sarcocystis spp. from rodents | PS |
| SgraupaukR | Reverse | GTCGTAGGTGCAAAGCATAACATC | PS |
| Species | GenBank Accession Numbers | Intraspecific Similarity (%) * | Interspecific Similarity, Comparing with Most Closely Related Species (%) * |
|---|---|---|---|
| S. cornixi | PP937501 | 98.6–100 | S. kutkienae 88.7–90.5 |
| S. halieti | PP937502–PP937512 | 96.3–100 | S. columbae 91.1–92.5 |
| S. kutkienae | PP937513, PP937514 | 99.0–100 | S. cornixi 88.4–89.1 |
| S. turdusi | PP937515 | 98.4–100 | S. wobeseri 84.8–86.1 |
| S. wobeseri | PP937516–PP937522 | 99.0–100 | S. calchasi 91.9–92.7 |
| Species | Genetic Region | Primer pairs | GenBank Acc No. | Percentage Intraspecific Similarity (Number of Sequences Determined in the Present Study) * | Percentage Interspecific Similarity, Comparing with Most Closely Related Species * |
|---|---|---|---|---|---|
| S. glareoli | 28S rRNA | GsSglaF1/ GsSglaR1 and SgraupaukF/ SgraupaukR | PP938236– PP938265 | 99.8–100 (30) | S. jamaicensis 99.6–99.7 |
| S. glareoli | ITS1 | GsSglaF1/ GsSglaR1 | PP937483–P937498 | 100 (16) | S. sp. Rod3 98.5 |
| S. sp. Rod3 | 28S rRNA | GsSglaF1/ GsSglaR1 and SgraupaukF/ SgraupaukR | PP938266– PP938268 | 100 (3) | S. jamaicensis 99.8 |
| S. sp. Rod3 | ITS1 | GsSglaF1/ GsSglaR1 | PP937499– PP937500 | 100 (2) | S. glareoli 98.5 |
| S. sp. Rod4 | 28S rRNA | SgraupaukF/ SgraupaukR | PP938269, PP938270 | 100 (2) | S. sp. Rod5 97.7 |
| S. sp. Rod5 | 28S rRNA | SgraupaukF/ SgraupaukR | PP938271 | not applicable (1) | S. cf. strixi and S. sp. Rod4 97.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šukytė, T.; Juozaitytė-Ngugu, E.; Švažas, S.; Butkauskas, D.; Prakas, P. The Genetic Identification of Numerous Apicomplexan Sarcocystis Species in Intestines of Common Buzzard (Buteo buteo). Animals 2024, 14, 2391. https://doi.org/10.3390/ani14162391
Šukytė T, Juozaitytė-Ngugu E, Švažas S, Butkauskas D, Prakas P. The Genetic Identification of Numerous Apicomplexan Sarcocystis Species in Intestines of Common Buzzard (Buteo buteo). Animals. 2024; 14(16):2391. https://doi.org/10.3390/ani14162391
Chicago/Turabian StyleŠukytė, Tautvilė, Evelina Juozaitytė-Ngugu, Saulius Švažas, Dalius Butkauskas, and Petras Prakas. 2024. "The Genetic Identification of Numerous Apicomplexan Sarcocystis Species in Intestines of Common Buzzard (Buteo buteo)" Animals 14, no. 16: 2391. https://doi.org/10.3390/ani14162391






