Regulatory Effects of Alhagi Honey Small-Molecule Sugars on Growth Performance and Intestinal Microbiota of Lambs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. AHAS Extraction
2.2. Component Separation of AHAS
2.3. Structural Evaluation of AHAS
2.3.1. Assessment of Monosaccharide Content
2.3.2. Spectral Valuation by Fourier Transforms Infrared (FT-IR)
2.4. Animal Experimental Design
2.5. Effect of AHAS on Lambs’ Growth Performance
2.6. Effect of AHAS on Levels of Immunoglobulin, Cytokines, and Oxidation Indices
2.7. Analysis of 16S rRNA Sequencing and Extraction of Fecal DNA
2.8. SCFAs Concentrations Determined in the Fecal Content
2.9. Statistical Evaluation/Analysis
3. Results
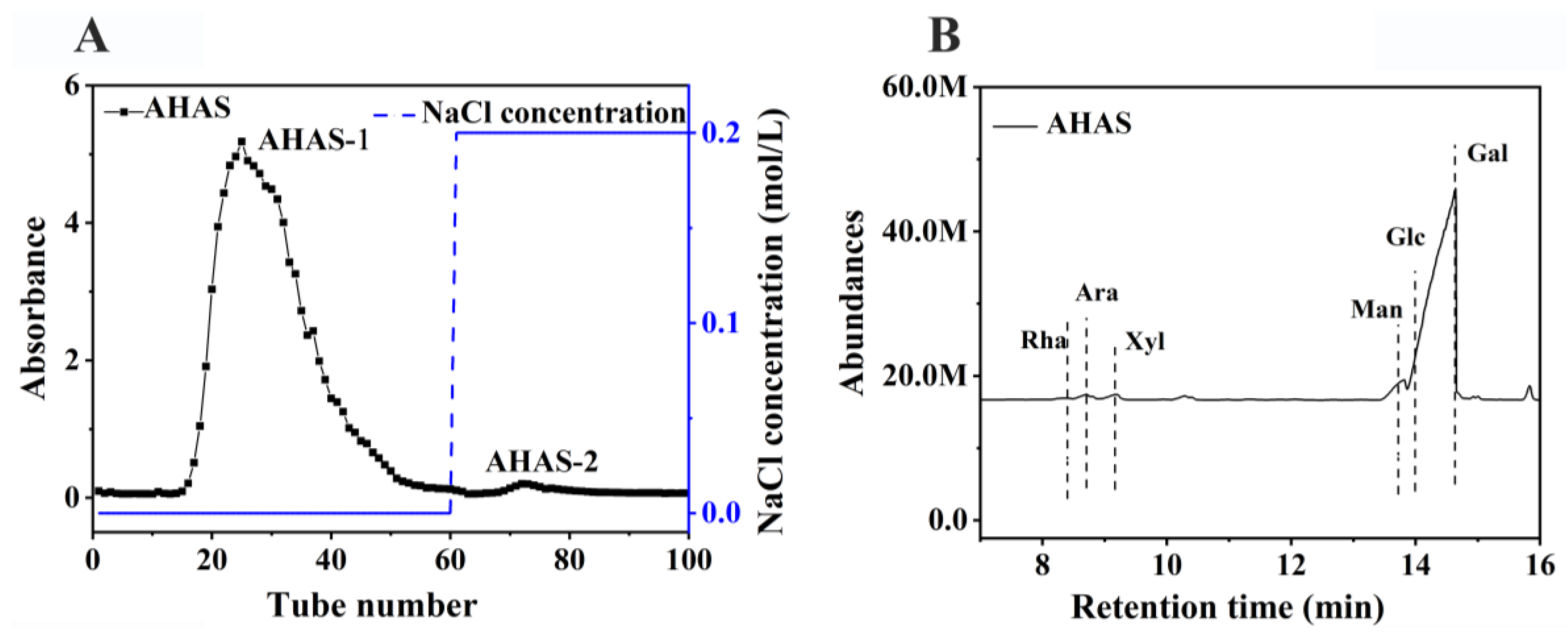
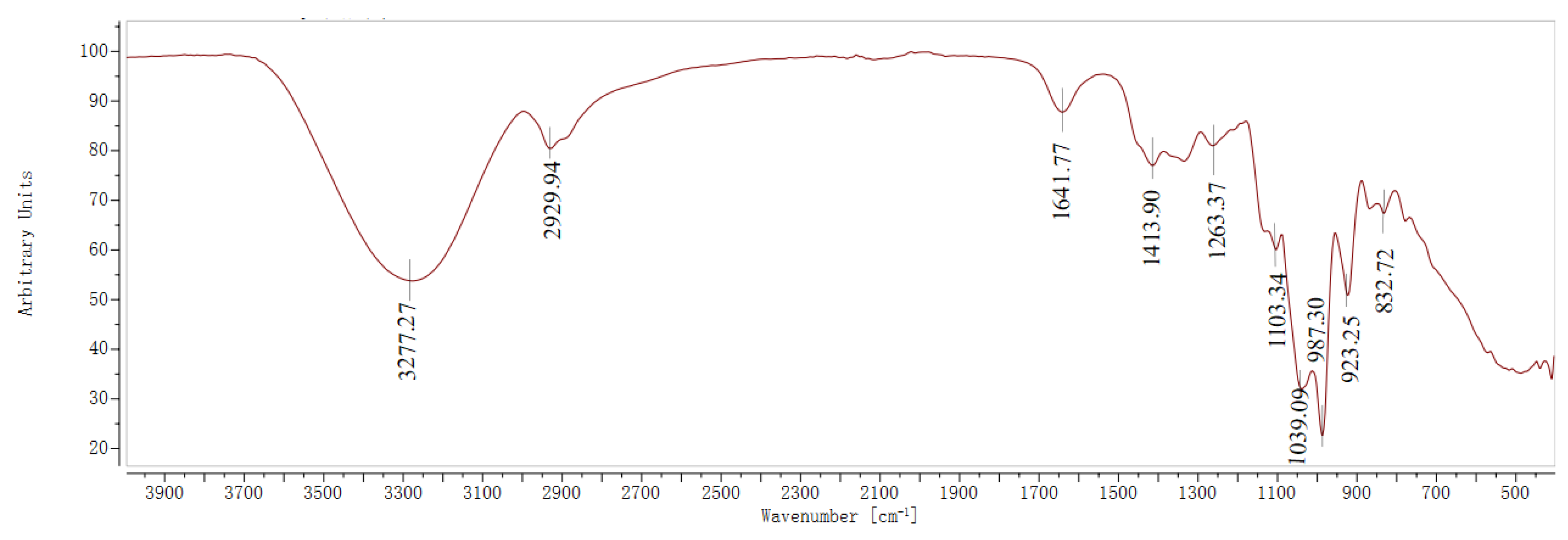
3.1. AHAS Primary Structure
3.1.1. AHAS Isolation and Monosaccharide Composition
3.1.2. Infrared Spectra of the AHAS
3.2. Effect of AHAS on Average Weight and Daily Gain of Lambs
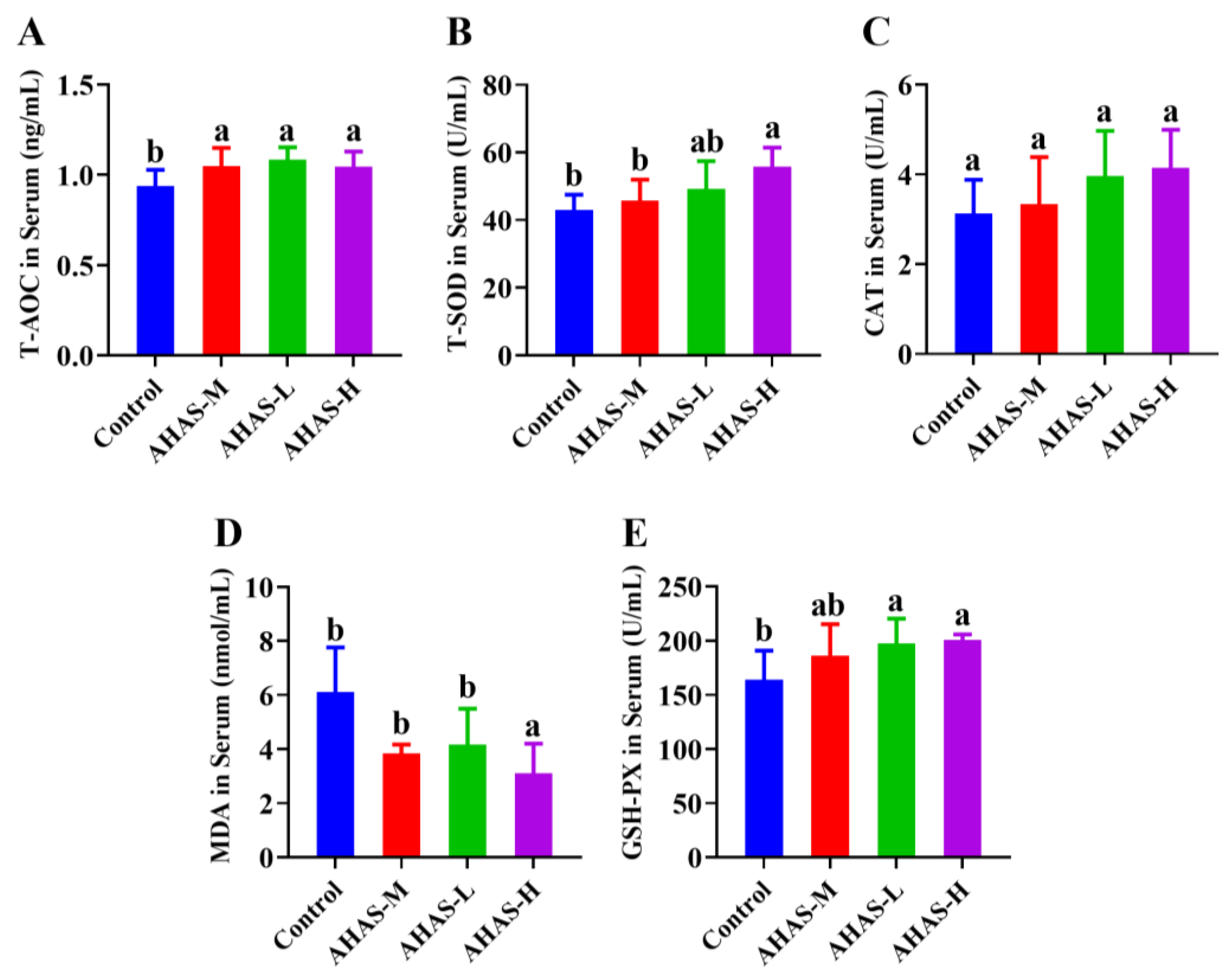
3.3. Effect of AHAS on the Antioxidant Status of Lambs
3.4. AHAS Effect on Lamb Serum Immunoglobulins
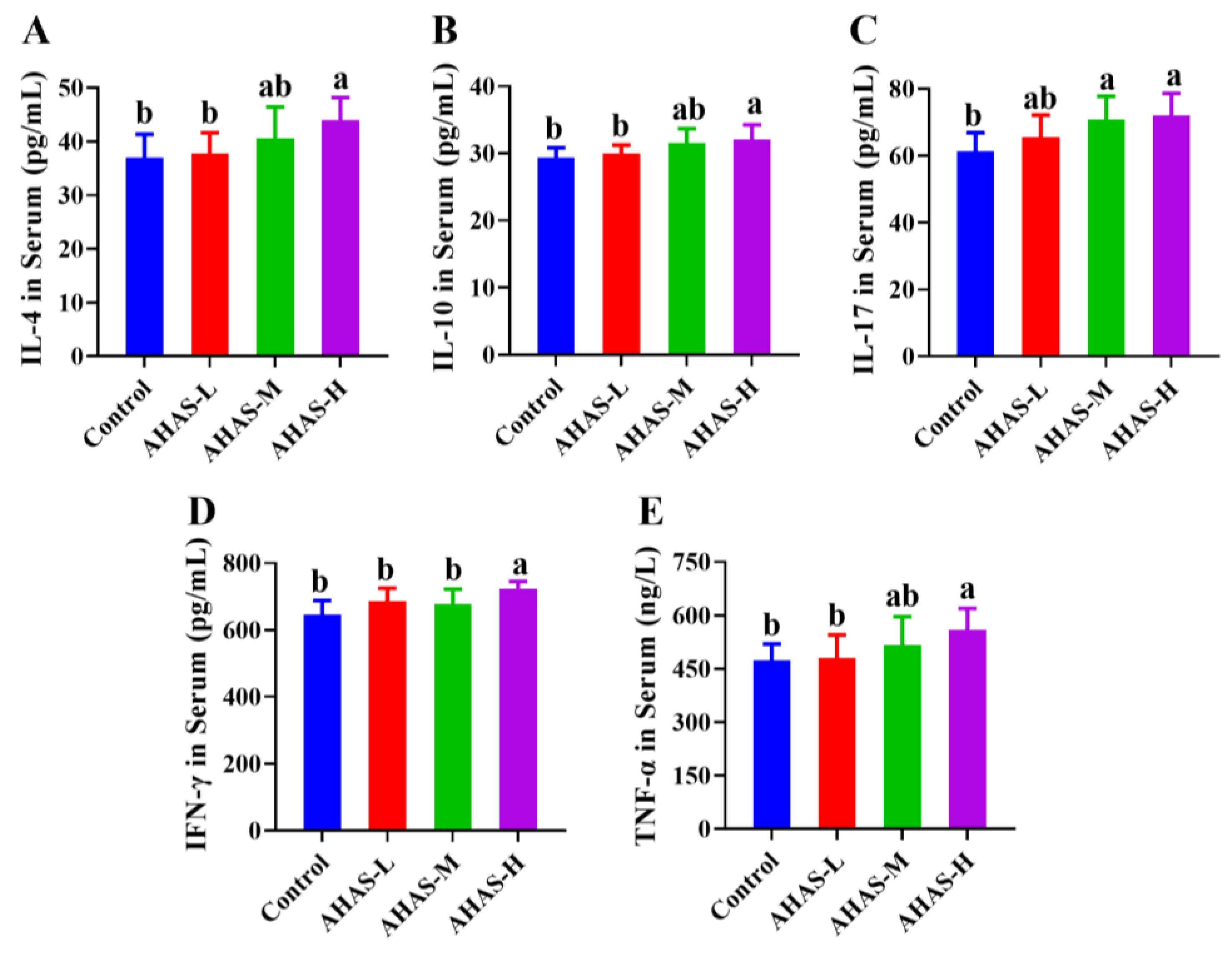
3.5. Effect of AHAS on Serum Cytokine Levels in Lambs
3.6. AHAS Effects on Lamb Diversity and Abundance of Intestinal Microbiota
3.6.1. Species Accumulation Curves and ASV/OTU
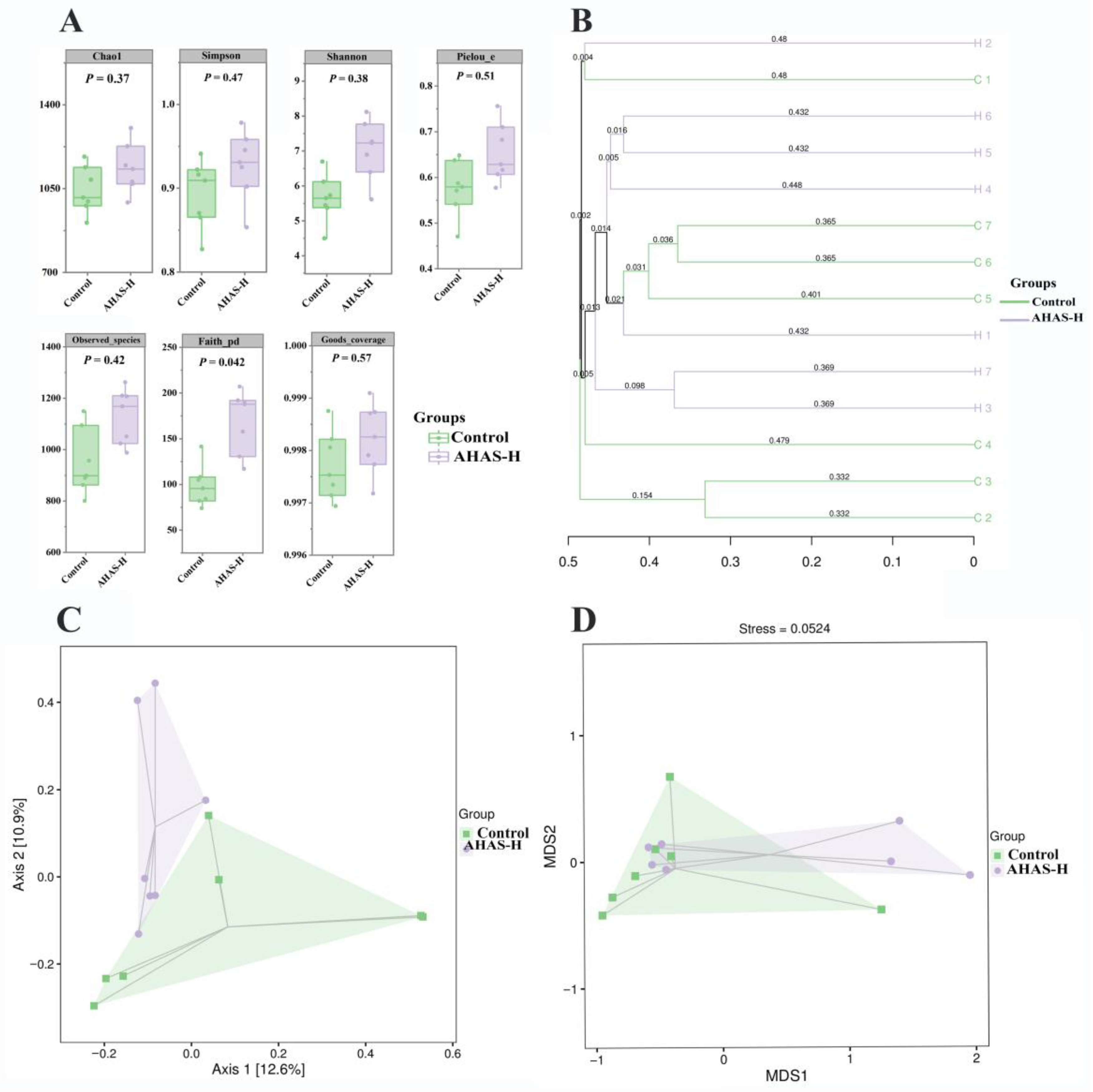
3.6.2. Alpha and Beta (α and β) Diversity Analysis
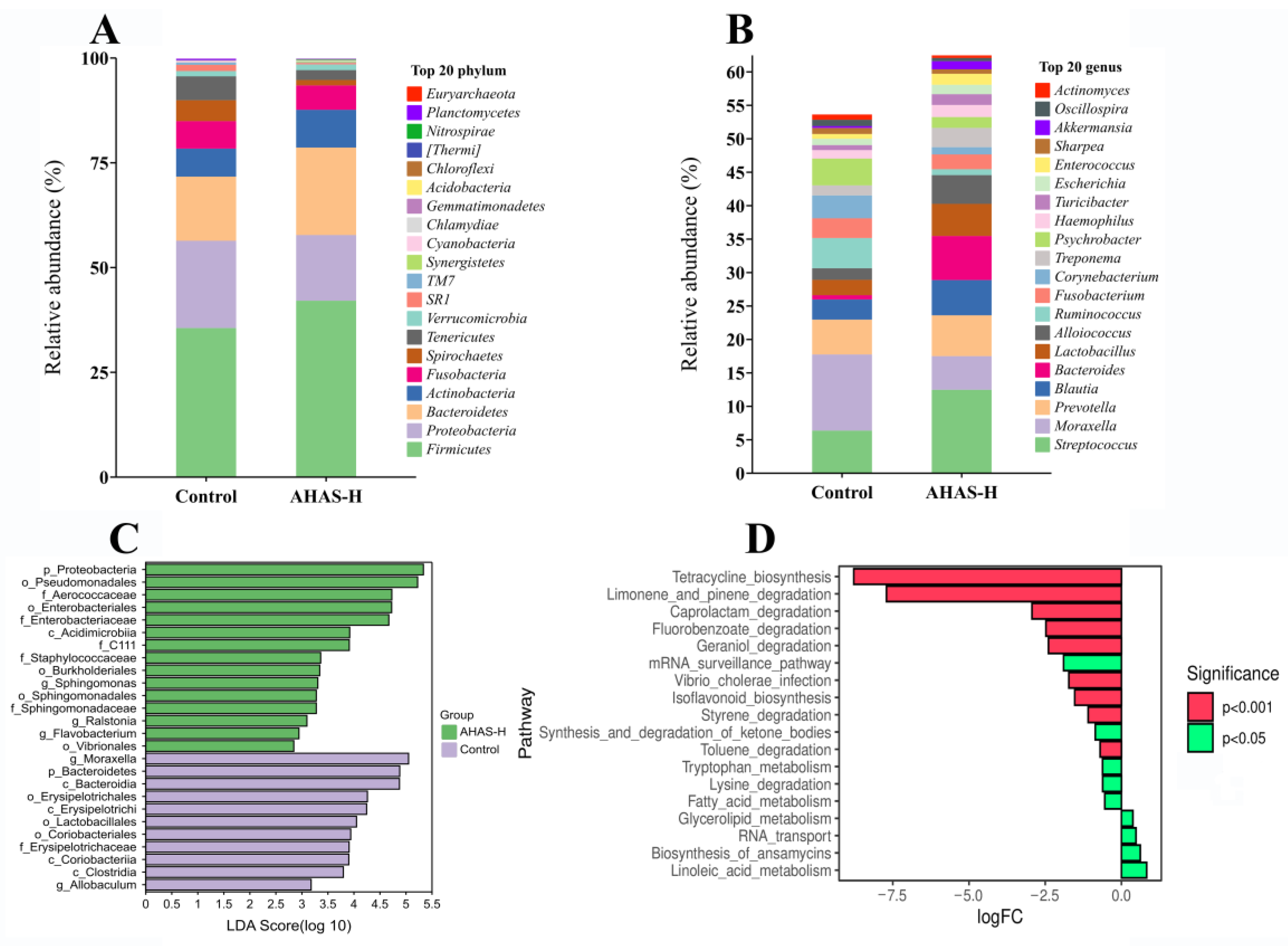
3.7. AHAS Impact on the Microbial Community Shape and Composition
3.8. Effect of AHAS on SCFAs in Lambs
4. Discussion
4.1. Growth Performance
4.2. Antioxidant Status
4.3. Immune Responses
4.4. Effects of AHAS on Fecal Microbiota Dynamics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, L.N.; Zhang, H.H.; Wang, F.; Yin, Y.X.; Yang, L.Y.; Chen, J.S. Research Note: Effects of polysaccharide-enriched Acanthopanax senticosus extract on growth performance, immune function, antioxidation, and ileal microbial populations in broiler chickens. Poult. Sci. 2021, 100, 101028. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.P.; Fan, Y.X.; Ma, T.W.; Wang, Z.; TanTai, W.J.; Nie, H.T.; Guo, Y.X.; Yu, X.Q.; Sun, L.W.; Wang, F. Carcass traits, meat quality, antioxidant status and antioxidant gene expression in muscle and liver of Hu lambs fed perilla seed. J. Anim. Physiol. Anim. Nutr. 2018, 102, e828–e837. [Google Scholar] [CrossRef]
- Bollen, C.; Louwagie, E.; Verstraeten, N.; Michiels, J.; Ruelens, P. Environmental, mechanistic and evolutionary landscape of antibiotic persistence. EMBO Rep. 2023, 24, e57309. [Google Scholar] [CrossRef]
- Xiao, J.; Guo, W.; Han, Z.; Xu, Y.; Xing, Y.; Phillips, C.J.C.; Shi, B. The Effects of Housing on Growth, Immune Function and Antioxidant Status of Young Female Lambs in Cold Conditions. Animals 2024, 14, 518. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, Z.; Guo, J.; Wang, W.; Duan, Y.; Hao, X.; Wang, R.; An, X.; Qi, J. Effect of wheat bran feruloyl oligosaccharides on the performance, blood metabolites, antioxidant status and rumen fermentation of lambs. Small Rumin. Res. 2019, 175, 65–71. [Google Scholar] [CrossRef]
- Chen, H.; Guo, B.; Yang, M.; Luo, J.; Hu, Y.; Qu, M.; Song, X. Response of Growth Performance, Blood Biochemistry Indices, and Rumen Bacterial Diversity in Lambs to Diets Containing Supplemental Probiotics and Chinese Medicine Polysaccharides. Front. Vet. Sci. 2021, 8, 681389. [Google Scholar] [CrossRef]
- GGuo, G.; Yang, W.; Fan, C.; Lan, R.; Gao, Z.; Gan, S.; Yu, H.; Yin, F.; Wang, Z. The effects of fucoidan as a dairy substitute on diarrhea rate and intestinal barrier function of the large intestine in weaned lambs. Front. Vet. Sci. 2022, 9, 1007346. [Google Scholar] [CrossRef] [PubMed]
- Quijada, N.M.; Bodas, R.; Lorenzo, J.M.; Schmitz-Esser, S.; Rodríguez-Lázaro, D.; Hernández, M. Dietary Supplementation with Sugar Beet Fructooligosaccharides and Garlic Residues Promotes Growth of Beneficial Bacteria and Increases Weight Gain in Neonatal Lambs. Biomolecules 2020, 10, 1179. [Google Scholar] [CrossRef] [PubMed]
- Wusiman, A.; Xu, S.; Ni, H.; Gu, P.; Liu, Z.; Zhang, Y.; Qiu, T.; Hu, Y.; Liu, J.; Wu, Y.; et al. Immunomodulatory effects of Alhagi honey polysaccharides encapsulated into PLGA nanoparticles. Carbohydr. Polym. 2019, 211, 217–226. [Google Scholar] [CrossRef]
- Jian, L.J.; Chang, J.M.; Ablise, M.; Li, G.R.; He, J.W. Isolation, purification, and structural elucidation of polysaccharides from Alhagi-honey. J. Asian Nat. Prod. Res. 2014, 16, 783–789. [Google Scholar] [CrossRef]
- Wusiman, A.; He, J.; Zhu, T.; Liu, Z.; Gu, P.; Hu, Y.; Liu, J.; Wang, D. Macrophage immunomodulatory activity of the cationic polymer modified PLGA nanoparticles encapsulating Alhagi honey polysaccharide. Int. J. Biol. Macromol. 2019, 134, 730–739. [Google Scholar] [CrossRef]
- Song, J.; Chen, Y.; Lv, Z.; Taoerdahong, H.; Li, G.; Li, J.; Zhao, X.; Jin, X.; Chang, J. Structural characterization of a polysaccharide from Alhagi honey and its protective effect against inflammatory bowel disease by modulating gut microbiota dysbiosis. Int. J. Biol. Macromol. 2024, 259, 128937. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhao, X.; Bo, J.; Lv, Z.; Li, G.; Chen, Y.; Liang, J.; Zhang, C.; Jin, X.; Liu, C.; et al. A polysaccharide from Alhagi honey protects the intestinal barrier and regulates the Nrf2/HO-1-TLR4/MAPK signaling pathway to treat alcoholic liver disease in mice. J. Ethnopharmacol. 2024, 321, 117552. [Google Scholar] [CrossRef]
- Cai, G.; Mao, N.; Gu, P.; Zhu, T.; He, J.; Peng, S.; Yang, Y.; Liu, Z.; Hu, Y.; Wang, D. Effects of Alhagi Honey Polysaccharides as Feed Supplement on Intestine Function and Microbiome, Immune Function, and Growth Performance in Chicken. Int. J. Mol. Sci. 2022, 23, 14332. [Google Scholar] [CrossRef] [PubMed]
- Mutailifu, P.; Nuerxiati, R.; Lu, C.; Huojiaaihemaiti, H.; Abuduwaili, A.; Yili, A. Extraction, purification, and characterization of polysaccharides from Alhagi pseudoalhagi with antioxidant and hypoglycemic activities. Process Biochem. 2022, 121, 339–348. [Google Scholar] [CrossRef]
- Cai, G.; Wusiman, A.; Gu, P.; Mao, N.; Xu, S.; Zhu, T.; He, J.; Liu, Z.; Wang, D. Supplementation of Alhagi honey polysaccharides contributes to the improvement of the intestinal immunity regulating the structure of intestinal flora in mice. Food Funct. 2021, 12, 9693–9707. [Google Scholar] [CrossRef]
- Li, Y.R.; Liu, S.T.; Gan, Q.; Zhang, J.; Chen, N.; Han, C.F.; Geng, W.J.; Wang, B.X.; Han, N.; Jia, S.R.; et al. Four polysaccharides isolated from Poria cocos mycelium and fermentation broth supernatant possess different activities on regulating immune response. Int. J. Biol. Macromol. 2023, 226, 935–945. [Google Scholar] [CrossRef]
- Wang, S.; Li, G.; Zhang, X.; Wang, Y.; Qiang, Y.; Wang, B.; Zou, J.; Niu, J.; Wang, Z. Structural characterization and antioxidant activity of Polygonatum sibiricum polysaccharides. Carbohydr. Polym. 2022, 291, 119524. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, J.; Yue, Y.; Wu, Y.; Zhang, W.; Xia, W.; Wu, M. Purification, structure identification and immune activity of a neutral polysaccharide from Cynanchum auriculatum. Int. J. Biol. Macromol. 2023, 237, 124142. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Zhu, M.J. A sensitive GC/MS detection method for analyzing microbial metabolites short chain fatty acids in fecal and serum samples. Talanta 2019, 196, 249–254. [Google Scholar] [CrossRef]
- Olech, M.; Cybulska, J.; Nowacka-Jechalke, N.; Szpakowska, N.; Masłyk, M.; Kubiński, K.; Martyna, A.; Zdunek, A.; Kaczyński, Z. Novel polysaccharide and polysaccharide-peptide conjugate from Rosa rugosa Thunb. pseudofruit—Structural characterisation and nutraceutical potential. Food Chem. 2023, 409, 135264. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Zhang, Z.F.; Gao, Z.M.; Huang, Y.; Wu, Z.Q. Physicochemical properties and cell-based bioactivity of Pu’erh tea polysaccharide conjugates. Int. J. Biol. Macromol. 2017, 104, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, X.; Yi, J.; Kang, Q.; Hao, L. Cognition of polysaccharides from confusion to clarity: When the next “omic” will come? Crit. Rev. Food Sci. Nutr. 2023, 63, 4728–4743. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Zhou, X.; Hu, X.; Chen, Y.; Xie, J. Advances in the regulation of natural polysaccharides on human health: The role of apoptosis/autophagy pathway. Crit. Rev. Food Sci. Nutr. 2023, 63, 3931–3942. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Wang, J.; Zhou, S.X.; Huang, D.C.; Qi, G.H. Ginger polysaccharides enhance intestinal immunity by modulating gut microbiota in cyclophosphamide-induced immunosuppressed mice. Int. J. Biol. Macromol. 2022, 223, 1308–1319. [Google Scholar] [CrossRef]
- De Brito, G.F.; Ponnampalam, E.N.; Hopkins, D.L. The Effect of Extensive Feeding Systems on Growth Rate, Carcass Traits, and Meat Quality of Finishing Lambs. Compr. Rev. Food Sci. Food Saf. 2017, 16, 23–38. [Google Scholar] [CrossRef]
- Li, S.; Guo, Y.; Guo, X.; Shi, B.; Ma, G.; Yan, S.; Zhao, Y. Effects of Artemisia ordosica Crude Polysaccharide on Antioxidant and Immunity Response, Nutrient Digestibility, Rumen Fermentation, and Microbiota in Cashmere Goats. Animals 2023, 13, 3575. [Google Scholar] [CrossRef]
- Ölmez, M.; Riaz, R.; Karadağoğlu, Ö.; Şahin, T.; Şerbetçi, İ.; Yılmaz, B.; Uysal, S.; Yörük, M.A. Effect of SOD-Rich Melon Supplement on Performance, Serum Biochemical, Antioxidant and Meat Quality Characteristics of Tuj Lambs. Agriculture 2023, 13, 625. [Google Scholar] [CrossRef]
- Franco, E.P.D.; Contesini, F.J.; Lima da Silva, B.; Alves de Piloto Fernandes, A.M.; Wielewski Leme, C.; Goncalves Cirino, J.P.; Bueno Campos, P.R.; de Oliveira Carvalho, P. Enzyme-assisted modification of flavonoids from Matricaria chamomilla: Antioxidant activity and inhibitory effect on digestive enzymes. J. Enzyme Inhib. Med. Chem. 2020, 35, 42–49. [Google Scholar] [CrossRef]
- Chen, J.; Long, L.; Jiang, Q.; Kang, B.; Li, Y. Effects of dietary supplementation of Lycium barbarum polysaccharides on growth performance, immune status, antioxidant capacity and selected microbial populations of weaned piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1106–1115. [Google Scholar] [CrossRef]
- Hao, X.; Wang, P.; Ren, Y.; Liu, G.; Zhang, J.; Leury, B.; Zhang, C. Effects of Astragalus membranaceus roots supplementation on growth performance, serum antioxidant and immune response in finishing lambs. Asian-Australas. J. Anim. Sci. 2020, 33, 965–972. [Google Scholar] [CrossRef]
- Gkoliou, G.; Agathangelidis, A.; Karakatsoulis, G.; Lalayanni, C.; Papalexandri, A.; Medina, A.; Genuardi, E.; Chlichlia, K.; Hatjiharissi, E.; Papaioannou, M.; et al. Differences in the immunoglobulin gene repertoires of IgG versus IgA multiple myeloma allude to distinct immunopathogenetic trajectories. Front. Oncol. 2023, 13, 1123029. [Google Scholar] [CrossRef] [PubMed]
- Keyt, B.A.; Baliga, R.; Sinclair, A.M.; Carroll, S.F.; Peterson, M.S. Structure, Function, and Therapeutic Use of IgM Antibodies. Antibodies 2020, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Glassman, C.R.; Mathiharan, Y.K.; Jude, K.M.; Su, L.; Panova, O.; Lupardus, P.J.; Spangler, J.B.; Ely, L.K.; Thomas, C.; Skiniotis, G.; et al. Structural basis for IL-12 and IL-23 receptor sharing reveals a gateway for shaping actions on T versus NK cells. Cell 2021, 184, 983–999. [Google Scholar] [CrossRef]
- Gärtner, Y.; Bitar, L.; Zipp, F.; Vogelaar, C.F. Interleukin-4 as a therapeutic target. Pharmacol. Ther. 2023, 242, 108348. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef]
- Han, J.; Wu, M.; Liu, Z. Dysregulation in IFN-γ signaling and response: The barricade to tumor immunotherapy. Front. Immunol. 2023, 14, 1190333. [Google Scholar] [CrossRef] [PubMed]
- El-Gindy, Y.M.; Abu Hafsa, S.H.; El-Deeb, N.M. The expression of liver TNF-α gene, liver and small intestine histology of thermal stressed growing rabbits affected by allicin and lycopene. J. Therm. Biol. 2023, 113, 103521. [Google Scholar] [CrossRef] [PubMed]
- Moheteer, A.; Li, J.; Abulikemu, X.; Lakho, S.A.; Meng, Y.; Zhang, J.; Khand, F.M.; Leghari, A.; Abula, S.; Guo, Q.; et al. Preparation and activity study of Ruoqiang jujube polysaccharide copper chelate. Front. Pharmacol. 2024, 14, 1347817. [Google Scholar] [CrossRef]
- Zhang, L.; Piao, X. Different dietary protein sources influence growth performance, antioxidant capacity, immunity, fecal microbiota and metabolites in weaned piglets. Anim. Nutr. 2022, 8, 71–81. [Google Scholar] [CrossRef]
- Manafu, Z.; Zhang, Z.; Malajiang, X.; Abula, S.; Guo, Q.; Wu, Y.; Wusiman, A.; Bake, B. Effects of Alhagi camelorum Fisch polysaccharide from different regions on growth performance and gastrointestinal microbiota of sheep lambs. Front. Pharmacol. 2024, 15, 1379394. [Google Scholar] [CrossRef]
- Dankwa, A.S.; Humagain, U.; Ishaq, S.L.; Yeoman, C.J.; Clark, S.; Beitz, D.C.; Testroet, E.D. Bacterial communities in the rumen and feces of lactating Holstein dairy cows are not affected when fed reduced-fat dried distillers’ grains with solubles. Animal 2021, 15, 100281. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Alwali, A.Y.; Parkinson, E.I. Small molecule inducers of actinobacteria natural product biosynthesis. J. Ind. Microbiol. Biotechnol. 2023, 50, 19. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. Biomed. Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A new functional genus with potential probiotic properties? Gut Microbes. 2021, 13, 1875796. [Google Scholar] [CrossRef] [PubMed]
- Tett, A.; Pasolli, E.; Masetti, G.; Ercolini, D.; Segata, N. Prevotella diversity, niches and interactions with the human host. Nat. Rev. Microbiol. 2021, 19, 585–599. [Google Scholar] [CrossRef]
- O’Callaghan, J.; O’Toole, P.W. Lactobacillus: Host-microbe relationships. Curr. Top. Microbiol. Immunol. 2013, 358, 119–154. [Google Scholar]
- Wang, Q.; Cai, R.; Huang, A.; Wang, X.; Qu, W.; Shi, L.; Li, C.; Yan, H. Comparison of Oropharyngeal Microbiota in Healthy Piglets and Piglets with Respiratory Disease. Front. Microbiol. 2018, 9, 3218. [Google Scholar] [CrossRef]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes. 2021, 13, 1907272. [Google Scholar] [CrossRef]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Peng, K.; Yu, Y.; Liang, C.; Xie, H.; Guo, Y.; Zhou, S.; Zheng, M.B.W.; Zheng, P.; Yang, P. Propionic acid regulates immune tolerant properties in B Cells. J. Cell Mol. Med. 2022, 26, 2766–2776. [Google Scholar] [CrossRef] [PubMed]
- iccolai, E.; Baldi, S.; Ricci, F.; Russo, E.; Nannini, G.; Menicatti, M.; Poli, G.; Taddei, A.; Bartolucci, G.; Calabrò, A.S.; et al. Evaluation and comparison of short chain fatty acids composition in gut diseases. World J. Gastroenterol. 2019, 25, 5543–5558. [Google Scholar] [CrossRef] [PubMed]


| Indicator | Control | AHAS-L | AHAS-M | AHAS-H |
|---|---|---|---|---|
| Initial body weight (kg) | 2.15 ± 0.09 | 2.10 ± 0.27 | 2.07 ± 0.24 | 2.17 ± 0.36 |
| Final body weight (kg) | 4.54 ± 1.24 b | 4.77 ± 0.68 ab | 4.98 ± 0.62 ab | 5.57 ± 0.97 a |
| Average daily gain (g/d) | 120.31 ± 12.24 b | 133.28 ± 20.04 ab | 147.07 ± 15.15 ab | 170.8 ± 15.43 a |
| Initial body height (cm) | 28.69 ± 2.07 | 29.58 ± 1.41 | 28.90 ± 2.06 | 29.33 ± 1.46 |
| Final body height (cm) | 39.72 ± 1.28 c | 43.14 ± 1.70 bc | 45.97 ± 1.55 b | 48.59 ± 2.92 a |
| Initial body slope length(cm) | 28.63 ± 3.04 | 28.88 ± 1.28 | 28.83 ± 1.89 | 29.72 ± 1.34 |
| Final body slope length(cm) | 40.88 ± 3.15 c | 43.47 ± 2.70 bc | 42.22 ± 2.35 b | 46.31 ± 2.22 a |
| Initial bust length(cm) | 29.87 ± 1.83 | 29.24 ± 1.12 | 28.96 ± 1.53 | 29.91 ± 0.74 |
| Final bust length (cm) | 40.49 ± 2.93 c | 44.25 ± 2.44 b | 44.35 ± 1.69 b | 49.58 ± 3.40 a |
| Initial pipe circumference length (cm) | 3.38 ± 0.37 | 3.25 ± 0.286 | 3.34 ± .163 | 3.25 ± 0.19 |
| Final pipe circumference length (cm) | 4.32 ± 0.17 | 4.36 ± 0.19 | 4.68 ± 0.24 | 4.82 ± 0.17 |
| Items | Control | AHAS-H |
|---|---|---|
| Acetic acid (µmol/mL) | 2320.04 ± 324.79 b | 3126.91 ± 312.47 a |
| Butyric acid (µmol/mL) | 325.12 ± 56.55 b | 517.71 ± 169.01 a |
| Isovaleric acid (µmol/mL) | 68.32 ± 15.53 | 84.06 ± 17.83 |
| Valeric acid (µmol/mL) | 57.92 ± 17.18 b | 106.82 ± 23.54 a |
| Caproic acid (µmol/mL) | 0.7235 ± 0.42 | 2.008 ± 0.58 |
| Propionic acid (µmol/mL) | 819.49 ± 69.51 b | 966.46 ± 82.58 a |
| Isobutyric acid (µmol/mL) | 76.71± 24.07 | 108.15 ± 12.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Kudereti, T.; Wusiman, A.; Abula, S.; He, X.; Li, J.; Yang, Y.; Guo, Q.; Guo, Q. Regulatory Effects of Alhagi Honey Small-Molecule Sugars on Growth Performance and Intestinal Microbiota of Lambs. Animals 2024, 14, 2402. https://doi.org/10.3390/ani14162402
Li J, Kudereti T, Wusiman A, Abula S, He X, Li J, Yang Y, Guo Q, Guo Q. Regulatory Effects of Alhagi Honey Small-Molecule Sugars on Growth Performance and Intestinal Microbiota of Lambs. Animals. 2024; 14(16):2402. https://doi.org/10.3390/ani14162402
Chicago/Turabian StyleLi, Jianlong, Tuerhong Kudereti, Adelijiang Wusiman, Saifuding Abula, Xiaodong He, Jiaxin Li, Yang Yang, Qianru Guo, and Qingyong Guo. 2024. "Regulatory Effects of Alhagi Honey Small-Molecule Sugars on Growth Performance and Intestinal Microbiota of Lambs" Animals 14, no. 16: 2402. https://doi.org/10.3390/ani14162402
APA StyleLi, J., Kudereti, T., Wusiman, A., Abula, S., He, X., Li, J., Yang, Y., Guo, Q., & Guo, Q. (2024). Regulatory Effects of Alhagi Honey Small-Molecule Sugars on Growth Performance and Intestinal Microbiota of Lambs. Animals, 14(16), 2402. https://doi.org/10.3390/ani14162402





