Morphologic, Proliferative, and Cytogenetic Changes during In Vitro Propagation of Cat Adipose Tissue-Derived Mesenchymal Stromal/Stem Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. cAT-MSC Isolation and Culture
2.2. Morphology
2.3. Colony Forming Units–Fibroblasts (CFU-F)
2.4. Doubling Time (DT)
2.5. Trilineage Differentiation Assay
2.6. Immunophenotype
2.7. Cytogenetic Analysis
2.8. Statistical Analysis
3. Results
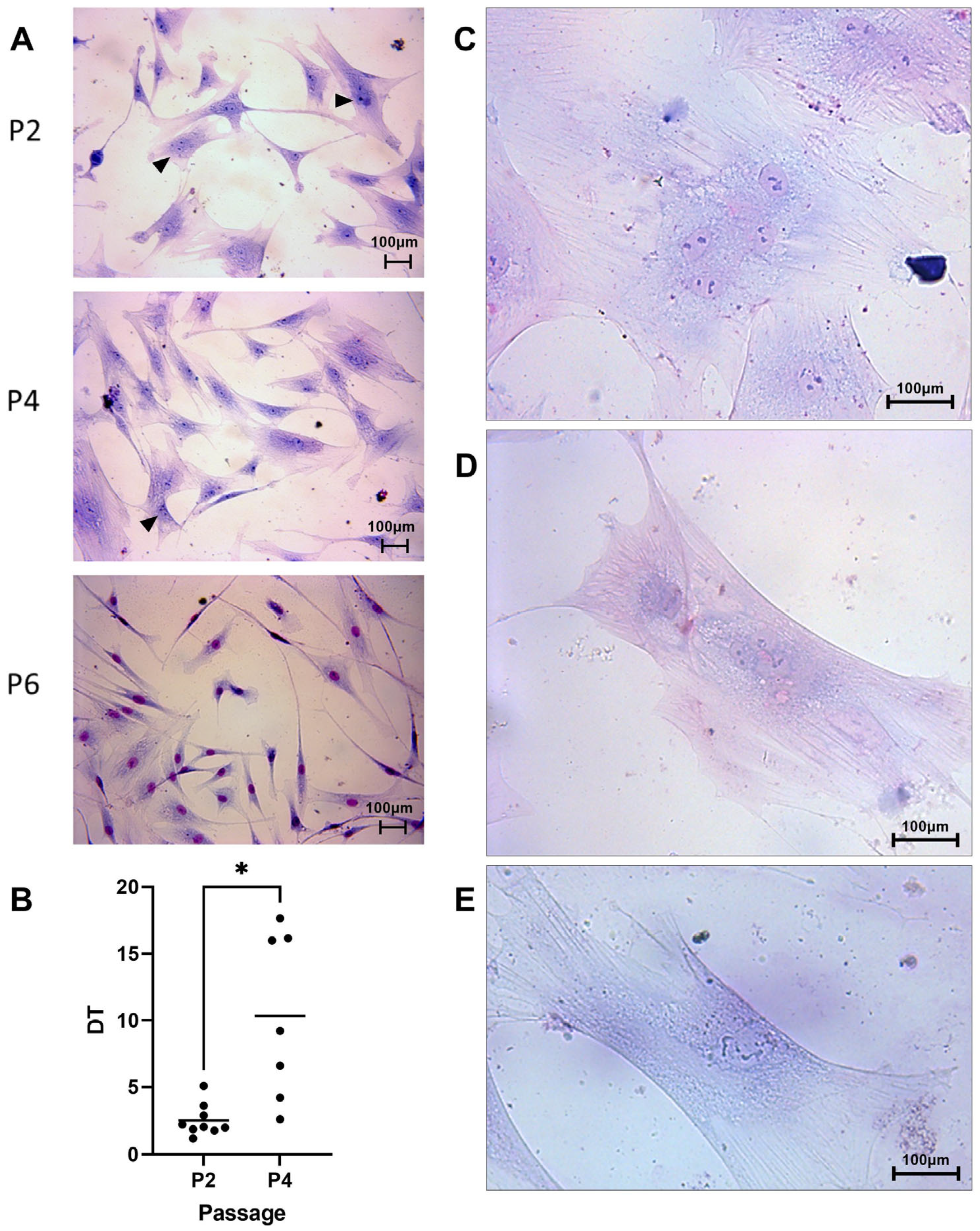
3.1. Cell Morphology and Proliferation
3.1.1. Cell Morphology
3.1.2. Cell Proliferation
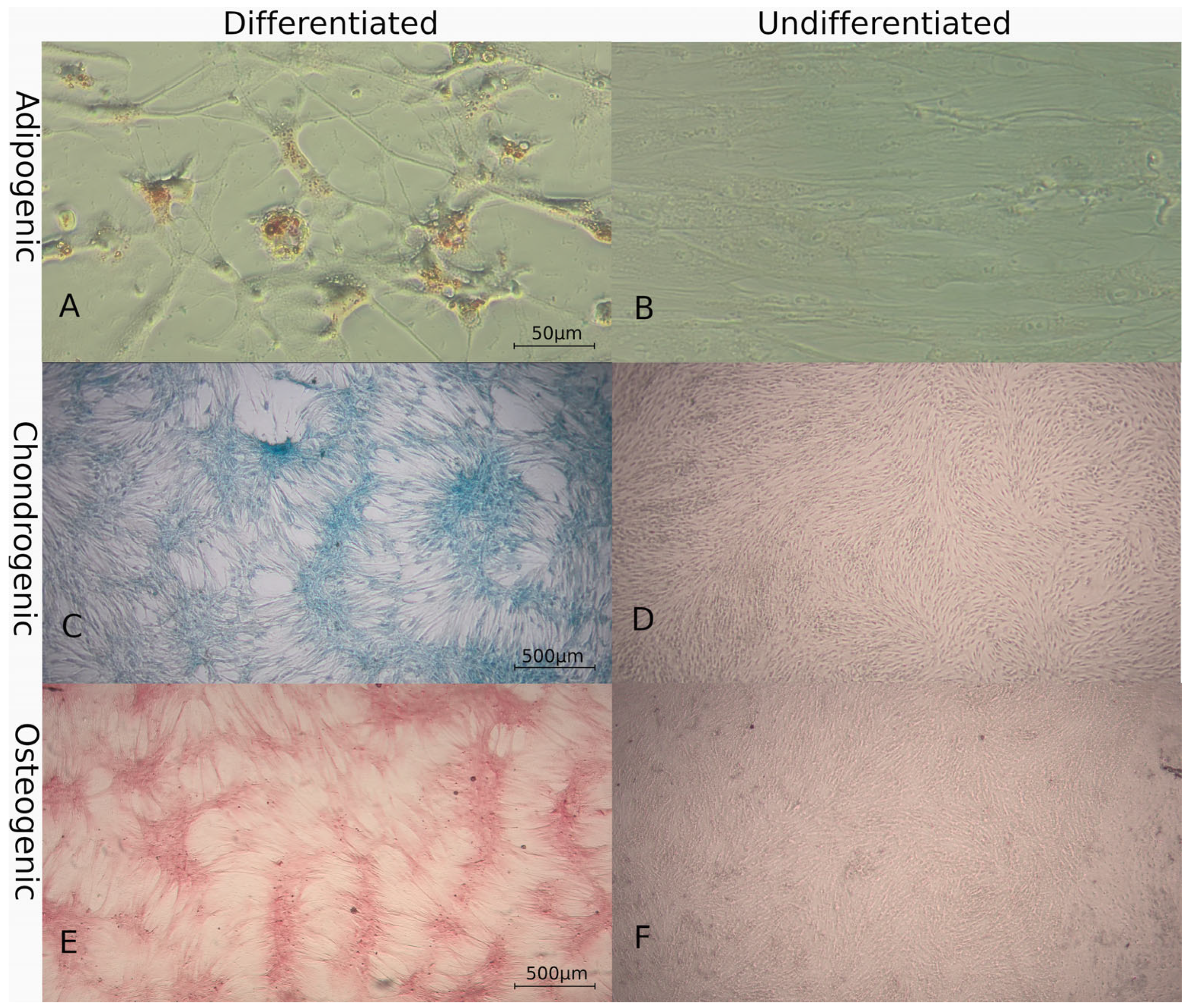
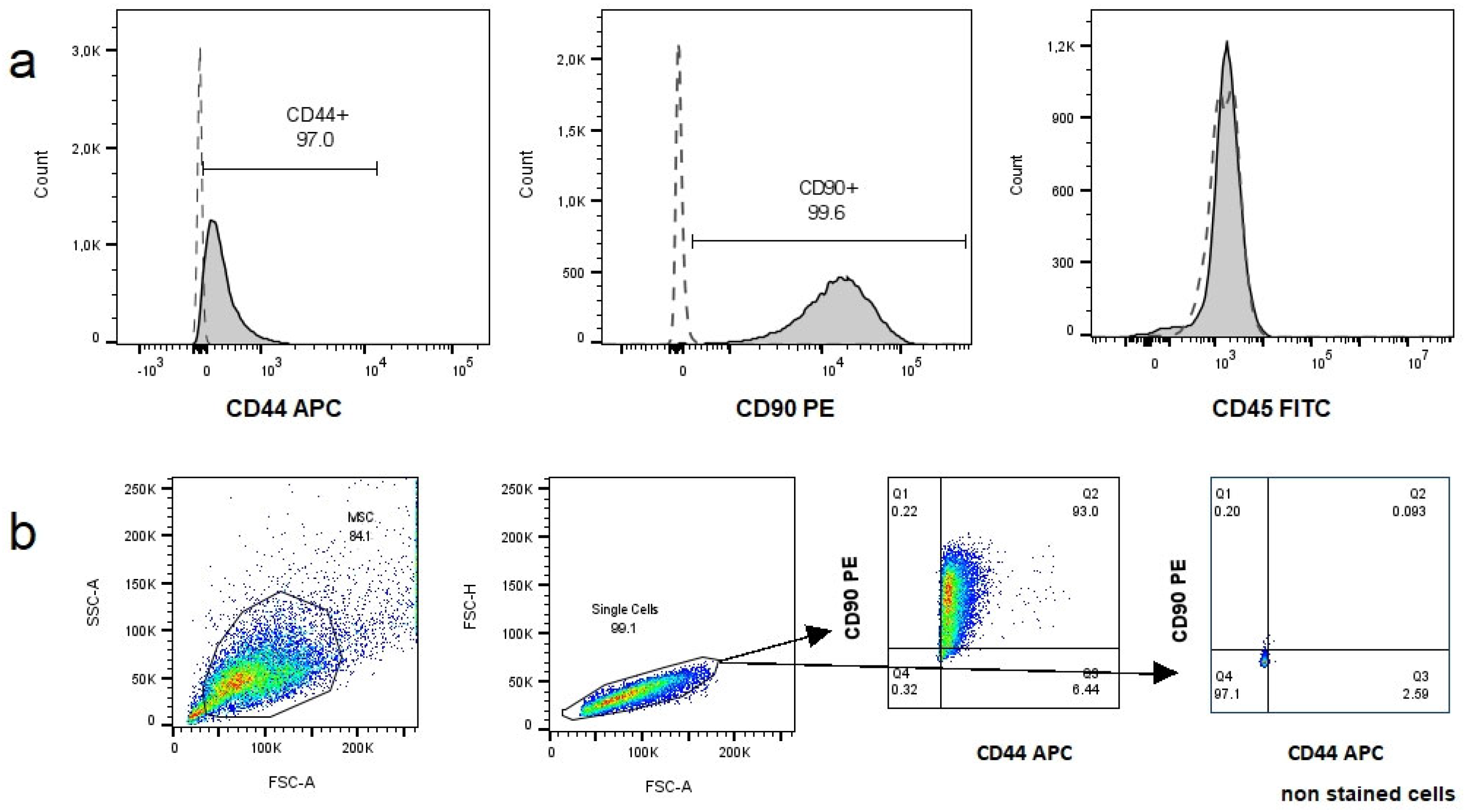
3.2. Characterization of MSCs
3.2.1. Multilineage Differentiation
3.2.2. Immunophenotype
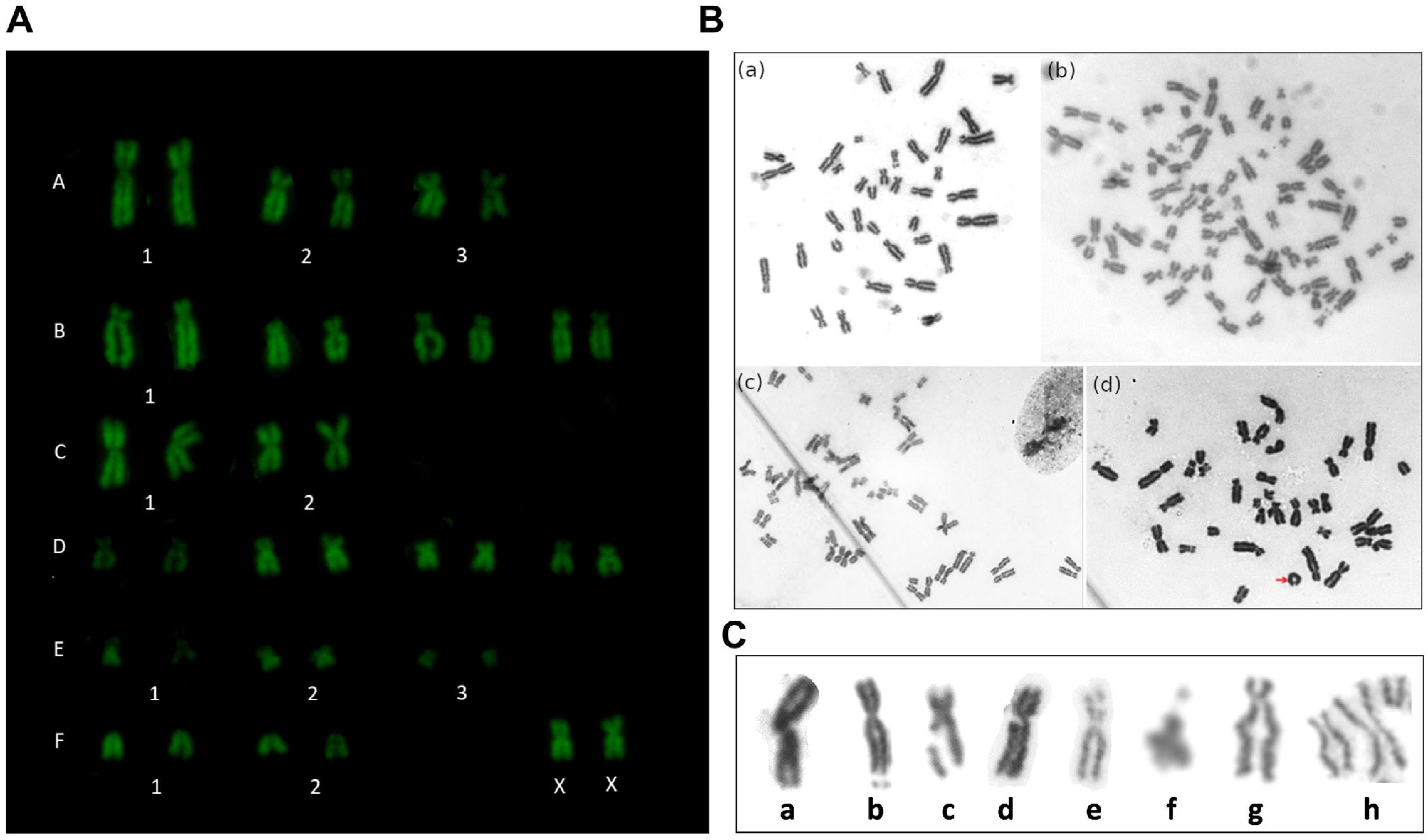
3.3. Cytogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, A.M.; Dow, S.W. Concise Review: Stem Cell Trials Using Companion Animal Disease Models. Stem Cells 2016, 34, 1709–1729. [Google Scholar] [CrossRef]
- Quimby, J.M.; Webb, T.L.; Habenicht, L.M.; Dow, S.W. Safety and Efficacy of Intravenous Infusion of Allogeneic Cryopreserved Mesenchymal Stem Cells for Treatment of Chronic Kidney Disease in Cats: Results of Three Sequential Pilot Studies. Stem Cell Res. Ther. 2013, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Quimby, J.M.; Borjesson, D.L. Mesenchymal Stem Cell Therapy in Cats: Current Knowledge and Future Potential. J. Feline Med. Surg. 2018, 20, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Voga, M.; Kovač, V.; Majdic, G. Comparison of Canine and Feline Adipose-Derived Mesenchymal Stem Cells/Medicinal Signaling Cells With Regard to Cell Surface Marker Expression, Viability, Proliferation, and Differentiation Potential. Front. Vet. Sci. 2021, 7, 610240. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.A.; Walker, N.J.; Napoli, E.; Borjesson, D.L. Evaluation of Senescence in Mesenchymal Stem Cells Isolated from Equine Bone Marrow, Adipose Tissue, and Umbilical Cord Tissue. Stem Cells Dev. 2012, 21, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Arzi, B.; Kol, A.; Murphy, B.; Walker, N.J.; Wood, J.A.; Clark, K.; Verstraete, F.J.M.; Borjesson, D.L. Feline Foamy Virus Adversely Affects Feline Mesenchymal Stem Cell Culture and Expansion: Implications for Animal Model Development. Stem Cells Dev. 2015, 24, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Neri, S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int. J. Mol. Sci. 2019, 20, 2406. [Google Scholar] [CrossRef] [PubMed]
- Borgonovo, T.; Vaz, I. Genetic Evaluation of Mesenchymal Stem Cells by G-Banded Karyotyping in a Cell Technology Center. Rev. Bras. Hematol. Hemoter. 2014, 36, 202–207. [Google Scholar] [CrossRef]
- Pan, Q.; Fouraschen, S.M.; de Ruiter, P.E.; Dinjens, W.N.; Kwekkeboom, J.; Tilanus, H.W.; van der Laan, L.J. Detection of Spontaneous Tumorigenic Transformation during Culture Expansion of Human Mesenchymal Stromal Cells. Exp. Biol. Med. 2014, 239, 105–115. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Z.-B.; Song, Y.-P.; Han, Z.C. Safety of Mesenchymal Stem Cells for Clinical Application. Stem Cells Int. 2012, 2012, 652034. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.E.C.; Harrison, N.J.; Maltby, E.; Smith, K.; Moore, H.D.; Shaw, P.J.; Heath, P.R.; Holden, H.; Andrews, P.W. Adaptation to Culture of Human Embryonic Stem Cells and Oncogenesis in Vivo. Nat. Biotechnol. 2007, 25, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.E.; Zaffaroni, N.; Novara, F.; Cometa, A.M.; Avanzini, M.A.; Moretta, A.; Montagna, D.; Maccario, R.; Villa, R.; Daidone, M.G.; et al. Human Bone Marrow–Derived Mesenchymal Stem Cells Do Not Undergo Transformation after Long-Term In Vitro Culture and Do Not Exhibit Telomere Maintenance Mechanisms. Cancer Res. 2007, 67, 9142–9149. [Google Scholar] [CrossRef] [PubMed]
- Lamm, N.; Ben-David, U.; Golan-Lev, T.; Storchová, Z.; Benvenisty, N.; Kerem, B. Genomic Instability in Human Pluripotent Stem Cells Arises from Replicative Stress and Chromosome Condensation Defects. Cell Stem Cell 2016, 18, 253–261. [Google Scholar] [CrossRef]
- Neri, S.; Bourin, P.; Peyrafitte, J.-A.; Cattini, L.; Facchini, A.; Mariani, E. Human Adipose Stromal Cells (ASC) for the Regeneration of Injured Cartilage Display Genetic Stability after In Vitro Culture Expansion. PLoS ONE 2013, 8, e77895. [Google Scholar] [CrossRef] [PubMed]
- Afonso Cornélio, D.; Batistuzzo de Medeiros, S.R.; Cornélio, D.A.; Medeiros, S.R.B. de Genetic Evaluation of Mesenchymal Stem Cells. Rev. Bras. Hematol. Hemoter. 2014, 36, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.C.; Fierro, F.A.; Ko, E.M.; Walker, N.J.; Arzi, B.; Tepper, C.G.; Dahlenburg, H.; Cicchetto, A.; Kol, A.; Marsh, L.; et al. Human and Feline Adipose-Derived Mesenchymal Stem Cells Have Comparable Phenotype, Immunomodulatory Functions, and Transcriptome. Stem Cell Res. Ther. 2017, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, A.J.; Claros, S.; Fernández, V.; Alcoholado, C.; Fariñas, F.; Moreno, A.; Becerra, J.; Andrades, J.A. Safety and Efficacy of the Mesenchymal Stem Cell in Feline Eosinophilic Keratitis Treatment. BMC Vet. Res. 2018, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Kang, K.K.; Oh, S.K.; Sung, S.E.; Kim, K.S.; Kwon, Y.S.; Yun, S. Isolation and Characterization of Feline Wharton’s Jelly-Derived Mesenchymal Stem Cells. Vet. Sci. 2021, 8, 24. [Google Scholar] [CrossRef]
- Thomas, P.; Holland, N.; Bolognesi, C.; Kirsch-Volders, M.; Bonassi, S.; Zeiger, E.; Knasmueller, S.; Fenech, M. Buccal Micronucleus Cytome Assay. Nat. Protoc. 2009, 4, 825–837. [Google Scholar] [CrossRef]
- Bolognesi, C.; Roggieri, P.; Ropolo, M.; Thomas, P.; Hor, M.; Fenech, M.; Nersesyan, A.; Knasmueller, S. Buccal Micronucleus Cytome Assay: Results of an Intra- and Inter-Laboratory Scoring Comparison. Mutagenesis 2015, 30, 545–555. [Google Scholar] [CrossRef]
- Gómez, M.C.; Qin, Q.; Biancardi, M.N.; Galiguis, J.; Dumas, C.; MacLean, R.A.; Wang, G.; Pope, C.E. Characterization and Multilineage Differentiation of Domestic and Black-Footed Cat Mesenchymal Stromal/Stem Cells from Abdominal and Subcutaneous Adipose Tissue. Cell. Reprogram. 2015, 17, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Algorta, A.; Artigas, R.; Rial, A.; Brandl, S.; Rodellar, C.; Benavides, U.; Maisonnave, J.; Yaneselli, K. Isolation and Characterization of Feline Dental Pulp Stem Cells. J. Feline Med. Surg. 2023, 25. [Google Scholar] [CrossRef] [PubMed]
- Yaneselli, K.; Filomeno, A.; Semiglia, G.; Arce, C.; Erickson, K. Allogeneic Stem Cell Transplantation for Bone Regeneration of a Nonunion Defect in a Canine. Vet. Med. Res. Rep. 2013, 4, 39–44. [Google Scholar]
- Cho, K.W.; Youn, H.Y.; Watari, T.; Tsujimoto, H.; Hasegawa, A.; Satoh, H. A Proposed Nomenclature of the Domestic Cat Karyotype. Cytogenet. Cell Genet. 1997, 79, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Udroiu, I.; Sgura, A. Cytogenetic Tests for Animal Production: State of the Art and Perspectives. Anim. Genet. 2017, 48, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Webb, T.L.; Quimby, J.M.; Dow, S.W. In Vitro Comparison of Feline Bone Marrow-Derived and Adipose Tissue-Derived Mesenchymal Stem Cells. J. Feline Med. Surg. 2012, 14, 165–168. [Google Scholar] [CrossRef]
- Martin, D.R.; Cox, N.R.; Hathcock, T.L.; Niemeyer, G.P.; Baker, H.J. Isolation and Characterization of Multipotential Mesenchymal Stem Cells from Feline Bone Marrow. Exp. Hematol. 2002, 30, 879–886. [Google Scholar] [CrossRef]
- Maciel, B.B.; Rebelatto, C.L.K.; Brofman, P.R.S.; Brito, H.F.V.; Patricio, L.F.L.; Cruz, M.A.; Locatelli-Dittrich, R. Morphology and Morphometry of Feline Bone Marrow-Derived Mesenchymal Stem Cells in Culture. Pesqui. Vet. Bras. 2014, 34, 1127–1134. [Google Scholar] [CrossRef]
- Vidal, M.A.; Kilroy, G.E.; Johnson, J.R.; Lopez, M.J.; Moore, R.M.; Gimble, J.M. Cell Growth Characteristics and Differentiation Frequency of Adherent Equine Bone Marrow-Derived Mesenchymal Stromal Cells: Adipogenic and Osteogenic Capacity. Vet. Surg. 2006, 35, 601–610. [Google Scholar] [CrossRef]
- Panasophonkul, S.; Samart, P.; Kongon, K.; Sathanawongs, A. Phenotypic Characteristics of Feline Adipose-Derived Stem Cells Affected by Cell Passage Number. Pol. J. Vet. Sci. 2017, 20, 651–660. [Google Scholar]
- Kim, H.-R.; Lee, J.; Byeon, J.S.; Gu, N.-Y.; Lee, J.; Cho, I.-S.; Cha, S.-H. Extensive Characterization of Feline Intra-Abdominal Adipose-Derived Mesenchymal Stem Cells. J. Vet. Sci. 2017, 18, 299–306. [Google Scholar] [CrossRef] [PubMed]
- LEE, B.-Y.; LI, Q.; SONG, W.-J.; CHAE, H.-K.; KWEON, K.; AHN, J.-O.; YOUN, H.-Y. Altered Properties of Feline Adipose-Derived Mesenchymal Stem Cells during Continuous in vitro cultivation. J. Vet. Med. Sci. 2018, 80, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.-J.; Ryu, H.-H.; Park, S.S.; Koyama, Y.; Kikuchi, M.; Woo, H.-M.; Kim, W.H.; Kweon, O.-K. Comparing the Osteogenic Potential of Canine Mesenchymal Stem Cells Derived from Adipose Tissues, Bone Marrow, Umbilical Cord Blood, and Wharton’s Jelly for Treating Bone Defects. J. Vet. Sci. 2012, 13, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Yaneselli, K.M.; Kuhl, C.P.; Terraciano, P.B.; de Oliveira, F.S.; Pizzato, S.B.; Pazza, K.; Magrisso, A.B.; Torman, V.; Rial, A.; Moreno, M.; et al. Comparison of the Characteristics of Canine Adipose Tissue-Derived Mesenchymal Stem Cells Extracted from Different Sites and at Different Passage Numbers. J. Vet. Sci. 2018, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal Cells from the Adipose Tissue-Derived Stromal Vascular Fraction and Culture Expanded Adipose Tissue-Derived Stromal/Stem Cells: A Joint Statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Guest, D.J.; Dudhia, J.; Smith, R.K.W.; Roberts, S.J.; Conzemius, M.; Innes, J.F.; Fortier, L.A.; Meeson, R.L. Position Statement: Minimal Criteria for Reporting Veterinary and Animal Medicine Research for Mesenchymal Stromal/Stem Cells in Orthopedic Applications. Front. Vet. Sci. 2022, 9, 817041. [Google Scholar] [CrossRef] [PubMed]
- Barkholt, L.; Flory, E.; Jekerle, V.; Lucas-samuel, S.; Ahnert, P.; Bisset, L.; Büscher, D.; Fibbe, W.; Foussat, A.; Kwa, M.; et al. Risk of Tumorigenicity in Mesenchymal Stromal Cell e Based Therapies—Bridging Scientific Observations and Regulatory Viewpoints. Cytotherapy 2013, 15, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Muntión, S.; Sánchez-Guijo, F.M.; Carrancio, S.; Villarón, E.; López, O.; Diez-Campelo, M.; San Miguel, J.F.; del Cañizo, M.C. Optimisation of Mesenchymal Stromal Cells Karyotyping Analysis: Implications for Clinical Use. Transfus. Med. 2012, 22, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Stultz, B.G.; McGinnis, K.; Thompson, E.E.; Lo Surdo, J.L.; Bauer, S.R.; Hursh, D.A. Chromosomal Stability of Mesenchymal Stromal Cells during in Vitro Culture. Cytotherapy 2016, 18, 336–343. [Google Scholar] [CrossRef]
- Nikitina, V.; Astrelina, T.; Nugis, V.; Ostashkin, A.; Karaseva, T.; Dobrovolskaya, E.; Usupzhanova, D.; Suchkova, Y.; Lomonosova, E.; Rodin, S.; et al. Clonal Chromosomal and Genomic Instability during Human Multipotent Mesenchymal Stromal Cells Long-Term Culture. PLoS ONE 2018, 13, e0192445. [Google Scholar] [CrossRef]
- Duailibi, M.T.; Kulikowski, L.D.; Duailibi, S.E.; Lipay, M.V.N.; Melaragno, M.I.; Ferreira, L.M.; Vacanti, J.P.; Yelick, P.C. Cytogenetic Instability of Dental Pulp Stem Cell Lines. J. Mol. Histol. 2012, 43, 89–94. [Google Scholar] [CrossRef]
- Simons, A.; Shaffer, L.G.; Hastings, R.J. Cytogenetic Nomenclature: Changes in the ISCN 2013 Compared to the 2009 Edition. Cytogenet. Genome Res. 2013, 141, 1–6. [Google Scholar] [CrossRef]
- Blázquez-Prunera, A.; Díez, J.M.; Gajardo, R.; Grancha, S. Human Mesenchymal Stem Cells Maintain Their Phenotype, Multipotentiality, and Genetic Stability When Cultured Using a Defined Xeno-Free Human Plasma Fraction. Stem Cell Res. Ther. 2017, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Mitalipova, M.M.; Rao, R.R.; Hoyer, D.M.; Johnson, J.A.; Meisner, L.F.; Jones, K.L.; Dalton, S.; Stice, S.L. Preserving the Genetic Integrity of Human Embryonic Stem Cells. Nat. Biotechnol. 2005, 23, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, N.R.; Musio, A.; Vezzoni, P.; Simpson, A.H.R.W.; Noble, B.S.; McWhir, J. Physiologic Oxygen Enhances Human Embryonic Stem Cell Clonal Recovery and Reduces Chromosomal Abnormalities. Cloning Stem Cells 2006, 8, 16–23. [Google Scholar] [CrossRef]
- Rebuzzini, P.; Neri, T.; Mazzini, G.; Zuccotti, M.; Redi, C.A.; Garagna, S. Karyotype Analysis of the Euploid Cell Population of a Mouse Embryonic Stem Cell Line Revealed a High Incidence of Chromosome Abnormalities That Varied during Culture. Cytogenet. Genome Res. 2008, 121, 18–24. [Google Scholar] [CrossRef]
- Di Bucchianico, S.; Giardi, M.F.; De Marco, P.; Ottaviano, L.; Botti, D. Cytogenetic Stability of Chicken T-Cell Line Transformed with Marek’s Disease Virus: Atomic Force Microscope, a New Tool for Investigation. J. Mol. Recognit. 2011, 24, 608–618. [Google Scholar] [CrossRef]
- Philpott, S.M.; Buehring, G.C. Defective DNA Repair in Cells with Human T-Cell Leukemia/Bovine Leukemia Viruses: Role of Tax Gene. J. Natl. Cancer Inst. 1999, 91, 933–942. [Google Scholar] [CrossRef] [PubMed]
| Aneuploidy | Polyploidy | Fracture | Gap | Deletion | Duplication | Chromatid Separation | Total Number of Aberrations | |
|---|---|---|---|---|---|---|---|---|
| P2 (n = 266) | 3 (1.1%) | 13 (4.9%) | 18 (6.8%) | 5 (1.9%) | 6 (2.3%) | 9 (3.4%) | 9 (3.4%) | 63 (23.6%) |
| P4 (n = 61) | 2 (3.3%) | 1 (1.6%) | 1 (1.6%) | 2 (3.3%) | 0 | 2 (3.3%) | 4 (6.6%) | 12 (19.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algorta, A.; Artigas, R.; Rial, A.; Benavides, U.; Maisonnave, J.; Yaneselli, K. Morphologic, Proliferative, and Cytogenetic Changes during In Vitro Propagation of Cat Adipose Tissue-Derived Mesenchymal Stromal/Stem Cells. Animals 2024, 14, 2408. https://doi.org/10.3390/ani14162408
Algorta A, Artigas R, Rial A, Benavides U, Maisonnave J, Yaneselli K. Morphologic, Proliferative, and Cytogenetic Changes during In Vitro Propagation of Cat Adipose Tissue-Derived Mesenchymal Stromal/Stem Cells. Animals. 2024; 14(16):2408. https://doi.org/10.3390/ani14162408
Chicago/Turabian StyleAlgorta, Agustina, Rody Artigas, Analía Rial, Uruguaysito Benavides, Jacqueline Maisonnave, and Kevin Yaneselli. 2024. "Morphologic, Proliferative, and Cytogenetic Changes during In Vitro Propagation of Cat Adipose Tissue-Derived Mesenchymal Stromal/Stem Cells" Animals 14, no. 16: 2408. https://doi.org/10.3390/ani14162408
APA StyleAlgorta, A., Artigas, R., Rial, A., Benavides, U., Maisonnave, J., & Yaneselli, K. (2024). Morphologic, Proliferative, and Cytogenetic Changes during In Vitro Propagation of Cat Adipose Tissue-Derived Mesenchymal Stromal/Stem Cells. Animals, 14(16), 2408. https://doi.org/10.3390/ani14162408





