Evaluation of the Effectiveness of Eugenol and MS-222 as Anesthetics in Zebrafish in Repeated Exposures and Post-Anesthesia Behaviour
Abstract
:Simple Summary
Abstract
1. Introduction
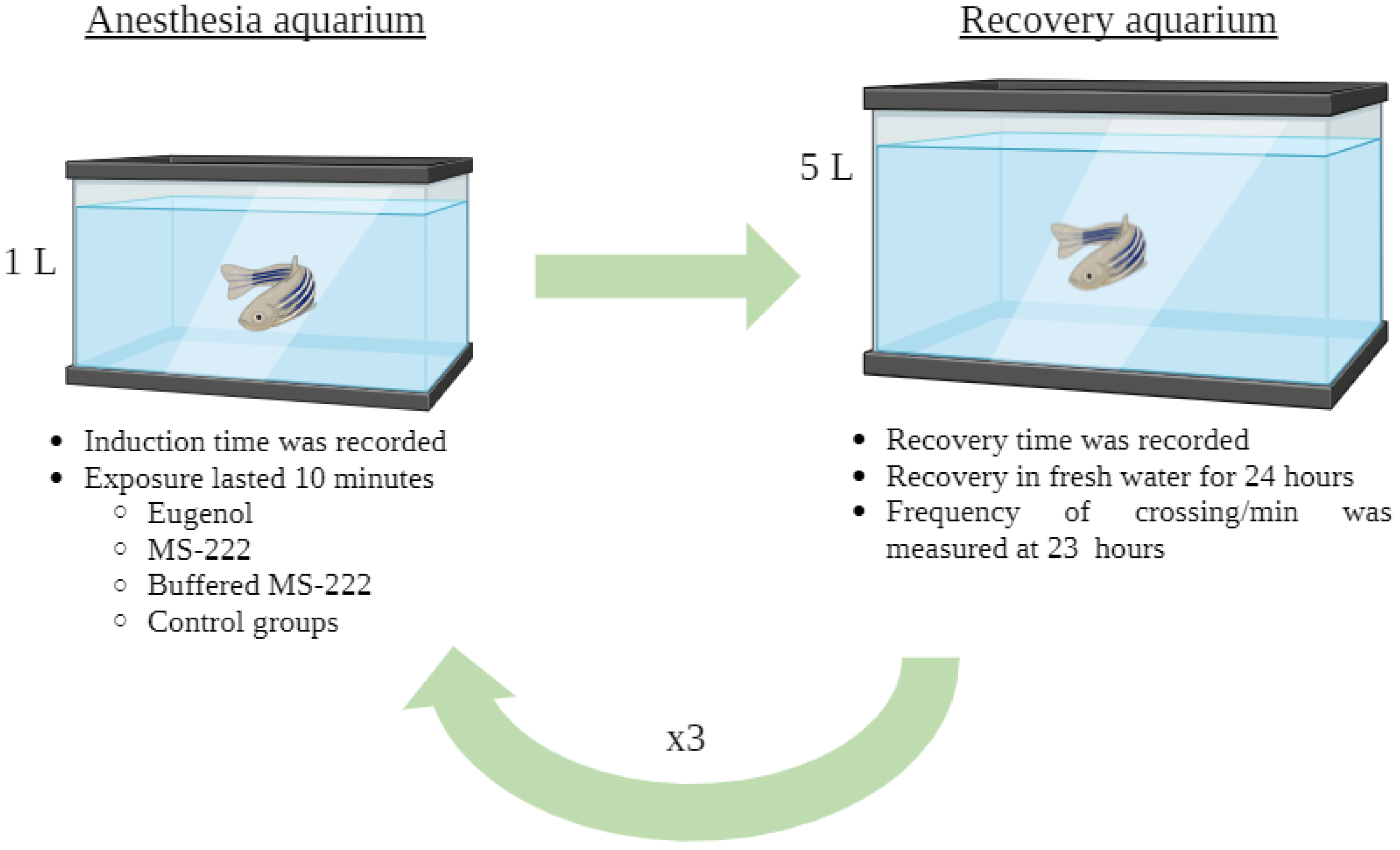
2. Materials and Methods
2.1. Animals and Husbandry
2.2. Experimental Design and Anesthetics Solution
2.3. Onset and Recovery from Light Anesthesia (Stage V)
2.4. Post-Anesthesia Behaviour Analysis
2.5. Statistical Analysis
3. Results
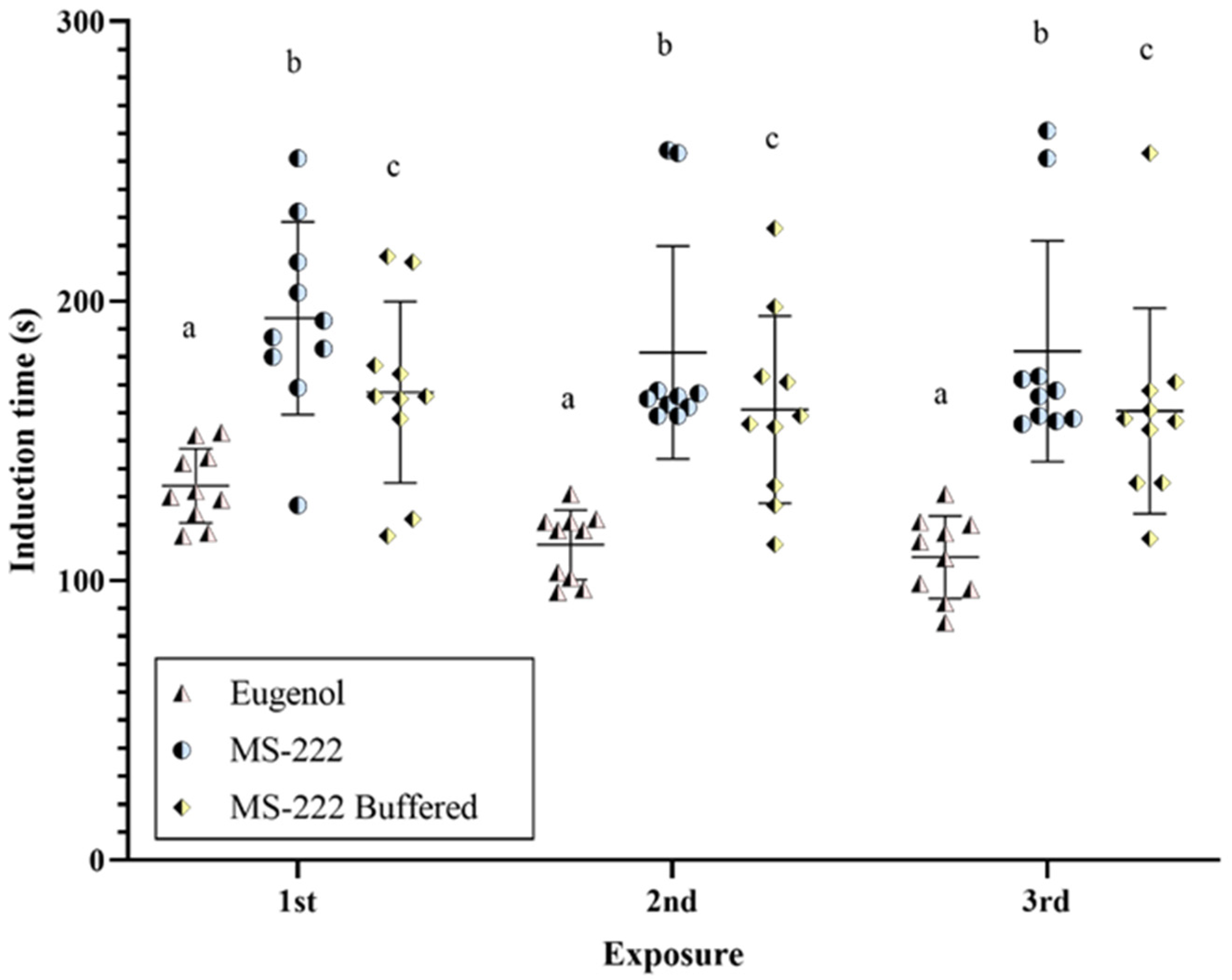
3.1. Onset of Light Anesthesia (Stage V)
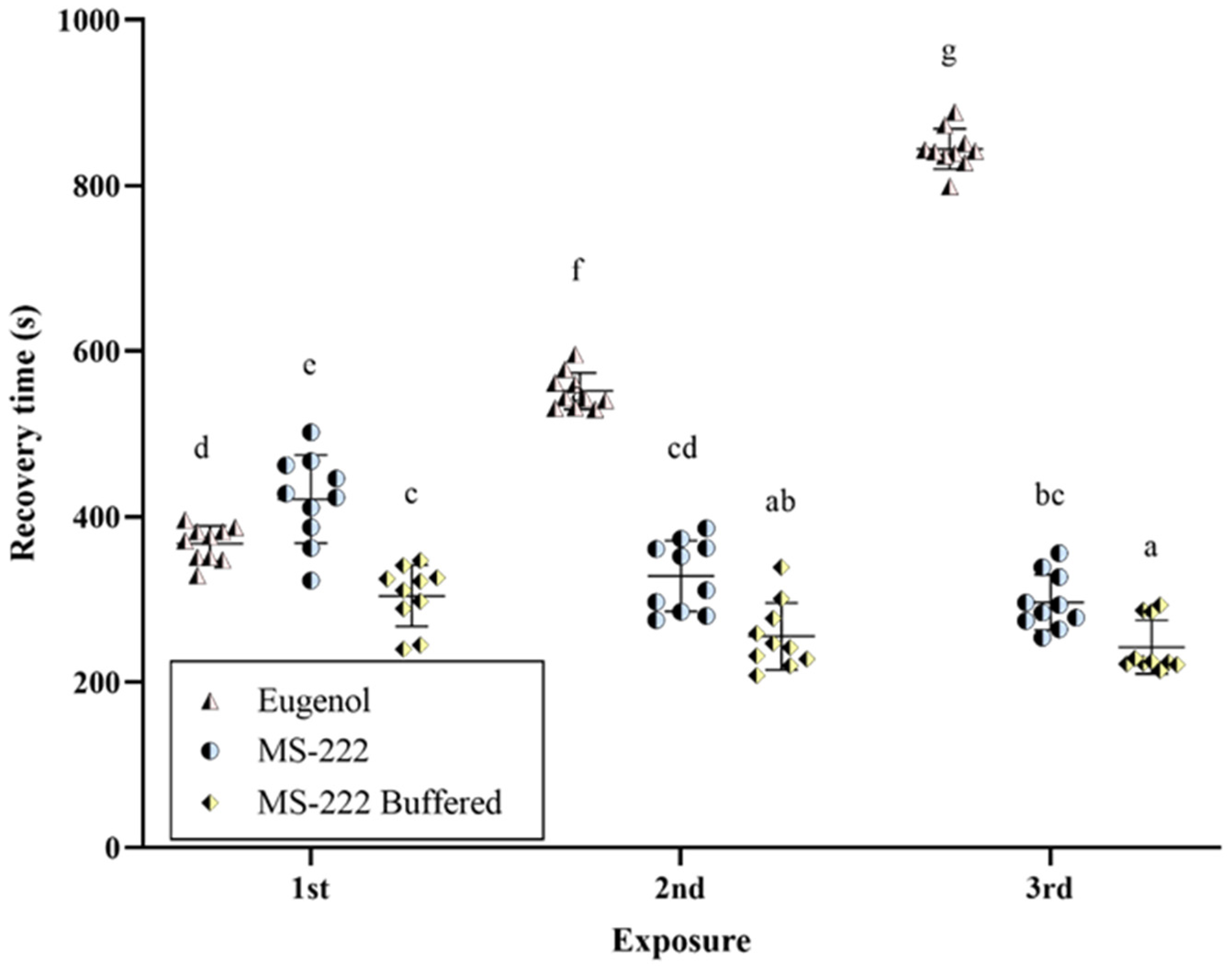
3.2. Recovery from Anesthesia
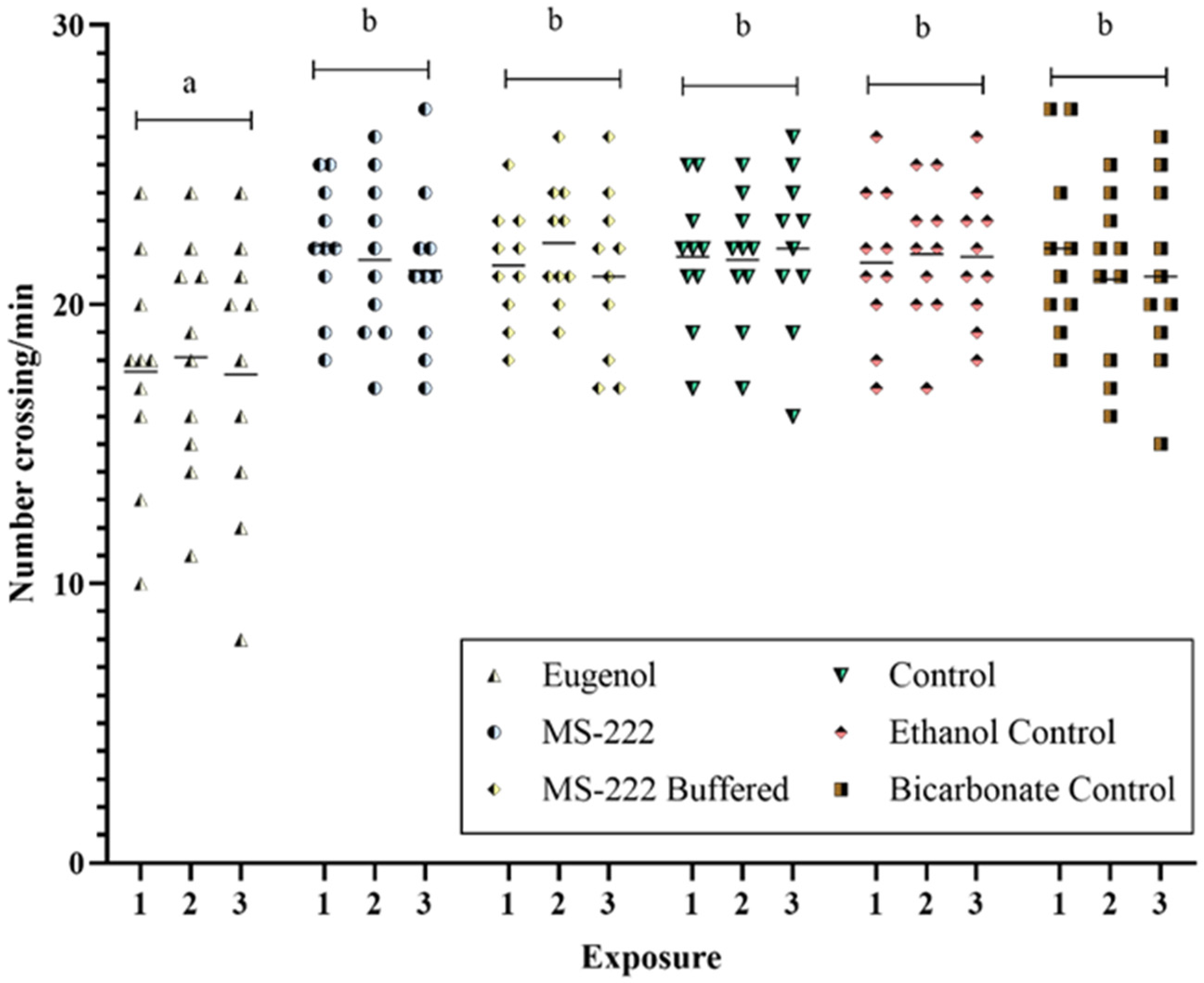
3.3. Post-Anesthesia Behaviour
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kinth, P.; Mahesh, G.; Panwar, Y. Mapping of Zebrafish Research: A Global Outlook. Zebrafish 2013, 10, 510–517. [Google Scholar] [CrossRef]
- Choi, T.-Y.; Choi, T.-I.; Lee, Y.-R.; Choe, S.-K.; Kim, C.-H. Zebrafish as an Animal Model for Biomedical Research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef]
- Sendra, M.; Pereiro, P.; Yeste, M.P.; Mercado, L.; Figueras, A.; Novoa, B. Size Matters: Zebrafish (Danio rerio) as a Model to Study Toxicity of Nanoplastics from Cells to the Whole Organism. Environ. Pollut. 2021, 268, 115769. [Google Scholar] [CrossRef]
- European Parliament. Directive 2010/63/EU of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, L276, 33–79. [Google Scholar]
- Hawkins, P.; Morton, D.B.; Burman, O.; Dennison, N.; Honess, P.; Jennings, M.; Lane, S.; Middleton, V.; Roughan, J.V.; Wells, S.; et al. A Guide to Defining and Implementing Protocols for the Welfare Assessment of Laboratory Animals: Eleventh Report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab. Anim. 2011, 45, 1–13. [Google Scholar] [CrossRef]
- Ehrlich, O.; Karamalakis, A.; Krylov, A.J.; Dudczig, S.; Hassell, K.L.; Jusuf, P.R. Clove Oil and AQUI-S Efficacy for Zebrafish Embryo, Larva, and Adult Anesthesia. Zebrafish 2019, 16, 451–459. [Google Scholar] [CrossRef]
- Matthews, M.; Varga, Z.M. Anesthesia and Euthanasia in Zebrafish. ILAR J. 2012, 53, 192–204. [Google Scholar] [CrossRef]
- Martins, T.; Diniz, E.; Félix, L.M.; Antunes, L. Evaluation of Anaesthetic Protocols for Laboratory Adult Zebrafish (Danio Rerio). PLoS ONE 2018, 13, e0197846. [Google Scholar] [CrossRef] [PubMed]
- Collymore, C. Anesthesia, Analgesia, and Euthanasia of the Laboratory Zebrafish. Zebrafish Biomed. Res. 2020, 403–413. [Google Scholar] [CrossRef]
- Sneddon, L.U. Clinical Anesthesia and Analgesia in Fish. J. Exot. Pet Med. 2012, 21, 32–43. [Google Scholar] [CrossRef]
- Martins, T.; Valentim, A.M.; Pereira, N.; Antunes, L.M. Anaesthesia and Analgesia in Laboratory Adult Zebrafish: A Question of Refinement. Lab. Anim. 2016, 50, 476–488. [Google Scholar] [CrossRef]
- Arnolds, D.E.W.; Zottoli, S.J.; Adams, C.E.; Dineen, S.M.; Fevrier, S.; Guo, Y.; Pascal, A.J. Physiological Effects of Tricaine on the Supramedullary/Dorsal Neurons of the Cunner, Tautogolabrus Adspersus. Biol. Bull. 2002, 203, 188–189. [Google Scholar] [CrossRef]
- Murray, M.J. Fish Surgery. Semin. Avian Exot. Pet Med. 2002, 11, 246–257. [Google Scholar] [CrossRef]
- Carter, K.M.; Woodley, C.M.; Brown, R.S. A Review of Tricaine Methanesulfonate for Anesthesia of Fish. Rev. Fish Biol. Fish. 2010, 21, 51–59. [Google Scholar] [CrossRef]
- Readman, G.D.; Owen, S.F.; Murrell, J.C.; Knowles, T.G. Do Fish Perceive Anaesthetics as Aversive? PLoS ONE 2013, 8, e73773. [Google Scholar] [CrossRef] [PubMed]
- Topic Popovic, N.; Strunjak-Perovic, I.; Coz-Rakovac, R.; Barisic, J.; Jadan, M.; Persin Berakovic, A.; Sauerborn Klobucar, R. Tricaine Methane-Sulfonate (MS-222) Application in Fish Anaesthesia. J. Appl. Ichthyol. 2012, 28, 553–564. [Google Scholar] [CrossRef]
- Congleton, J.L. Stability of Some Commonly Measured Blood-Chemistry Variables in Juvenile Salmonids Exposed to a Lethal Dose of the Anaesthetic MS-222. Aquac. Res. 2006, 37, 1146–1149. [Google Scholar] [CrossRef]
- Velíšek, J.; Stejskal, V.; Kouřil, J.; Svobodová, Z. Comparison of the Effects of Four Anaesthetics on Biochemical Blood Profiles of Perch. Aquac. Res. 2009, 40, 354–361. [Google Scholar] [CrossRef]
- Wong, D.; von Keyserlingk, M.A.G.; Richards, J.G.; Weary, D.M. Conditioned Place Avoidance of Zebrafish (Danio rerio) to Three Chemicals Used for Euthanasia and Anaesthesia. PLoS ONE 2014, 9, e88030. [Google Scholar] [CrossRef]
- Ferreira, J.M.; Jorge, S.; Félix, L.; Morello, G.M.; Olsson, I.A.S.; Valentim, A.M. Behavioural Aversion and Cortisol Level Assessment When Adult Zebrafish Are Exposed to Different Anaesthetics. Biology 2022, 11, 1433. [Google Scholar] [CrossRef]
- Javahery, S.; Nekoubin, H.; Moradlu, A.H. Effect of Anaesthesia with Clove Oil in Fish (Review). Fish Physiol. Biochem. 2012, 38, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Hoseini, S.M.; Vatnikov, Y.A.; Nikishov, A.A.; Kulikov, E.V. Thymol as a New Anesthetic in Common Carp (Cyprinus carpio): Efficacy and Physiological Effects in Comparison with Eugenol. Aquaculture 2018, 495, 376–383. [Google Scholar] [CrossRef]
- Corso, M.N.; Marques, L.S.; Gracia, L.F.G.; Rodrigues, R.B.; Barcellos, L.J.G.; Streit, D.P., Jr. Effects of Different Doses of Eugenol on Plasma Cortisol Levels and the Quality of Fresh and Frozen-Thawed Sperm in South American Catfish (Rhamdia quelen). Theriogenology 2019, 125, 135–139. [Google Scholar] [CrossRef]
- Barbas, L.A.L.; Torres, M.F.; da Costa, B.M.P.; Feitosa, M.J.M.; Maltez, L.C.; Amado, L.L.; Toda, Y.P.S.; dos Santos Batista, P.; Cabral, D.A.C.; Hamoy, M. Eugenol Induces Body Immobilization yet Evoking an Increased Neuronal Excitability in Fish during Short-Term Baths. Aquat. Toxicol. 2021, 231, 105734. [Google Scholar] [CrossRef]
- Davis, D.J.; Klug, J.; Hankins, M.; Holly, D.M.; Monticelli, S.R.; Song, A.; Gillespie, C.H.; Bryda, E.C. Effects of clove oil as a euthanasia agent on blood collection efficiency and serum cortisol levels in Danio rerio. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 564–567. [Google Scholar]
- Grush, J.; Noakes, D.L.G.; Moccia, R.D. The Efficacy of Clove Oil As An Anesthetic for the Zebrafish, Danio rerio (Hamilton). Zebrafish 2004, 1, 46–53. [Google Scholar] [CrossRef]
- Test No. 236: Fish Embryo Acute Toxicity (FET) Test. OECD Guidelines for the Testing of Chemicals, Section 2, 2013. Available online: https://www.oecd-ilibrary.org/environment/test-no-236-fish-embryo-acute-toxicity-fet-test_9789264203709-en;jsessionid=qUEq2vCzsqJse-xNgL-GuneAadgf3BggeF8PtSqz.ip-10-240-5-26 (accessed on 10 July 2024).
- Johnson, R.; Wolf, J.; Braunbeck, T. OECD guidance document for the diagnosis of endocrine-related histopathology of fish gonads. Organ. Econ. Co-Oper. Dev. 2009, 96, 1–96. [Google Scholar]
- Chambel, J.; Pinho, R.; Sousa, R.; Ferreira, T.; Baptista, T.; Severiano, V.; Mendes, S.; Pedrosa, R. The Efficacy of MS-222 as Anaesthetic Agent in Four Freshwater Aquarium Fish Species. Aquac. Res. 2013, 46, 1582–1589. [Google Scholar] [CrossRef]
- Sánchez-Vázquez, F.J.; Terry, M.I.; Felizardo, V.O.; Vera, L.M. Daily Rhythms of Toxicity and Effectiveness of Anesthetics (MS222 and Eugenol) in Zebrafish (Danio Rerio). Chronobiol. Int. 2011, 28, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Collymore, C.; Tolwani, A.; Lieggi, C.; Rasmussen, S. Efficacy and safety of 5 anesthetics in adult zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Science 2014, 53, 198–203. [Google Scholar]
- Ross, L.G.; Ross, B. The Nature of Anaesthesia, Sedation and Analgesia. Anaesth. Sedative Tech. Aquat. Anim. 2008, 41–51. [Google Scholar] [CrossRef]
- Valentim, A.M.; Félix, L.M.; Carvalho, L.; Diniz, E.; Antunes, L.M. A New Anaesthetic Protocol for Adult Zebrafish (Danio rerio): Propofol Combined with Lidocaine. PLoS ONE 2016, 11, e0147747. [Google Scholar] [CrossRef]
- Zang, L.; Shimada, Y.; Nishimura, Y.; Tanaka, T.; Nishimura, N. Repeated Blood Collection for Blood Tests in Adult Zebrafish. J. Vis. Exp. 2015. [Google Scholar] [CrossRef]
- Falcão, K.V.G.; de Azevedo, R.D.S.; de Lima, L.R.A.; de Souza Bezerra, R. A Rapid Protocol for Inducing Acute Pancreatitis in Zebrafish Models. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 283, 109958. [Google Scholar] [CrossRef]
- Huang, W.-C.; Hsieh, Y.-S.; Chen, I.-H.; Wang, C.-H.; Chang, H.-W.; Yang, C.-C.; Ku, T.-H.; Yeh, S.-R.; Chuang, Y.-J. Combined Use of MS-222 (Tricaine) and Isoflurane Extends Anesthesia Time and Minimizes Cardiac Rhythm Side Effects in Adult Zebrafish. Zebrafish 2010, 7, 297–304. [Google Scholar] [CrossRef]
- Bowker, J.D.; Trushenski, J.T.; Gaikowski, M.P.; Straus, D.L. (Eds.) Guide to Using Drugs, Biologics, and Other Chemicals in Aquaculture; American Fisheries Society Fish Culture Section: Bethesda, MD, USA, 2019. [Google Scholar]
- Food and Drug Administration. Guidance for Industry: Concerns Related to the Use of Clove Oil as an Anesthetic for Fish. Rockville 2007. Available online: https://www.fda.gov/media/69954/download (accessed on 10 July 2024).
- European Commission. Commission Implementing Regulation (EU) 2022/1452 of 1 September 2022 concerning the authorisation of 3-ethylcyclopentan-1,2-dione, 4-hydroxy-2,5-dimethylfuran-3(2H)- one, 4,5-dihydro-2-methylfuran-3(2H)-one, eugenol, 1-methoxy-4-(prop-1(trans)-enyl)benzene, α-pentylcinnamaldehyde, α-hexylcinnamaldehyde and 2-acetylpyridine as feed additives for certain animal species. Off. J. Eur. Union 2022, L228, 17–29. [Google Scholar]
- Sladky, K.K.; Swanson, C.R.; Stoskopf, M.K.; Loomis, M.R.; Lewbart, G.A. Comparative Efficacy of Tricaine Methanesulfonate and Clove Oil for Use as Anesthetics in Red Pacu (Piaractus brachypomus). Am. J. Vet. Res. 2001, 62, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Neiffer, D.L.; Stamper, M.A. Fish Sedation, Anesthesia, Analgesia, and Euthanasia: Considerations, Methods, and Types of Drugs. ILAR J. 2009, 50, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Valentim, A.; Pereira, N.; Antunes, L.M. Anaesthetics and Analgesics Used in Adult Fish for Research: A Review. Lab. Anim. 2018, 53, 325–341. [Google Scholar] [CrossRef]
- Guénette, S.A.; Uhland, F.C.; Hélie, P.; Beaudry, F.; Vachon, P. Pharmacokinetics of Eugenol in Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2007, 266, 262–265. [Google Scholar] [CrossRef]
- Gerlai, R.; Lee, V.; Blaser, R. Effects of Acute and Chronic Ethanol Exposure on the Behavior of Adult Zebrafish (Danio rerio). Pharmacol. Biochem. Behav. 2006, 85, 752–761. [Google Scholar] [CrossRef]
- Agues-Barbosa, T.; da Silva Junior, F.C.; Gomes-de-Lima, J.N.; Batistuzzo de Medeiros, S.R.; Luchiari, A.C. Behavioral Genetics of Alcohol’s Effects in Three Zebrafish (Danio rerio) Populations. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 114, 110495. [Google Scholar] [CrossRef] [PubMed]
- Chance, R.J.; Cameron, G.A.; Fordyce, M.; Noguera, P.; Wang, T.; Collins, C.; Secombes, C.J.; Collet, B. Effects of Repeated Anaesthesia on Gill and General Health of Atlantic Salmon, (Salmo salar). J. Fish Biol. 2018, 93, 1069–1081. [Google Scholar] [CrossRef]
- Mitjana, O.; Bonastre, C.; Insua, D.; Falceto, M.V.; Esteban, J.; Josa, A.; Espinosa, E. The Efficacy and Effect of Repeated Exposure to 2-Phenoxyethanol, Clove Oil and Tricaine Methanesulphonate as Anesthetic Agents on Juvenile Angelfish (Pterophyllum scalare). Aquaculture 2014, 433, 491–495. [Google Scholar] [CrossRef]
- Smith, D.A.; Smith, S.A.; Holladay, S.D. Effect of Previous Exposure to Tricaine Methanesulfonate on Time to Anesthesia in Hybrid Tilapias. J. Aquat. Anim. Health 1999, 11, 183–186. [Google Scholar] [CrossRef]
- Weber, R.A.; Pérez-Maceira, J.J.; Peleteiro, J.B.; García-Martín, L.; Aldegunde, M. Effects of Acute Exposure to 2-Phenoxyethanol, Clove Oil, MS-222, and Metomidate on Primary and Secondary Stress Responses in Senegalese Sole (Solea senegalensis Kaup 1858). Aquaculture 2011, 321, 108–112. [Google Scholar] [CrossRef]
- Stockman, J.; Weber, E.S.P.; Kass, P.H.; Pascoe, P.J.; Paul-Murphy, J. Physiologic and Biochemical Measurements and Response to Noxious Stimulation at Various Concentrations of MS-222 in Koi (Cyprinus carpio). Vet. Anaesth. Analg. 2013, 40, 35–47. [Google Scholar] [CrossRef]
- Stenger, V.G.; Maren, T.H. The Pharmacology of MS 222 (Ethyl-m-Aminobenzoate) in Squalus acanthias. Comp. Gen. Pharmacol. 1974, 5, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Gressler, L.T.; Parodi, T.V.; Riffel, A.P.K.; DaCosta, S.T.; Baldisserotto, B. Immersion Anaesthesia with Tricaine Methanesulphonate or Propofol on Different Sizes and Strains of Silver Catfish Rhamdia Quelen. J. Fish Biol. 2012, 81, 1436–1445. [Google Scholar] [CrossRef]
- Tsang, B.; Zahid, H.; Ansari, R.; Lee, R.C.-Y.; Partap, A.; Gerlai, R. Breeding Zebrafish: A Review of Different Methods and a Discussion on Standardization. Zebrafish 2017, 14, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, C.-Q.; Fan, Y.-Q.; Xie, Y.-S.; Yin, Z.-F.; Xu, Z.-J.; Zhang, H.-L.; Cao, J.-T.; Han, Z.-H.; Wang, Y.; et al. The Evaluation of Rapid Cooling As an Anesthetic Method for the Zebrafish. Zebrafish 2014, 11, 71–75. [Google Scholar] [CrossRef]
- Xue, Y.-J.; Chang, C.-C.; Lai, J.-M.; Wang, J.H. Determining the Tranquilization Dose and Residue of Tricaine Methanesulfonate (MS-222) in Sea Bass Lates Calcarifer Tissue. Fish. Sci. 2017, 83, 625–633. [Google Scholar] [CrossRef]
- Kiessling, A.; Johansson, D.; Zahl, I.H.; Samuelsen, O.B. Pharmacokinetics, Plasma Cortisol and Effectiveness of Benzocaine, MS-222 and Isoeugenol Measured in Individual Dorsal Aorta-Cannulated Atlantic Salmon (Salmo salar) Following Bath Administration. Aquaculture 2009, 286, 301–308. [Google Scholar] [CrossRef]
- Attili, S.; Hughes, S.M. Anaesthetic Tricaine Acts Preferentially on Neural Voltage-Gated Sodium Channels and Fails to Block Directly Evoked Muscle Contraction. PLoS ONE 2014, 9, e103751. [Google Scholar] [CrossRef] [PubMed]
- Ramlochansingh, C.; Branoner, F.; Chagnaud, B.P.; Straka, H. Efficacy of Tricaine Methanesulfonate (MS-222) as an Anesthetic Agent for Blocking Sensory-Motor Responses in Xenopus Laevis Tadpoles. PLoS ONE 2014, 9, e101606. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-M.; Lee, K.; Im, S.-T.; Go, E.J.; Kim, Y.H.; Park, C.-K. Co-Application of Eugenol and QX-314 Elicits the Prolonged Blockade of Voltage-Gated Sodium Channels in Nociceptive Trigeminal Ganglion Neurons. Biomolecules 2020, 10, 1513. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala-Soldado, N.; Mora-Medina, R.; Molina-López, A.M.; Lora-Benítez, A.J.; Moyano-Salvago, R. Evaluation of the Effectiveness of Eugenol and MS-222 as Anesthetics in Zebrafish in Repeated Exposures and Post-Anesthesia Behaviour. Animals 2024, 14, 2418. https://doi.org/10.3390/ani14162418
Ayala-Soldado N, Mora-Medina R, Molina-López AM, Lora-Benítez AJ, Moyano-Salvago R. Evaluation of the Effectiveness of Eugenol and MS-222 as Anesthetics in Zebrafish in Repeated Exposures and Post-Anesthesia Behaviour. Animals. 2024; 14(16):2418. https://doi.org/10.3390/ani14162418
Chicago/Turabian StyleAyala-Soldado, Nahúm, Rafael Mora-Medina, Ana María Molina-López, Antonio Jesús Lora-Benítez, and Rosario Moyano-Salvago. 2024. "Evaluation of the Effectiveness of Eugenol and MS-222 as Anesthetics in Zebrafish in Repeated Exposures and Post-Anesthesia Behaviour" Animals 14, no. 16: 2418. https://doi.org/10.3390/ani14162418




