Recent Developments in the Application of Filamentous Fungus Aspergillus oryzae in Ruminant Feed
Abstract
:Simple Summary
Abstract
1. Introduction
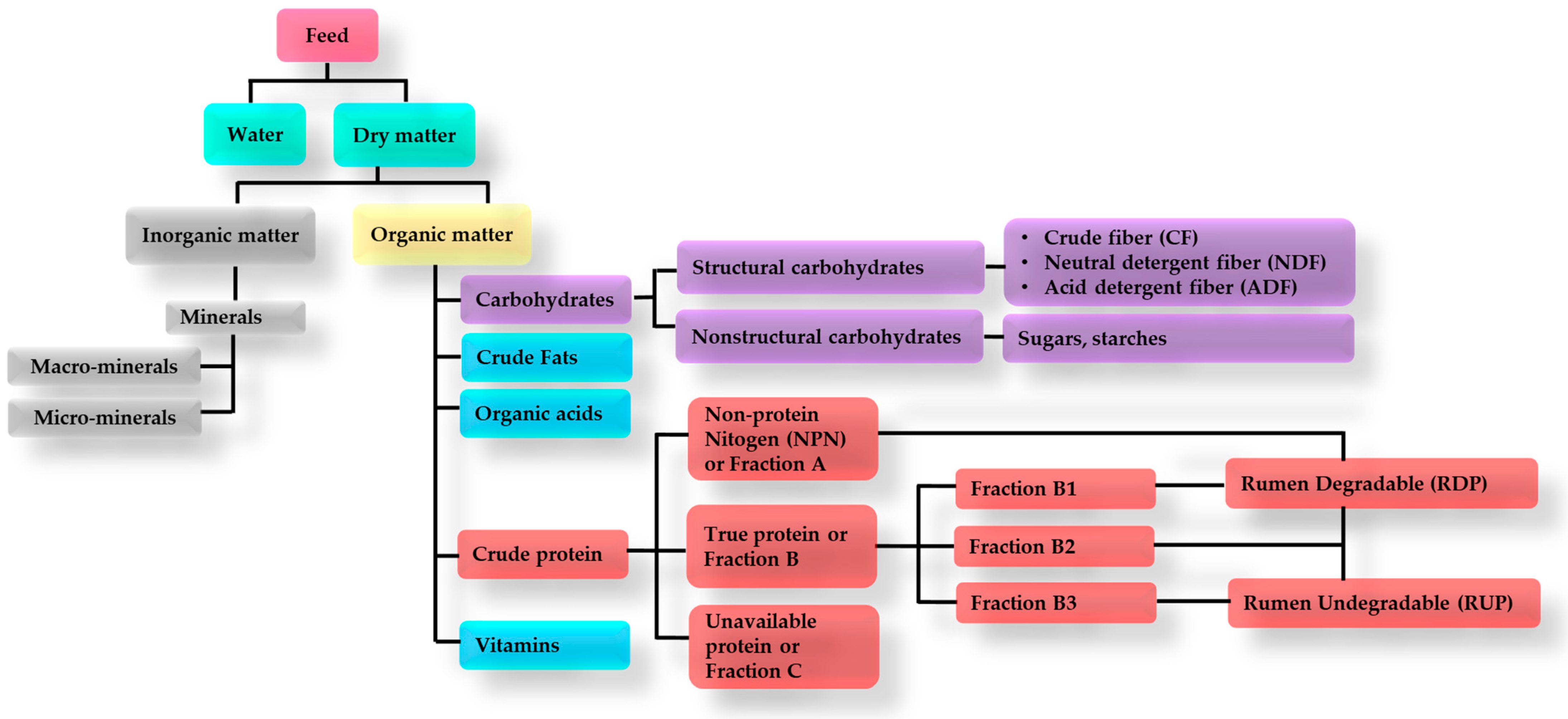
2. Ruminant Feed Ingredients, Supplements, and Their Efficiency
2.1. Feed Ingredients and Supplements
2.2. Feed Efficiency
2.3. Protein Supplementation in Ruminant Feed
2.4. Microbial Supplementation in Ruminant Feed
3. Filamentous Fungi Cultivation, Composition, and Characteristics
3.1. Filamentous Fungi Cultivation
3.2. Aspergillus Oryzae Cultivation Potentials
3.2.1. Ethanol Industry Residues for A. oryzae Cultivation
3.2.2. Pulp and Paper Industry Residues for A. oryzae Cultivation
3.2.3. Food Industry Residues for A. oryzae Cultivation
3.3. Nutritional Profile of Filamentous Fungi
| Glucose | Vinasse | BYW | SSL60 | SNL50 | SSL60 | POPE | SPW | SBM | RSM | FM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AO | NI | RO | AO | NI | RO | AO | AO | AO | RO | AA | AO | RO | ||||
| Crude protein | 45.7 | 62.2 | 50.6 | 44.7 | 57.6 | 50.9 | 43.8 | 44.4 | 46.3 | 44.9 | 39.6 | 45.7 | 49.7 | 43.3 | 33.7 | 31.8 |
| Essential amino acids % CP | ||||||||||||||||
| Arginine | 5.5 | 5.4 | 3.6 | 4.5 | 3.6 | 3.7 | 4.7 | 5.2 | 5.2 | 4.3 | 10.4 | 7.4 | 6.0 | 6.1 | ||
| Histidine | 1.9 | 2.12 | 1.8 | 1.7 | 1.5 | 1.5 | 1.5 | 7.5 | 6.3 | 8.0 | 3.6 | 2.7 | 2.6 | 2.5 | ||
| Isoleucine | 3.2 | 3.46 | 3.2 | 3.1 | 2.8 | 3.1 | 2.6 | 5.7 | 5.6 | 4.3 | 6.3 | 5.4 | 6.4 | 4.6 | 4.0 | 3.9 |
| Leucine | 5.3 | 5.95 | 5.0 | 5.5 | 4.7 | 4.8 | 5.7 | 8.7 | 8.3 | 8.1 | 8 | 8.8 | 7.8 | 7.4 | 6.7 | 7.1 |
| Lysine | 5.9 | 6.5 | 5.0 | 4.8 | 4.2 | 4.5 | 5.2 | 2.8 | 2.6 | 2.9 | 7.2 | 15.5 | 17.8 | 6.1 | 5.3 | 7.4 |
| Methionine | 1.2 | 1.5 | 1.3 | 1.3 | 1.1 | 1.2 | 1.0 | 1.5 | 1.9 | 2.9 | 1.5 | 2.4 | 3.1 | 1.4 | 2.0 | 2.6 |
| Phenylalanine | 3.2 | 3.3 | 3.1 | 3.2 | 2.7 | 2.9 | 3.6 | 3.8 | 3.5 | 3.7 | 5.2 | 11.1 | 9.4 | 5.0 | 3.9 | 3.9 |
| Threonine | 3.7 | 3.9 | 3.2 | 3.8 | 3.1 | 3.2 | 2.9 | 12. | 12.4 | 11.2 | 4.8 | 4.6 | 5.7 | 4.1 | 4.3 | 4.1 |
| Valine | 4.1 | 4.4 | 3.6 | 3.8 | 3.5 | 3.6 | 4.0 | 7.9 | 7.8 | 7.7 | 7.1 | 4.6 | 6.2 | 4.8 | 5.0 | 4.8 |
| Sum of AAs | 34.2 | 36.5 | 29.9 | 31.6 | 27.3 | 28.4 | 31.5 | 55.2 | 53.6 | 53.2 | 54.1 | 52.4 | 56.5 | 43.5 | 40.0 | 42.6 |
| Fatty acid content g/Kg DM | ||||||||||||||||
| Lipid % | 6.9 | 4.0 | 17.2 | 7.0 | 5.5 | 3.5 | 11.4 | 7.4 | 5.7 | 1.9 | 1.1 | 9.8 | ||||
| Myristic acid C14:0 | 0.3 | 0.2 | 1.1 | 0.3 | 0.3 | 0.5 | 0.2 | 0.2 | 0.6 | 1.2 | 0.0 | 0.0 | 0.0 | |||
| Palmitic acid C16:0 | 13.8 | 5.0 | 34.9 | 13.9 | 6.1 | 12.5 | 18.1 | 14.8 | 12.5 | 54.4 | 1.3 | 0.8 | 1.4 | |||
| Palmitoleic acid C16:1 | 1.1 | 1.1 | 4.8 | 2.2 | 1.4 | 3.5 | 1.0 | 0.4 | 0.4 | 3.3 | 0.0 | 0.1 | 0.0 | |||
| Stearic acid C18:0 | 4.1 | 1.2 | 8.8 | 5.4 | 1.8 | 3.4 | 6.0 | 3.7 | 4.6 | 3.7 | 0.5 | 0.3 | 0.8 | |||
| Oleic acid C18:1 | 23.7 | 20.9 | 43.7 | 23.7 | 14.1 | 15.3 | 24.1 | 16.5 | 22.3 | 30.0 | 2.8 | 10.7 | 4.2 | |||
| Linoleic acid C18:2 | 24.8 | 10.3 | 65.2 | 22.6 | 10.4 | 18.8 | 50.6 | 31.3 | 3.8 | 6.7 | 6.8 | 3.8 | 3.3 | |||
| Linolenic acid C18:3 | 0.2 | 0.8 | 0.8 | 0.2 | 0.3 | 0.3 | 0.4 | 0.3 | 7.2 | 0.9 | 1.8 | 12.3 | ||||
| Macronutrient content g/kg DM | ||||||||||||||||
| Calcium | 1.0 | 1.7 | 3.0 | 23.8 | 58.5 | 26.4 | 56.3 | 2.9 | 3.5 | 1.8 | 0.2 | 0.2 | 3.4 | 8.3 | 4.5 | |
| Potassium | 11.3 | 9.3 | 1.3 | 12.0 | 15.8 | 8.9 | 9.3 | 11.2 | 12.8 | 7.4 | 1.4 | 0.5 | 21.2 | 12.3 | 10.3 | |
| Phosphorus | 12.4 | 17.5 | 21.2 | 9.1 | 8.9 | 16.4 | 27.5 | 17.2 | 16.7 | 20.5 | 1.6 | 2.0 | 6.2 | 11.4 | 8.0 | |
| Magnesium | 0.6 | 1.1 | 0.4 | 0.9 | 0.8 | 1.2 | 3.2 | 3.6 | 3.9 | 2.6 | 2.9 | 4.9 | 4.3 | |||
| Sodium | 0.3 | 0.3 | 0.3 | 1.1 | 1.3 | 1.6 | 3.4 | 4.5 | 4.8 | 3.2 | 0.4 | 0.2 | 0.0 | 0.4 | 1.0 | |
| Sulfur | 4.6 | 4.7 | 3.1 | 4.7 | 4.8 | 3.9 | 4.6 | 5.9 | 3.6 | |||||||
4. Protein Digestion in Ruminants
5. Single-Cell Protein in Ruminant Feed
5.1. The Effect of Aspergillus Oryzae Supplementation on Ruminal Digestion
5.1.1. Effect on Dry Matter Digestibility
5.1.2. Effect on Rumen Microbiota
5.2. Effect of Aspergillus Oryzae Supplementation on Ruminant Products
| Figure | Concentration | Form of Addition | Subject Animals | Variables | Findings | Ref. |
|---|---|---|---|---|---|---|
| AO | 3 g/d | Added with 5.6% tallow | 28 Holstein cows | Milk production, dry matter intake | Did not stimulate any variables. | [153] |
| AO extract, Amaferm® | 1 g/d | Supplemented into 85% concentrate diet | 48 lambs | Growth and carcass characteristics | ADG increased. | [232] |
| SC with AO | 0.5 g/d SC plus 3 g/d AO | Whole-shelled corn or high-moisture corn | 48 lambs | Growth and carcass characteristics | BW increased. | [232] |
| SC with AO | 10 g/d SC plus 3 g/d AO | Supplemented into diet (60% rolled barley and 40% timothy hay) | 40 dairy cows | DMI and milk yield and composition | Higher daily milk production, better weight gain. | [25] |
| AO | 3 g/d | Inclusion in silage and TMR | 7 dairy cows | Milk yield and composition | Increased yield, 4% FCM. | [217] |
| AO and/or SC | 3.5 g/d | Supplemented into the basal diet | 80 multiparous lactating cows | Lactation performance | Increased feed intake and daily milk production. | [26] |
| AO | 113 g/day | Supplemented into the basal diet with 35% whole cottonseed | 108 Holstein cows | Milk production | Decreased milk production and feed intake. | [174] |
| AO culture | 3 g/d | Supplemented into steam-flaked and steam-rolled corn | 32 multiparous Holstein cows | Milk yield and compositions | Increased protein and SNF percentage. | [218] |
| AO extract | 3 g/d | Supplemented into the TMR plus 136 g of rice mill by-product | 110 multiparous lactating cows | Rumen metabolites and milk production | Increased SNF percentage, lower blood urea N concentrations. | [216] |
| AO extract | 5 g/day | Supplemented into the TMR | 282 multiparous Holstein cows | Milk production and composition | Lesser concentration and yield of milk true protein compared to control group. | [227] |
| AO extract with yeast culture | 56 g/d (yeast culture) plus 3 g/d (A. oryzae) | Supplemented into the TMR | 521 Pluriparous Holstein cows | Milk yield and composition | Lower percentages of lactose and SNF. | [233] |
| AO extract containing α-amylase activity | 12 g/d | Supplemented into the TMR | 150 lactating cows from 45 commercial dairy herds | Milk production and composition | Slight milk yield increase, lower milk fat percentage. | [234] |
| AO fermentation extract | 15 g/d | Supplemented into the TMR (alfalfa hay and steam-flaked corn) | 33 Holstein cows (22 multiparous and 11 primiparous) | Productive variables in transition dairy cows | Increased milk production, decreased plasma non-esterified fatty acids. | [221] |
| AO fermentation extract | 15 g/d | Supplemented into the TMR (corn silage and rolled corn) | 2455 multiparous Holstein cows | Milk yield and compositions | Decreased milk yield, increased milk fat content. | [221] |
| AO fermentation extract | 3 g/d | Supplemented into the TMR | 210 early-lactation Holstein cows | Milk yield and compositions | Increased milk production, increased FCM 3.5%. | [171] |
| AO fermented culture | 10% (w/w) levels | Added to a commercial concentrate (GT-03) | 15 Garut sheep | Dry matter intake | Increased protein intake and organic matter. | [235] |
| AO fermentation extract | 1.5 g/d | Supplemented into the TMR | 64 lactating cows | Digestibility of CP, NDF, and DM | Increased DMI. | [162] |
| AO fermentation extract non-ionic surfactant | 100 g/d (prepartum) 150 g/d (postpartum) | Supplemented into the TMR | 40 Holstein dairy cows | Dry matter intake (DMI) and milk production | Greater DMI in the transitional period, increased milk fat content. | [164] |
| AO fermentation extract | 2 g/d | Top-dressed on texturized starter ration | 52 bull calves | Growth rate at weaning age and rumen development | Tended to increase ADG. | [165] |
| AO culture | 3 g/d | Supplemented into the diet (70% concentrate and 30% forage) | 2 dry and 2 lactating cows | Nutrient utilization | Increased rumen and total tract digestibility of fiber fractions. | [77] |
| AO fermentation extract | 3 g/d | Top-dressing at the morning feeding plus 87 g of ground sorghum | 46 lactating cows (20 primiparous and 26 multiparous) | Milk production and composition | Increased milk production. | [166] |
6. Perspectives for Circular Bioeconomy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. The Future of Food and Agriculture: Trends and Challenges; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Keating, B.A.; Herrero, M.; Carberry, P.S.; Gardner, J.; Cole, M.B. Food wedges: Framing the global food demand and supply challenge towards 2050. Glob. Food Secur. 2014, 3, 125–132. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.C.; Smith, R.G.; Schipanski, M.E.; Atwood, L.W.; Mortensen, D.A. Agriculture in 2050: Recalibrating Targets for Sustainable Intensification. BioScience 2017, 67, 386–391. [Google Scholar] [CrossRef]
- Giller, K.E.; Delaune, T.; Silva, J.V.; Descheemaeker, K.; van de Ven, G.; Schut, A.G.; van Ittersum, M.K. The future of farming: Who will produce our food? Food Secur. 2021, 13, 1073–1099. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2012. [Google Scholar]
- Food and Agriculture Organization of the United Nations. The State of Food and Agriculture 2009; United Nations: New York, NY, USA, 2009. [Google Scholar]
- van Hal, O.; Weijenberg, A.; de Boer, I.; van Zanten, H. Accounting for feed-food competition in environmental impact assessment: Towards a resource efficient food-system. J. Clean. Prod. 2019, 240, 118241. [Google Scholar] [CrossRef]
- Voora, V.; Larrea, C.; Bermudez, S. Global Market Report: Soybeans; JSTOR: Winnipeg, MB, Canada, 2020. [Google Scholar]
- Rezende, V.T.; Ali, S.; Bonaudo, T.; Gameiro, A.H. Brazilian soybeans as feed for livestock in Europe: An insight into the nitrogen flows. Reg. Environ. Chang. 2023, 23, 33. [Google Scholar] [CrossRef]
- Oecd, Food, and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook 2024–2033; OECD-FAO: Rome, Italy, 2024. [Google Scholar]
- Lima, M.; Junior, C.A.d.S.; Rausch, L.; Gibbs, H.K.; Johann, J.A. Demystifying sustainable soy in Brazil. Land Use Policy 2019, 82, 349–352. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Transforming the Livestock Sector through the Sustainable Development Goals; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Avelar, Z.; Rodrigues, R.M.; Pereira, R.N.; Vicente, A.A. Future food proteins—Trends and perspectives. In Future Foods; Academic Press: Cambridge, MA, USA, 2022; pp. 267–285. [Google Scholar]
- Schneider, F.; Tarawali, S. Sustainable Development Goals and livestock systems. Rev. Sci. Tech. 2021, 40, 585–595. [Google Scholar] [CrossRef]
- Bratosin, B.C.; Darjan, S.; Vodnar, D.C. Single cell protein: A potential substitute in human and animal nutrition. Sustainability 2021, 13, 9284. [Google Scholar] [CrossRef]
- Nasseri, A.T.; Rasoul-Amini, S.; Morowvat, M.H.; Ghasemi, Y. Single cell protein: Production and process. Am. J. Food Technol. 2011, 6, 103–116. [Google Scholar] [CrossRef]
- Sharif, M.; Zafar, M.H.; Aqib, A.I.; Saeed, M.; Farag, M.R.; Alagawany, M. Single cell protein: Sources, mechanism of production, nutritional value and its uses in aquaculture nutrition. Aquaculture 2021, 531, 735885. [Google Scholar] [CrossRef]
- Bentley, R. From miso, sake and shoyu to cosmetics: A century of science for kojic acid. Nat. Prod. Rep. 2006, 23, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Spalvins, K.; Ivanovs, K.; Blumberga, D. Single cell protein production from waste biomass: Review of various agricultural by-products. Agron. Res. 2018, 147, 409–418. [Google Scholar]
- Kong, F.; Lu, N.; Liu, Y.; Zhang, S.; Jiang, H.; Wang, H.; Li, S. Aspergillus oryzae and Aspergillus niger co-cultivation extract affects in vitro degradation, fermentation characteristics, and bacterial composition in a diet-specific manner. Animals 2021, 11, 1248. [Google Scholar] [CrossRef] [PubMed]
- Podversich, F.; Tarnonsky, F.; Bollatti, J.; da Silva, G.M.; Schulmeister, T.M.; Martinez, J.V.; Heredia, D.C.; Ipharraguerre, I.R.; Dubeux, J.C.B.; Ferrareto, L.; et al. 327 Effects of Inclusion of Amaferm on Animal Performance, Chewing Activity, and Nutrient Digestibility of Backgrounding Beef Heifers Fed Either a Sorghum Silage- or a Byproducts-based Diet. J. Anim. Sci. 2021, 99 (Suppl. S3), 181–182. [Google Scholar] [CrossRef]
- Beharka, A.A.; Nagaraja, T.J. Effect of Aspergillus oryzae extract alone or in combination with antimicrobial compounds on ruminal bacteria. J. Dairy Sci. 1998, 81, 1591–1598. [Google Scholar] [CrossRef]
- Chiquette, J. Saccharomyces cerevisiae and Aspergillus oryzae, used alone or in combination, as a feed supplement for beef and dairy cattle. Can. J. Anim. Sci. 1995, 75, 405–415. [Google Scholar] [CrossRef]
- Sallam, S.M.; Abdelmalek, M.L.; Kholif, A.E.; Zahran, S.M.; Ahmed, M.H.; Zeweil, H.S.; Olafadehan, O.A. The effect of Saccharomyces cerevisiae live cells and Aspergillus oryzae fermentation extract on the lactational performance of dairy cows. Anim. Biotechnol. 2020, 31, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, E.; Lawrence, R. Microbial fermentation for reduction of antinutritional factors. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 239–260. [Google Scholar]
- Lukuyu, B.A.; Gachuiri, C.K.; Lukuyu, M.N.; Lusweti, C.; Mwendia, S.W. Feeding Dairy Cattle in East Africa; East Africa Dairy Development Project: Kampala, Uganda, 2012. [Google Scholar]
- Harris, L.E. International Feed Descriptions, International Feed Names, and Country Feed Names; International Network of Feed Information Centers: Logan, UT, USA, 1980. [Google Scholar]
- Gerritse, R.G. Movement of Nutrients from Onsite Wastewater Systems in Soils; Citeseer: Princeton, NJ, USA, 2002. [Google Scholar]
- Annison, E.F.; Bryden, W.L. Perspectives on ruminant nutrition and metabolism I. Metab. Rumen. 1998, 11, 173–198. [Google Scholar]
- National Research Council. Nutrient Requirements of Beef Cattle, Seventh Rev; The National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Committee on Animal Nutrition Board on Agriculture; Natural Resources National Research Council. Nutrient Requirements of Dairy Cattle, Seventh Revised Addition 2001; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Palmquist, D.L. The role of dietary fats in efficiency of ruminants. J. Nutr. 1994, 124 (Suppl. S8), 1377S–1382S. [Google Scholar] [PubMed]
- Muller, L.; Fales, S. Supplementation of cool-season grass pastures for dairy cattle. In Grass for Dairy Cattle; CABI Publishing: Oxfordshire, UK, 1998; pp. 335–350. [Google Scholar]
- Rearte, D.H.; Pieroni, G.A. Supplementation of Temperate Pastures; International Grassland Congress: Leipzig, Germany, 2021. [Google Scholar]
- Freer, M. Nutrient Requirements of Domesticated Ruminants; CSIRO Publishing: Clayton South, Australia, 2007. [Google Scholar]
- Bargo, F.; Muller, L.; Kolver, E.; Delahoy, J. Invited review: Production and digestion of supplemented dairy cows on pasture. J. Dairy Sci. 2003, 86, 1–42. [Google Scholar] [CrossRef]
- Schroeder, G.; Delahoy, J.; Vidaurreta, I.; Bargo, F.; Gagliostro, G.; Muller, L. Milk fatty acid composition of cows fed a total mixed ration or pasture plus concentrates replacing corn with fat. J. Dairy Sci. 2003, 86, 3237–3248. [Google Scholar] [CrossRef] [PubMed]
- Zinn, R.A. and A.P. Jorquera, Feed value of supplemental fats used in feedlot cattle diets. Eterinary Clin. N. Am. Food Anim. Pract. 2007, 23, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Stanko, R. Dietary fats as reproductive nutraceuticals in beef cattle. J. Anim. Sci. 2000, 77, 1–12. [Google Scholar] [CrossRef]
- Chiba, L.I.; Peo, E.R.; Lewis, A.J.; Brumm, M.C.; Fritschen, R.D.; Crenshaw, J.D. Effect of dietary fat on pig performance and dust levels in modified-open-front and environmentally regulated confinement buildings. J. Anim. Sci. 1985, 61, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, S.; Palanisamy, V.; Balasubramaniam, M.; Palanisamy, S.; Jaganathan, M.; Kannan, T.A. Effect of nonstructural carbohydrates on production performance, rumen metabolism and rumen health in lambs fed with isocaloric and isonitrogenous complete diets. Trop. Anim. Health Prod. 2024, 56, 181. [Google Scholar] [CrossRef]
- Sniffen, C.J.; O’Connor, J.D.; Van Soest, P.J.; Fox, D.G.; Russell, J.B. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. J. Anim. Sci. 1992, 70, 3562–3577. [Google Scholar] [CrossRef]
- Felini, R.; Cavallini, D.; Buonaiuto, G.; Bordin, T. Assessing the impact of thermoregulatory mineral supplementation on thermal comfort in lactating Holstein cows. Vet. Anim. Sci. 2024, 24, 100363. [Google Scholar] [CrossRef] [PubMed]
- Hinton, D.G. Supplementary Feeding of Sheep and Beef Cattle; Landlinks Press: Collingwood, Australia, 2007. [Google Scholar]
- Engels, E. Voedingsnavorsing met die weidende dier. S. Afr. J. Anim. Sci. 1983, 13, 292–298. [Google Scholar]
- Nel, J.; Van Niekerk, B. The value of protein and energy-rich supplements in the maintenance of Merino sheep grazing sour grassveld. Proc. S. Afr. J. Anim. Sci. 1970, 9, 155. [Google Scholar]
- Chesson, A. Impact of Biotechnology on Livestock Feeds and Feeding; Mededelingen van de Faculteit Landbouwwetenschappen Rijksuniversiteit Gent: Gent, Belgium, 1992. [Google Scholar]
- Beauchemin, K.A. Invited review: Current perspectives on eating and rumination activity in dairy cows. J. Dairy Sci. 2018, 101, 4762–4784. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, A.; Heinrichs, B.; Cavallini, D.; Fustini, M.; Formigoni, A. Limiting total mixed ration availability alters eating and rumination patterns of lactating dairy cows. JDS Commun. 2021, 2, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.G. Protected proteins and amino acids for ruminants. In Biotechnology in Animal Feeds and Animal Feeding; Wiley online Library: Hoboken, NJ, USA, 1995; p. 141. [Google Scholar]
- Bercovici, D.; Gaertner, H.F.; Puigserver, A.J. Poly-L-lysine and multioligo (L-methionyl) poly-L-lysine as nutritional sources of essential amino acids. J. Agric. Food Chem. 1989, 37, 873–877. [Google Scholar] [CrossRef]
- Wallace, R.; Frumholtz, P.; Walker, N.D. Breakdown of N-terminally modified peptides and an isopeptide by rumen microorganisms. Appl. Environ. Microbiol. 1993, 59, 3147–3149. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.; Wallace, R.; Ørskov, E. Control of rate and extent of protein degradation. In Physiological Aspects of Digestion and Metabolism in Ruminants; Elsevier: Amsterdam, The Netherlands, 1991; pp. 541–592. [Google Scholar]
- Kaufmann, W.; Lüpping, W. Protected proteins and protected amino acids for ruminants. In Protein Contribution of Feedstuffs for Ruminants; Elsevier: Amsterdam, The Netherlands, 1982; pp. 36–75. [Google Scholar]
- Chalmers, M.I.; Cuthbertson, D.; Synge, R. Ruminal ammonia formation in relation to the protein requirement of sheep: I. Duodenal administration and heat processing as factors influencing fate of casein supplements. J. Agric. Sci. 1954, 44, 254–262. [Google Scholar] [CrossRef]
- Craig, W.M.; Broderick, G.A. Effect of heat treatment on true digestibility in the rat, in vitro proteolysis and available lysine content of cottonseed meal protein. J. Anim. Sci. 1981, 52, 292–301. [Google Scholar] [CrossRef]
- Hurrell, R.; Finot, P. Food processing on protein digestibility and amino acid availability. In Nutritional Adequacy, Nutrient Availability and Needs; Experientia Supplementum; Mauron, J., Ed.; Birkhäuser: Basel, Switzerland, 1983; pp. 233–246. [Google Scholar]
- Tamminga, S. Protein degradation in the forestomachs of ruminants. J. Anim. Sci. 1979, 49, 1615–1630. [Google Scholar] [CrossRef]
- Seo, J.K.; Kim, S.-W.; Kim, M.H.; Upadhaya, S.D.; Kam, D.K.; Ha, J.K. Direct-fed microbials for ruminant animals. Asian-Australas. J. Anim. Sci. 2010, 23, 1657–1667. [Google Scholar] [CrossRef]
- Barton, M.D. Antibiotic use in animal feed and its impact on human health. Nutr. Res. Rev. 2000, 13, 279–299. [Google Scholar] [CrossRef]
- Fon, F.; Nsahlai, I. Effect of direct-fed microbial consortia on ruminal fermentation of maize stover in sheep. Small Rumin. Res. 2013, 111, 71–75. [Google Scholar] [CrossRef]
- Miles, R.D.; Boot Walla, S.M. Direct-fed microbials in animal production. A review. J. Vet. Med. Anim. Health 1991, 5, 117–132. [Google Scholar]
- Yoon, I.; Stern, M. Influence of direct-fed microbials on ruminal microbial fermentation and performance of ruminants: A review. Asian-Australas. J. Anim. Sci. 1995, 8, 533–555. [Google Scholar] [CrossRef]
- Oetzel, G.; Emery, K.; Kautz, W.; Nocek, J. Direct-fed microbial supplementation and health and performance of pre-and postpartum dairy cattle: A field trial. J. Dairy Sci. 2007, 90, 2058–2068. [Google Scholar] [CrossRef]
- Puniya, A.K.; Salem, A.Z.M.; Kumar, S.; Dagar, S.S.; Griffith, G.W.; Puniya, M.; Ravella, S.R.; Kumar, N.; Dhewa, T.; Kumar, R. Role of live microbial feed supplements with reference to anaerobic fungi in ruminant productivity: A review. J. Integr. Agric. 2015, 14, 550–560. [Google Scholar] [CrossRef]
- Nocek, J.; Kautz, W.; Leedle, J.; Block, E. Direct-fed microbial supplementation on the performance of dairy cattle during the transition period. J. Dairy Sci. 2003, 86, 331–335. [Google Scholar] [CrossRef]
- Kung, L., Jr. Direct-fed microbials for dairy cows and enzymes for lactating dairy cows: New theories and applications. In 2001 Pennsylvania State Dairy Cattle Nutrition Workshop; Pennsylvania State Extension: Grantville, PA, USA, 2001; pp. 22–42. [Google Scholar]
- Lee, Y.-K.; Puong, K.-Y.; Ouwehand, A.C.; Salminen, S. Displacement of bacterial pathogens from mucus and Caco-2 cell surface by lactobacilli. J. Med. Microbiol. 2003, 52, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Frizzo, L.; Soto, L.; Zbrun, M.; Bertozzi, E.; Sequeira, G.; Armesto, R.R.; Rosmini, M. Lactic acid bacteria to improve growth performance in young calves fed milk replacer and spray-dried whey powder. Anim. Feed. Sci. Technol. 2010, 157, 159–167. [Google Scholar] [CrossRef]
- Holzapfel, W.; Geisen, R.; Schillinger, U. Biological preservation of foods with reference to protective cultures, bacteriocins and food-grade enzymes. Int. J. Food Microbiol. 1995, 24, 343–362. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.A.; Beauchemin, K.A.; Alazzeh, A.Y.; Baah, J.; Teather, R.M.; Stanford, K. The use of direct fed microbials to mitigate pathogens and enhance production in cattle. Can. J. Anim. Sci. 2011, 91, 193–211. [Google Scholar] [CrossRef]
- Elghandour, M.M.; Chagoyán JC, V.; Salem, A.Z.; Kholif, A.E.; Castañeda JS, M.; Camacho, L.M.; Cerrillo-Soto, M.A. Effects of Saccharomyces cerevisiae at direct addition or pre-incubation on in vitro gas production kinetics and degradability of four fibrous feeds. Ital. J. Anim. Sci. 2014, 13, 3075. [Google Scholar] [CrossRef]
- Martin, S.A.; Streeter, M. Effect of malate on in vitro mixed ruminal microorganism fermentation. J. Anim. Sci. 1995, 73, 2141–2145. [Google Scholar] [CrossRef] [PubMed]
- Frumholtz, P.; Newbold, C.; Wallace, R. Influence of Aspergillus oryzae fermentation extract on the fermentation of a basal ration in the rumen simulation technique (Rusitec). J. Agric. Sci. 1989, 113, 169–172. [Google Scholar] [CrossRef]
- Gomez-Alarcon, R.; Dudas, C.; Huber, J. Influence of cultures of Aspergillus oryzae on rumen and total tract digestibility of dietary components. J. Dairy Sci. 1990, 73, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Wikandari, R.; Hasniah, N.; Taherzadeh, M.J. The role of filamentous fungi in advancing the development of a sustainable circular bioeconomy. Bioresour. Technol. 2022, 345, 126531. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Mahboubi, A.; Lennartsson, P.R.; Taherzadeh, M.J. Waste biorefineries using filamentous ascomycetes fungi: Present status and future prospects. Bioresour. Technol. 2016, 215, 334–345. [Google Scholar] [CrossRef]
- Fazenda, M.L.; Seviour, R.; McNeil, B.; Harvey, L.M. Submerged culture fermentation of “higher fungi”: The macrofungi. Adv. Appl. Microbiol. 2008, 63, 33–103. [Google Scholar] [PubMed]
- El-Enshasy, H.A. Chapter 9—Filamentous Fungal Cultures–Process Characteristics, Products, and Applications. In Bioprocessing for Value-Added Products from Renewable Resources; Yang, S.-T., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 225–261. [Google Scholar]
- Gomi, K. ASPERGILLUS|Aspergillus oryzae. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 92–96. [Google Scholar]
- Uwineza, C.; Mahboubi, A.; Atmowidjojo, A.; Ramadhani, A.; Wainaina, S.; Millati, R.; Wikandari, R.; Niklasson, C.; Taherzadeh, M.J. Cultivation of edible filamentous fungus Aspergillus oryzae on volatile fatty acids derived from anaerobic digestion of food waste and cow manure. Bioresour. Technol. 2021, 37, 125410. [Google Scholar] [CrossRef]
- Karimi, S.; Soofiani, N.M.; Mahboubi, A.; Ferreira, J.A.; Lundh, T.; Kiessling, A.; Taherzadeh, M.J. Evaluation of Nutritional Composition of Pure Filamentous Fungal Biomass as a Novel Ingredient for Fish Feed. Fermentation 2021, 7, 152. [Google Scholar] [CrossRef]
- Karimi, S.; Soofiani, N.M.; Lundh, T.; Mahboubi, A.; Kiessling, A.; Taherzadeh, M.J. Evaluation of filamentous fungal biomass cultivated on vinasse as an alternative nutrient source of fish feed: Protein, lipid, and mineral composition. Fermentation 2019, 5, 99. [Google Scholar] [CrossRef]
- Asadollahzadeh, M.; Ghasemian, A.; Saraeia, A.; Resalati, H.; Taherzadeh, M.J. Production of fungal biomass protein by filamentous fungi cultivation on liquid waste streams from pulping process. BioResources 2018, 13, 5013–5031. [Google Scholar] [CrossRef]
- Jin, B.; Yan, X.; Yu, Q.; van Leeuwen, J. A comprehensive pilot plant system for fungal biomass protein production and wastewater reclamation. Adv. Environ. Res. 2002, 6, 179–189. [Google Scholar] [CrossRef]
- Sar, T.; Larsson, K.; Fristedt, R.; Undeland, I.; Taherzadeh, M.J. Demo-scale production of protein-rich fungal biomass from potato protein liquor for use as innovative food and feed products. Food Biosci. 2022, 47, 101637. [Google Scholar] [CrossRef]
- Duru, C.C.; Uma, N.U. Production of fungal biomass from cormel process waste-water of cocoyam (Xanthosoma sagittifolium (L) Schott) using Aspergillus oryzae obtained from cormel flour. J. Sci. Food Agric. 2003, 83, 850–857. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Lennartsson, P.R.; Taherzadeh, M.J. Production of ethanol and biomass from thin stillage using food-grade Zygomycetes and Ascomycetes filamentous fungi. Energies 2014, 7, 3872–3885. [Google Scholar] [CrossRef]
- te Biesebeke, R.; Ruijter, G.; Rahardjo, Y.S.; Hoogschagen, M.J.; Heerikhuisen, M.; Levin, A.; Punt, P.J. Aspergillus oryzae in solid-state and submerged fermentations: Progress report on a multi-disciplinary project. FEMS Yeast Res. 2002, 2, 245–248. [Google Scholar] [CrossRef]
- Machida, M. Progress of Aspergillus oryzae genomics. Adv. Appl. Microbiol. 2002, 51, 81–106. [Google Scholar] [PubMed]
- Zhang, Z.Y.; Jin, B.; Bai, Z.H.; Wang, X.Y. Production of fungal biomass protein using microfungi from winery wastewater treatment. Bioresour. Technol. 2008, 99, 3871–3876. [Google Scholar] [CrossRef]
- Rousta, N.; Hellwig, C.; Wainaina, S.; Lukitawesa, L.; Agnihotri, S.; Rousta, K.; Taherzadeh, M.J. Filamentous Fungus Aspergillus oryzae for Food: From Submerged Cultivation to Fungal Burgers and Their Sensory Evaluation—A Pilot Study. Foods 2021, 10, 2774. [Google Scholar] [CrossRef] [PubMed]
- Sar, T.; Ferreira, J.A.; Taherzadeh, M.J. Conversion of fish processing wastewater into fish feed ingredients through submerged cultivation of Aspergillus oryzae. Syst. Microbiol. Biomanufacturing 2021, 1, 100–110. [Google Scholar] [CrossRef]
- Parchami, M.; Ferreira, J.A.; Taherzadeh, M.J. Starch and protein recovery from brewer’s spent grain using hydrothermal pretreatment and their conversion to edible filamentous fungi–A brewery biorefinery concept. Bioresour. Technol. 2021, 337, 125409. [Google Scholar] [CrossRef]
- Naili, B.; Sahnoun, M.; Bejar, S.; Kammoun, R. Optimization of submerged Aspergillus oryzae S2 α-amylase production. Food Sci. Biotechnol. 2016, 25, 185–192. [Google Scholar] [CrossRef]
- Jin, B.; Yu, Q.; Leeuwen, J.v. A bioprocessing mode for simultaneous fungal biomass protein production and wastewater treatment using an external air-lift bioreactor. J. Chem. Technol. Biotechnol. 2001, 76, 1041–1048. [Google Scholar] [CrossRef]
- Jin, B.; Zepf, F.; Bai, Z.; Gao, B.; Zhu, N. A biotech-systematic approach to select fungi for bioconversion of winery biomass wastes to nutrient-rich feed. Process Saf. Environ. Prot. 2016, 103, 60–68. [Google Scholar] [CrossRef]
- Ravinder, R.; Venkateshwar Rao, L.; Ravindra, P. Studies on Aspergillus oryzae mutants for the production of single cell proteins from deoiled rice bran. Food Technol. Biotechnol. 2003, 41, 243–246. [Google Scholar]
- Melnichuk, N.; Braia, M.J.; Anselmi, P.A.; Meini, M.-R.; Romanini, D. Valorization of two agroindustrial wastes to produce alpha-amylase enzyme from Aspergillus oryzae by solid-state fermentation. Waste Manag. 2020, 106, 155–161. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Karimi, K.; Taherzadeh, M.J. Integrated process for protein, pigments, and biogas production from baker’s yeast wastewater using filamentous fungi. Bioresour. Technol. 2021, 337, 125356. [Google Scholar]
- Yousufi, M.K. To determine protein content of single cell protein produced by using various combinations of fruit wastes and two standard food fungi. Int. J. Adv. Biotechnol. Res. 2012, 3, 533–536. [Google Scholar]
- Bátori, V.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Ethanol and protein from ethanol plant by-products using edible fungi Neurospora intermedia and Aspergillus oryzae. BioMed Res. Int. 2015, 2015, 176371. [Google Scholar] [CrossRef]
- Mahboubi, A.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Value-added products from dairy waste using edible fungi. Waste Manag. 2016, 59, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Szendefy, J.; Szakacs, G.; Christopher, L. Potential of solid-state fermentation enzymes of Aspergillus oryzae in biobleaching of paper pulp. Enzym. Microb. Technol. 2006, 39, 1354–1360. [Google Scholar] [CrossRef]
- Sar, T.; Ozturk, M.; Taherzadeh, M.J.; Ferreira, J.A. New insights on protein recovery from olive oil mill wastewater through bioconversion with edible filamentous fungi. Processes 2020, 8, 1210. [Google Scholar] [CrossRef]
- Rousta, N.; Ferreira, J.A.; Taherzadeh, M.J. Production of L-carnitine-enriched edible filamentous fungal biomass through submerged cultivation. Bioengineered 2021, 12, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Filho, P.F.S.; Nair, R.B.; Andersson, D.; Lennartsson, P.R.; Taherzadeh, M.J. Vegan-mycoprotein concentrate from pea-processing industry byproduct using edible filamentous fungi. Fungal Biol. Biotechnol. 2018, 5, 1–10. [Google Scholar]
- Majumdar, S.; Bhowal, J. Studies on production and evaluation of biopigment and synthetic dye decolorization capacity of laccase produced by A. oryzae cultivated on agro-waste. Bioprocess Biosyst. Eng. 2021, 45, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Serba, E.; Pimenov, N.; Mochalina, P.; Overchenko, M.; Borscheva, Y.; Sharikov, A.; Rimareva, L. Production of Aspergillus oryzae RCAM 01133 biomass with increased protein and polysaccharides content using by-products of food industry. Agron. Res. 2020, 18, 290–300. [Google Scholar]
- National Research Council. Nutrient Requirements of Dairy Cattle: 2001; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Adhikari, S.; Schop, M.; de Boer, I.J.M.; Huppertz, T. Protein Quality in Perspective: A Review of Protein Quality Metrics and Their Applications. Nutrients 2022, 14, 947. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, J.P.F. Amino Acids in Animal Nutrition, 2nd ed.; CABI: Wallingford, UK, 2003. [Google Scholar]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single Cell Protein—State-of-the-Art, Industrial Landscape and Patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, R.; Sabapathy, S.N.; Bawa, A.S. Functional and Edible Uses of Soy Protein Products. Compr. Rev. Food Sci. Food Saf. 2008, 7, 14–28. [Google Scholar] [CrossRef]
- Bach, A.; Calsamiglia, S.; Stern, M.D. Nitrogen metabolism in the rumen. J. Dairy Sci. 2005, 88, E9–E21. [Google Scholar] [CrossRef]
- Pérez-Barbería, F.J. The Ruminant: Life History and Digestive Physiology of a Symbiotic Animal. In Sustainable and Environmentally Friendly Dairy Farms; Springer International Publishing: Cham, Switzerland, 2020; pp. 19–45. [Google Scholar]
- Santos, J.; Bilby, T.; Thatcher, W.; Staples, C.; Silvestre, F. Long Chain Fatty Acids of Diet as Factors Influencing Reproduction in Cattle. Reprod. Domest. Anim. 2008, 43 (Suppl. S2), 23–30. [Google Scholar] [CrossRef]
- Rousta, N.; Aslan, M.; Akbas, M.Y.; Ozcan, F.; Sar, T.; Taherzadeh, M.J. Effects of fungal based bioactive compounds on human health: Review paper. Crit. Rev. Food Sci. Nutr. 2024, 64, 7004–7027. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B. Application of Probiotic and Prebiotic in Animals Production: A Review. Environ. Ecol. 2013, 31, 873–876. [Google Scholar]
- Barker, T.W.; Drouliscos, N.J.; Worgan, J.T. Composition and nutritional evaluation of Aspergillus oryzae biomass grown on palm oil processing effluents. J. Sci. Food Agric. 1981, 32, 1014–1020. [Google Scholar] [CrossRef]
- Sauvant, D.; Perez, J.M.; Tran, G. Tables of Composition and Nutritional Value of Feed Materials: Pigs, Poultry, Cattle, Sheep, Goats, Rabbits, Horses and Fish; Wageningen Academic Publishers: Wageningen, The Netherlands, 2004. [Google Scholar]
- Schwab, C.G.; Broderick, G.A. A 100-Year Review: Protein and amino acid nutrition in dairy cows. J. Dairy Sci. 2017, 100, 10094–10112. [Google Scholar] [CrossRef] [PubMed]
- Vaga, M. Investigating Ruminal Nitrogen Metabolism. Ph.D. Thesis, Swedish University of Agricultural Sciences, Umeå, Sweden, 2017. [Google Scholar]
- Reynal, S.M.; Broderick, G.A. Effect of Dietary Level of Rumen-Degraded Protein on Production and Nitrogen Metabolism in Lactating Dairy Cows*. J. Dairy Sci. 2005, 88, 4045–4064. [Google Scholar] [CrossRef] [PubMed]
- Mjoun, K.; Kalscheur, K.; Hippen, A.; Schingoethe, D. Ruminal degradability and intestinal digestibility of protein and amino acids in soybean and corn distillers grains products. J. Dairy Sci. 2010, 93, 4144–4154. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, U.; Sniffen, C.J.; Stern, M.D.; Van Soest, P.J. Evaluation of a mathematical model of rumen digestion and an in vitro simulation of rumen proteolysis to estimate the rumen-undegraded nitrogen content of feedstuffs. Br. J. Nutr. 2007, 50, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Paz, H.; Klopfenstein, T.; Hostetler, D.; Fernando, S.; Castillo-Lopez, E.; Kononoff, P. Ruminal degradation and intestinal digestibility of protein and amino acids in high-protein feedstuffs commonly used in dairy diets. J. Dairy Sci. 2014, 97, 6485–6498. [Google Scholar] [CrossRef]
- Ørskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 2009, 92, 499–503. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Kumar, S.; Kumari, R.; Kumar, K.; Walli, T.K. Roasting and formaldehyde method to make bypass protein for ruminants and its importance: A review. Indian J. Anim. Sci. 2015, 85, 223–230. [Google Scholar] [CrossRef]
- Sahraei Belverdy, M.; Alamouti, A.A.; Khadem, A.A.; González, J.; Carro, M.D.; Kianmehr, M.H.; Azizi, M.H. Encapsulation of soybean meal with fats enriched in palmitic and stearic acids: Effects on rumen-undegraded protein and in vitro intestinal digestibility. Arch. Anim. Nutr. 2019, 73, 158–169. [Google Scholar] [CrossRef]
- Nia, S.A.M.; Ingalls, J.R. Effect of heating on canola meal protein degradation in the rumen and digestion in the lower gastrointestinal tract of steers. Can. J. Anim. Sci. 1992, 72, 83–88. [Google Scholar] [CrossRef]
- Ljøkjel, K.; Harstad, O.M.; Skrede, A. Effect of heat treatment of soybean meal and fish meal on amino acid digestibility in mink and dairy cows. Anim. Feed. Sci. Technol. 2000, 84, 83–95. [Google Scholar] [CrossRef]
- Barchiesi-Ferrari, C.; Anrique, R. Ruminal degradability of dry matter and crude protein from moist dehulled lupin and extruded rapeseed meal. Chil. J. Agric. Res. 2011, 71, 430. [Google Scholar] [CrossRef]
- Rossi, F.; Fiorentini, L.; Masoero, F.; Piva, G. Effect of fat coating on rumen degradation and intestinal digestibility of soybean meal. Anim. Feed. Sci. Technol. 1999, 81, 309–318. [Google Scholar] [CrossRef]
- Stanford, K.; McAllister, T.A.; Xu, Z.; Cheng, K.-J.; Pickard, M. Comparison of lignosulfonate-treated canola meal and soybean meal as rumen undegradable protein supplements for lambs. Can. J. Anim. Sci. 1995, 75, 371–377. [Google Scholar] [CrossRef]
- Brand, T.S.; van Zyl, J.H.C.; Dreyer, O. The effect of formaldehyde treatment of canola oilcake meal and sweet lupins on the in situ dry matter and crude protein digestibility. S. Afr. J. Anim. Sci. 2023, 53, 91–100. [Google Scholar] [CrossRef]
- Beever, R.E.; Burns, D. Phosphorus Uptake, Storage and Utilization by Fungi. Adv. Bot. Res. 1981, 8, 127–219. [Google Scholar]
- Driessen, F.; Ubbels, J.; Stadhouders. Continuous manufacture of yogurt. I. Optimal conditions and kinetics of the prefermentation process. Biotechnol. Bioeng. 1977, 19, 821–839. [Google Scholar] [CrossRef]
- Babel, W.; Pöhland, H.D.; Soyez, K. Single cell proteins. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley online Library: Hoboken, NJ, USA, 2000. [Google Scholar]
- Heinz, T.; Schadereit, R.; Henk, G. Untersuchungen zum Einsatz von Erdöldestillat-Futterhefe “Fermosin” in der Tierernährung. Arch. Anim. Nutr. 1979, 29, 81–91. [Google Scholar] [CrossRef]
- Lichtfield, J.H. The Production of Fungi. Single-Cell Protein I; The MIT Press: Cambridge, MA, USA, 1968. [Google Scholar]
- Madika, A.; Aliyu, M.S.; Tijjani, M.B.; Musa, B. Production of single cell protein from pineapple waste using Saccharomyces cerevisiae. Afr. J. Nat. Sci. 2019, 19, 10–16. [Google Scholar]
- Bajpai, P. Microorganisms Used for Single-Cell Protein Production, in Single Cell Protein Production from Lignocellulosic Biomass; Springer: Berlin/Heidelberg, Germany, 2017; pp. 21–30. [Google Scholar]
- White, A. Principles of Biochemistry, 2nd ed.; Blakiston Division, McGraw-Hill Book Company: New York, NY, USA, 1959. [Google Scholar]
- Goldberg, I. Single Cell Protein; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 1. [Google Scholar]
- Wang, Y.; McAllister, T. Rumen microbes, enzymes and feed digestion-a review. Asian-Australas. J. Anim. Sci. 2002, 15, 1659–1676. [Google Scholar] [CrossRef]
- Martin, S.; Nisbet, D.; Dean, R. Influence of a commerical yeast supplement on the in vitro ruminal fermentation. Nutr. Rep. Int. 1989, 40, 395–403. [Google Scholar]
- Fondevila, M.; Newbold, C.J.; Hotten, P.M.; Ørskov, E.R. A note on the effect of Aspergillus oryzae fermentation extract on the rumen fermentation of sheep given straw. Anim. Sci. Technol. 1990, 51, 422–425. [Google Scholar] [CrossRef]
- Varel, V.H.; Kreikemeier, K.K.; Jung, H.J.G.; Hatfield, R.D. In vitro stimulation of forage fiber degradation by ruminal microorganisms with Aspergillus oryzae fermentation extract. Appl. Environ. Microbiol. 1993, 59, 3171–3176. [Google Scholar] [CrossRef]
- Bertrand, J.; Grimes, L. Influence of tallow and Aspergillus oryzae fermentation extract in dairy cattle rations. J. Dairy Sci. 1997, 80, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Newbold, C.; Frumholtz, P.; Wallace, R. Influence of Aspergillus oryzae fermentation extract on rumen fermentation and blood constituents in sheep given diets of grass hay and barley. J. Agric. Sci. 1992, 119, 423–427. [Google Scholar] [CrossRef]
- Newbold, C.; McKain, N.; Wallace, R. Combined effects of Aspergillus oryzae fermentation extract and monensin on fermentation in the rumen simulation technique (Rusitec). J. Agric. Sci. 1993, 121, 241–246. [Google Scholar] [CrossRef]
- Takiya, C.S.; Calomeni, G.D.; Silva, T.H.; Vendramini, T.H.A.; Silva, G.G.; Consentini, C.E.C.; Bertoni, J.C.; Zilio, E.M.C.; Rennó, F.P. Increasing dietary doses of an Aspergillus oryzae extract with alpha-amylase activity on nutrient digestibility and ruminal fermentation of lactating dairy cows. Anim. Feed Sci. Technol. Health Care 2017, 228, 159–167. [Google Scholar] [CrossRef]
- Beharka, A.A.; Nagaraja, T.G. Effect of Aspergillus oryzae fermentation extract (Amaferm®) on in vitro fiber degradation. J. Dairy Sci. 1993, 76, 812–818. [Google Scholar] [CrossRef]
- Wiedmeier, R.; Arambel, M.; Walters, J. Effect of yeast culture and Aspergillus oryzae fermentation extract on ruminal characteristics and nutrient digestibility. J. Dairy Sci. 1987, 70, 2063–2068. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, Y.; Wang, Y.; Wang, C.; Liu, J. Effects of addition of Aspergillus oryzae culture and 2-hydroxyl-4-(methylthio) butanoic acid on milk performance and rumen fermentation of dairy cows. Anim. Sci. J. 2017, 88, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Di Francia, A.; Masucci, F.; De Rosa, G.; Varricchio, M.; Proto, V. Effects of Aspergillus oryzae extract and a Saccharomyces cerevisiae fermentation product on intake, body weight gain and digestibility in buffalo calves. Anim. Feed. Sci. Technol. Health Care 2008, 140, 67–77. [Google Scholar] [CrossRef]
- Sosa, A.; Galindo, J.; Tejido, M.L.; Diaz, A.; Martinez, M.E.; Saro, C.; Morand-Fehr, P. Effects of Aspergillus oryzae on in vitro ruminal fermentation. Options Mediterr. Série A Séminaires Mediterr. 2011, 175–179. [Google Scholar]
- Denigan, M.; Huber, J.; Alhadhrami, G.; Al-Dehneh, A. Influence of feeding varying levels of Amaferm® on performance of lactating dairy cows. J. Dairy Sci. 1992, 75, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Niver, J.W.; Tucker, R.; Mitchell, G., Jr. Fiber digestion in lambs fed an extract of Aspergillus oryzae. J. Anim. Sci. 1973, 37, 1446–1450. [Google Scholar] [CrossRef]
- Kim, H.; Chung, S.; Moon, Y.; Ha, J.; Seo, I.; Ahn, B.; Lee, S. Effect of yeast culture, fungal fermentation extract and non-ionic surfactant on performance of Holstein cows during transition period. Anim. Feed. Sci. Technol. 2006, 126, 23–29. [Google Scholar] [CrossRef]
- Yohe, T.; O’Diam, K.; Daniels, K.M. Growth, ruminal measurements, and health characteristics of Holstein bull calves fed an Aspergillus oryzae fermentation extract. J. Dairy Sci. 2015, 98, 6163–6175. [Google Scholar] [CrossRef]
- Gomez-Alarcon, R.; Huber, J.T.; Higginbotham, G.E.; Wiersma, F.; Ammon, D.; Taylor, B. Influence of feeding Aspergillus oryzae fermentation extract on the milk yields, eating patterns, and body temperatures of lactating cows. J. Anim. Sci. 1991, 69, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Chiquette, J. Evaluation of the protective effect of probiotics fed to dairy cows during a subacute ruminal acidosis challenge. Anim. Feed. Sci. Technol. 2009, 153, 278–291. [Google Scholar] [CrossRef]
- Varel, V.; Kreikemeier, K. Response to various amounts of Aspergillus oryzae fermentation extract on ruminal metabolism in cattle. J. Dairy Sci. 1994, 77, 3081–3086. [Google Scholar] [CrossRef]
- Yoon, I.; Stern, M. Effects of Saccharomyces cerevisiae and Aspergillus oryzae cultures on ruminal fermentation in dairy cows. J. Dairy Sci. 1996, 79, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Newbold, C.; Brock, R.; Wallace, R. Influence of autoclaved or irradiated Aspergillus oryzae fermentation extract on fermentation in the rumen simulation technique (Rusitec). J. Agric. Sci. 1991, 116, 159–162. [Google Scholar] [CrossRef]
- Kellems, R.; Lagerstedt, A.; Wallentine, M. Effect of feeding Aspergillus oryzae fermentation extract or Aspergillus oryzae plus yeast culture plus mineral and vitamin supplement on performance of Holstein cows during a complete lactation. J. Dairy Sci. 1990, 73, 2922–2928. [Google Scholar] [CrossRef]
- Wallentine, M.V.; Johnston, N.P.; Andrus, D.; Jones, R.; Huber, J.T.; Higginbotham, G. The effect of feeding an Aspergillus oryzae culture-vitamin mix on the performance of lactating dairy cows during periods of heat stress. J. Dairy Sci. 1986, 69 (Suppl. S1), 189. [Google Scholar]
- Harris, B., Jr.; Van Horn, H.H.; Manookian, K.E.; Marshall, S.P.; Taylor, M.J.; Wilcox, C.J. Sugarcane silage, sodium hydroxide-and steam pressure-treated sugarcane bagasse, corn silage, cottonseed hulls, sodium bicarbonate, and Aspergillis oryzae product in complete rations for lactating cows. J. Dairy Sci. 1983, 66, 1474–1485. [Google Scholar] [CrossRef]
- Van Horn, H.; Harris, B.; Taylor, M.; Bachman, K.; Wilcox, C. By-product feeds for lactating dairy cows: Effects of cottonseed hulls, sunflower hulls, corrugated paper, peanut hulls, sugarcane bagasse, and whole cottonseed with additives of fat, sodium bicarbonate, and Aspergillus oryzae product on milk production. J. Dairy Sci. 1984, 67, 2922–2938. [Google Scholar] [CrossRef]
- Varel, V.H.; Kreikemeier, K.K. Influence of feeding Aspergillus oryzae fermentation extract (Amaferm) on in situ fiber degradation, ruminal fermentation, and microbial protein synthesis in nonlactating cows fed alfalfa or bromegrass hay. J. Anim. Sci. 1994, 72, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Hovell, F.D.D.; Nǵambi, J.W.W.; Barber, W.P.; Kyle, D.J. The voluntary intake of hay by sheep in relation to its degradability in the rumen as measured in nylon bags. Anim. Sci. Technol. 1986, 42, 111–118. [Google Scholar] [CrossRef]
- Huber, J.; Higginbotham, G.; Ware, D. Influence of feeding Vitaferm, containing an enzyme-producing culture from Aspergillus oryzae, on performance of lactating cows. J. Dairy Sci. 1985, 68 (Suppl. S1), 122. [Google Scholar]
- Miranda, R.; Mendoza, M.; Bárcena-Gama, J.; González, M.; Ferrara, R.; Ortega, C.; Cobos, P. Effect of Saccharomyces cerevisiae or Aspergillus oryzae cultures and NDF level on parameters of ruminal fermentation. Anim. Feed. Sci. Technol. Health Care 1996, 63, 289–296. [Google Scholar] [CrossRef]
- Raper, K.; Fennell, D.I. The Genus Aspergillus. Mycologia. 1966, 58, 500–502. [Google Scholar]
- Firkins, J.; Weiss, W.; Eastridge, M.; Hull, B. Effects of feeding fungal culture extract and animal-vegetable fat on degradation of hemicellulose and on ruminal bacterial growth in heifers1, 2. J. Dairy Sci. 1990, 73, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Uwineza, C.; Bouzarjomehr, M.; Parchami, M.; Sar, T.; Taherzadeh, M.J.; Mahboubi, A. Evaluation of in vitro digestibility of Aspergillus oryzae fungal biomass grown on organic residue derived-VFAs as a promising ruminant feed supplement. J. Anim. Sci. Biotechnol. 2023, 14, 120. [Google Scholar] [CrossRef]
- Martin, S.; Nisbet, D. Effects of Aspergillus oryzae fermentation extract on fermentation of amino acids, bermudagrass and starch by mixed ruminal microorganisms in vitro. J. Anim. Sci. 1990, 68, 2142–2149. [Google Scholar] [CrossRef]
- Beharka, A.; Nagaraja, T.; Morrill, J. Performance and ruminal function development of young calves fed diets with Aspergillus oryzae fermentation extract. J. Dairy Sci. 1991, 74, 4326–4336. [Google Scholar] [CrossRef]
- Arambel, M.J.; Wiedmeier, R.D.; Walters, J.L. Influence of donor animal adaptation to added yeast culture and/or Aspergillus oryzae fermentation extract on in vitro rumen fermentation. Nutr. Rep. Int. 1987, 35, 433–437. [Google Scholar]
- Bryant, M.P. Nutritional requirements of the predominant rumen cellulolytic bacteria. Fed. Proc. 1973, 32, 1809–1813. [Google Scholar]
- Dehority, B.H. Scott, and P. Kowaluk, Volatile fatty acid requirements of cellulolytic rumen bacteria. J. Bacteriol. 1967, 94, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Dawson, K.; Newman, K.; Boling, J. Effects of microbial supplements containing yeast and lactobacilli on roughage-fed ruminal microbial activities. J. Anim. Sci. 1990, 68, 3392–3398. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.; Hemken, R.; Dawson, K.; Harmon, R.; Barker, K. Influence of addition of yeast culture supplement to diets of lactating cows on ruminal fermentation and microbial populations. J. Dairy Sci. 1988, 71, 2967–2975. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ha, J.; Cheng, K.-J. Influence of an anaerobic fungal culture administration on in vivo ruminal fermentation and nutrient digestion. Anim. Feed. Sci. Technol. Health Care 2000, 88, 201–217. [Google Scholar] [CrossRef]
- Autrey, K.; McCaskey, T.; Little, J. Cellulose digestibility of fibrous materials treated with Trichoderma viride cellulase. J. Dairy Sci. 1975, 58, 67–71. [Google Scholar] [CrossRef]
- Caldwell, D.R.; Bryant, M.P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl. Environ. Microbiol. 1966, 14, 794–801. [Google Scholar] [CrossRef]
- Russell, J.B.; Hespell, R.B. Microbial rumen fermentation. J. Dairy Sci. 1981, 64, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Kanegasaki, S.; Takahashi, H. Function of growth factors for rumen microorganisms I. Nutritional characteristics of Selenomonas ruminantium. J. Bacteriol. 1967, 93, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Linehan, B.; Scheifinger, C.; Wolin, M. Nutritional requirements of Selenomonas ruminantium for growth on lactate, glycerol, or glucose. Appl. Environ. Microbiol. 1978, 35, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Bercovitz, A.; Peleg, Y.; Battat, E.; Rokem, J.S.; Goldberg, I. Localization of pyruvate carboxylase in organic acid-producing Aspergillus strains. Appl. Environ. Microbiol. 1990, 56, 1594–1597. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, D.J.; Martin, S.A. Effect of dicarboxylic acids and Aspergillus oryzae fermentation extract on lactate uptake by the ruminal bacterium Selenomonas ruminantium. Appl. Environ. Microbiol. 1990, 56, 3515–3518. [Google Scholar] [CrossRef]
- Waldrip, H.; Martin, S. Effects of an Aspergillus oryzae fermentation extract and other factors on lactate utilization by the ruminal bacterium Megasphaera elsdenii. J. Anim. Sci. 1993, 71, 2770–2776. [Google Scholar] [CrossRef] [PubMed]
- Dennis, S.; Nagaraja, T.; Bartley, E. Effect of lasalocid or monensin on lactate-producing or using rumen bacteria. J. Anim. Sci. 1981, 52, 418–426. [Google Scholar] [CrossRef]
- Choudhury, P.K.; Jena, R.; Tomar, S.K.; Puniya, A.K. Reducing Enteric Methanogenesis through Alternate Hydrogen Sinks in the Rumen. Methane 2022, 1, 320–341. [Google Scholar] [CrossRef]
- Mwenya, B.; Santoso, B.; Sar, C.; Gamo, Y.; Kobayashi, T.; Arai, I.; Takahashi, J. Effects of including β1–4 galacto-oligosaccharides, lactic acid bacteria or yeast culture on methanogenesis as well as energy and nitrogen metabolism in sheep. Anim. Feed Sci. Technol. 2004, 115, 313–326. [Google Scholar] [CrossRef]
- Counotte, G.H.M.; Prins, R.A.; Janssen, R.H.A.M.; DeBie, M.J.A. Role of Megasphaera elsdenii in the fermentation of DL-[2-13C] lactate in the rumen of dairy cattle. Appl. Environ. Microbiol. 1981, 42, 649–655. [Google Scholar] [CrossRef]
- Slyter, L.L. Influence of acidosis on rumen function. J. Anim. Sci. 1976, 43, 910–929. [Google Scholar] [CrossRef] [PubMed]
- Górka, P.; Kowalski, Z.M.; Pietrzak, P.; Kotunia, A.; Kiljanczyk, R.; Flaga, J.; Holst, J.J.; Guilloteau, P.; Zabielski, R. Effect of sodium butyrate supplementation in milk replacer and starter diet on rumen development in calves. Development 2009, 4, 10–11. [Google Scholar]
- Malhi, M.; Gui, H.; Yao, L.; Aschenbach, J.R.; Gäbel, G.; Shen, Z. Increased papillae growth and enhanced short-chain fatty acid absorption in the rumen of goats are associated with transient increases in cyclin D1 expression after ruminal butyrate infusion. J. Dairy Sci. 2013, 96, 7603–7616. [Google Scholar] [CrossRef]
- Mould, F.; Ørskov, E.; Mann, S. Associative effects of mixed feeds. I. Effects of type and level of supplementation and the influence of the rumen fluid pH on cellulolysis in vivo and dry matter digestion of various roughages. Anim. Feed Sci. 1983, 10, 15–30. [Google Scholar]
- Stewart, C.S. Factors affecting the cellulolytic activity of rumen contents. Appl. Environ. Microbiol. 1977, 33, 497–502. [Google Scholar] [CrossRef]
- Oellermann, S.; Arambel, M.; Kent, B.; Walters, J. Effect of graded amounts of Aspergillus oryzae fermentation extract on ruminal characteristics and nutrient digestibility in cattle. J. Dairy Sci. 1990, 73, 2413–2416. [Google Scholar] [CrossRef]
- Hymes-Fecht, U.C.; Casper, D.P. Adaptation and withdrawal of feeding dried Aspergillus oryzae fermentation product to dairy cattle and goats on in vitro NDF digestibility of selected forage sources. Transl. Anim. Sci. 2021, 5, txab051. [Google Scholar] [CrossRef] [PubMed]
- Khattab, M.; El-Zaiat, H.; El Taw, A.A.; Matloup, O.; Morsy, A.; Abdou, M.; Ebeid, H.; Attia, M.; Sallam, S. Impact of lemongrass and galangal as feed additives on performance of lactating Barki goats. Int. J. Dairy Sci. 2017, 12, 184–189. [Google Scholar] [CrossRef]
- Kholif, A.; Matloup, O.; Morsy, T.; Abdo, M.; Abu Elella, A.; Anele, U.; Swanson, K. Rosemary and lemongrass herbs as phytogenic feed additives to improve efficient feed utilization, manipulate rumen fermentation and elevate milk production of Damascus goats. Livest. Sci. 2017, 204, 39–46. [Google Scholar] [CrossRef]
- Kholif, A.; Gouda, G.; Anele, U.; Galyean, M. Extract of Moringa oleifera leaves improves feed utilization of lactating Nubian goats. Small Rumin. Res. 2018, 158, 69–75. [Google Scholar] [CrossRef]
- Salem, A.Z.; Kholif, A.E.; Elghandour, M.M.; Buendía, G.; Mariezcurrena, M.D.; Hernandez, S.R.; Camacho, L.M. Influence of oral administration of Salix babylonica extract on milk production and composition in dairy cows. Ital. J. Anim. Sci. 2014, 13, 2978. [Google Scholar] [CrossRef]
- Morsy, T.A.; Kholif, A.E.; Kholif, S.M.; Kholif, A.M.; Sun, X.; Salem, A.Z.M. Effects of two enzyme feed additives on digestion and milk production in lactating Egyptian buffaloes. Ann. Anim. Sci. 2016, 16, 209–222. [Google Scholar] [CrossRef]
- Xiao, J.; Alugongo, G.; Chung, R.; Dong, S.; Li, S.; Yoon, I.; Wu, Z.; Cao, Z. Effects of Saccharomyces cerevisiae fermentation products on dairy calves: Ruminal fermentation, gastrointestinal morphology, and microbial community. J. Dairy Sci. 2016, 99, 5401–5412. [Google Scholar] [CrossRef]
- Martin, S.A.; Nisbet, D.J. Effect of direct-fed microbials on rumen microbial fermentation. J. Dairy Sci. 1992, 75, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Higginbotham, G.; Bath, D.L.; Butler, L. Effect of feeding an Aspergillus oryzae extract on milk production and related responses in a commercial dairy herd. J. Dairy Sci. 1993, 76, 1484–1489. [Google Scholar] [CrossRef]
- Chiou, P.W.-S.; Chen, C.-R.; Yu, B. Effects of Aspergillus oryzae fermentation extract on performance of lactating cows in the summer and winter in Taiwan. Asian-Australas. J. Anim. Sci. 2002, 15, 382–389. [Google Scholar] [CrossRef]
- Yu, P.; Huber, J.; Theurer, C.; Chen, K.; Nussio, L.; Wu, Z. Effect of steam-flaked or steam-rolled corn with or without Aspergillus oryzae in the diet on performance of dairy cows fed during hot weather. J. Dairy Sci. 1997, 80, 3293–3297. [Google Scholar] [CrossRef]
- Newbold, C. Probiotics as feed additives in ruminant diets. In Proceedings of the 51st Minnesota Nutrition Conference, Bloomington, Minnesota, 18–19 September 1990; pp. 102–118. [Google Scholar]
- Waldo, D.; Jorgensen, N. Forages for high animal production: Nutritional factors and effects of conservation. J. Dairy Sci. 1981, 64, 1207–1229. [Google Scholar] [CrossRef]
- Sucu, E.; Moore, C.; VanBaale, M.J.; Jensen, H.; Sanz-Fernandez, M.V.; Baumgard, L.H. Effects of feeding Aspergillus oryzae fermentation product to transition Holstein cows on performance and health. Can. J. Anim. Sci. 2018, 99, 237–243. [Google Scholar] [CrossRef]
- Sievert, S.; Shaver, R. Carbohydrate and Aspergillus oryzae effects on intake, digestion, and milk production by dairy cows. J. Dairy Sci. 1993, 76, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Alarcon, R.; Wiersma, F.; Ammon, D.; Higginbotham, G.E.; Huber, J.T. Effect of feeding Amaferm (Aspergillus oryzae extract) to cows in early lactation on milk yields and related parameters. J. Dairy Sci. 1988, 71 (Suppl. S1), 302. [Google Scholar]
- Chiou, P.; Chen, C.; Yu, B.J. Effects of Aspergillus oryzae fermentation extract on in situ degradation of feedstuffs. Asian-Australas. J. Anim. Sci. 2000, 13, 1076–1083. [Google Scholar] [CrossRef]
- Campos, M.R.; Herrera-Saldana, R.; Viniegas, G.G.; Diaz, C.M. The effect of Aspergillus niger and Aspergillus oryzae (Amaferm) as probiotics on in situ digestibility of a high fibre diet. J. Dairy Sci. 1990, 73 (Suppl. S1), 133. [Google Scholar]
- Linn, J.G. Factors affecting the composition of milk from dairy cows. In Designing Foods: Animal Product Options in the Marketplace; National Academies Press: Washington, DC, USA, 1988. [Google Scholar]
- Higginbotham, G.E.; Santos, J.E.; Juchem, S.O.; DePeters, E.J. Effect of feeding Aspergillus oryzae extract on milk production and rumen parameters. Livest. Prod. Sci. 2004, 86, 55–59. [Google Scholar] [CrossRef]
- Williams, P.; Newbold, C. Rumen probiosis: The effects of novel microorganisms on rumen fermentation and ruminant productivity. In 2th Feed Manufacturers Conference: Recent Advances in Animal Nutrition; Butterworth-Heinemann: Loughborough, UK, 1990; pp. 211–227. [Google Scholar]
- Williams, P.E.; Tait, C.; Innes, G.M.; Newbold, C.J. Effects of the inclusion of yeast culture (Saccharomyces cerevisiae plus growth medium) in the diet of dairy cows on milk yield and forage degradation and fermentation patterns in the rumen of steers. J. Anim. Sci. 1991, 69, 3016–3026. [Google Scholar] [CrossRef]
- Caton, J.S.; Erickson, D.O.; Carey, D.A.; Ulmer, D.L. Influence of Aspergillus oryzae fermentation extract on forage intake, site of digestion, in situ degradability, and duodenal amino acid flow in steers grazing cool-season pasture. J. Anim. Sci. 1993, 71, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, J.M.; Abney, M.D.; Galyean, M.L.; Rivera, J.D.; Hanson, K.C.; McLeod, K.R.; Harmon, D.L. Effects of a dietary Aspergillus oryzae extract containing α-amylase activity on performance and carcass characteristics of finishing beef cattle. J. Anim. Sci. 2007, 85, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Zerby, H.N.; Bard, J.L.; Loerch, S.C.; Kuber, P.S.; Radunz, A.E.; Fluharty, F.L. Effects of diet and Aspergillus oryzae extract or Saccharomyces cervisiae on growth and carcass characteristics of lambs and steers fed to meet requirements of natural markets. J. Anim. Sci. 2011, 89, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Higginbotham, G.; Collar, C.; Aseltine, M.; Bath, D. Effect of yeast culture and Aspergillus oryzae extract on milk yield in a commercial dairy herd. J. Dairy Sci. 1994, 77, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.; Tricarico, J. Case study: Effects of an Aspergillus oryzae extract containing α-amylase activity on lactational performance in commercial dairy herds. Prof. Anim. Sci. 2007, 23, 291–294. [Google Scholar] [CrossRef]
- Lubis, D.; Haryanto, B.; Wina, E.; Suhargiyantatmo, T. Feeding of Aspergillus oryzae fermentation culture (AOFC) to growing sheep: 2. Growth rate and feed efficiency. J. Ilmu Ternak Dan Vet. 2002, 7, 214–219. [Google Scholar]
| Substrate | Substrate Loading | pH | Temperature (°C) | CP% | Working Volume | Main Findings | Ref. |
|---|---|---|---|---|---|---|---|
| Synthetic medium VFAs | 3–18 g/L of mixture of acetic propionic, butyric, and caproic acid | 5–8 | 35 | <41 | 250 mL | The fungal growth inhibition increased with increasing acid concentration. | [83] |
| Winery wastewater treatment | 50 mL | 4–6 | 30 | 35.3 | 250 mL | To obtain the protein-rich fungal biomass, the biorefinery can be used in winery waste streams. | [93] |
| Oat flour | 20 g/L oat flour, 10 g/L sucrose, and 100 mL cooking oil | 5 | 35 | 37 | 26 L | - | [94] |
| Fish processing wastewater | 3 L salt brine | 5.5 | 35 | 54 | 4.5 L | A. oryzae has the potential to be cultivated on different wastewater streams. | [95] |
| Brewer spent grain | 14.6 g of wet BSG | 5.5 | 35 | 30.52 | 250 mL | The hydrothermal pretreatment can increase both solubilization of the substrate and the protein content of the produced biomass. | [96] |
| Black liquor | - | 5.5 | 35 | 47.6 | 4 L | Compared to other fungal strains, A. oryzae has the highest yield of production using black liquor. | [86] |
| Vinasse | 21 g/L for 5% vinasse solution | 5–6.5 | 35 | 44.7 | 250 mL | Vinasse which is diluted 95% (v/v) supports the highest growth rate. | [85] |
| Agro-industrial residuals | 14 g/L agro-industrial by-products and 11.5 g/L whey powder | 5.5 | 24 | - | 500 mL | The optimum volume of free air must be about five time higher than the volume of medium to achieve the highest rate of production. | [97] |
| Glucose | 30 g/L glucose | 5–6.5 | 35 | 45.7 | 250 mL | - | [84] |
| Starch-processing wastewater (SPW) | - | 5.2 | 38 | 46.7 | - | Semicontinuous process of fungal cultivation using SPW has a higher productivity. | [98] |
| Grape marc residue | 15 g of dried grape marc residues | - | 30 | 23 | 250 mL | The produced biomass can be a good feed supplement since it has more than 50% dry matter digestibility. | [99] |
| Wastewater of cocoyam | 200 mL | 4.5 | 35 | - | 500 mL | By the addition of urea, the protein level of the produced A. oryzae biomass will increase about two times. | [89] |
| VFAs from food waste and cow manure | 50 mL | 6 | 35 | Up to 41 | 250 mL | Using VFAs produced from food wastes as the substrate, protein-rich biomass production can be achieved. | [83] |
| Thin stillage | 50 mL of medium | 5.5 | 30–45 | 48 | 250 mL | A. oryzae can be preferable for cultivation on thin stillage compared to S. cerevisiae since it can consume pentose sugars. | [90] |
| Deoiled rice bran | 10 g | 3–7 | 36–45 | 43 | 500 mL | - | [100] |
| Soybean husk and flour mill | 1 g | 6 | 30 | 10 | Solid state fermentation | - | [101] |
| Baker yeast wastewater | 50 mL | 5.3 | 30 | 43.8 | 250 mL | Nitrogen supplementation increases the protein content by 12%. | [102] |
| Wheat kernel | - | - | 30–35 | - | SSF | For obtaining a higher biomass yield, pretreatment is essential. | [91] |
| Fruit wastes | - | - | 28 | 57.3 | - | A. oryzae fungal biomass grows optimally on pomegranate rind and pineapple skin. | [103] |
| Whole stillage | 1 L | 5 | 35 | 42.3 ± 1.7 | 2.5 L | - | [104] |
| Dairy wastes mixture | 100 mL of medium | 4–7 | 35 | 30–40 | 250 mL | High concentration of lactic acid does not have a negative impact on degradation of fat by A. oryzae. | [105] |
| Paper pulp | - | 6–9 | 30 | - | 500 mL | - | [106] |
| Olive oil mill wastewater | 50 mL of olive oil mill wastewater | 5.2 | 35 | 32.3 | 250 mL | Addition of nitrogen source decreased cultivation time and increased biomass protein content. | [107] |
| Glucose | 30 g/L glucose, 5 g/L yeast extract | 5.5 | 35 | 47 | 250 mL | A. oryzae has the potential to produce fungal biomass with high L-carnitine content. | [108] |
| Pea-processing by-product (PpB) | 3.5 L PpB dissolved in distilled water | 6.1–6.5 | 35 | 43.1 | 4.5 L | PpB can be applied for the production of protein-rich biomass. | [109] |
| Leaf and stalk | - | 5 | 30 | - | 150 mL | - | [110] |
| Food industry by-products | - | 6.5 | 30 | 58.9 | - | - | [111] |
| Treatment Methods | Protein Sources | Treatment Conditions | Digestibility Treatments | Effect | Ref. |
|---|---|---|---|---|---|
| Heat treatment | Rapeseed meal (RM) | Mixed with water and heated at 110 °C for 30 min | In situ and in vitro intestinal analysis | RUP increased from 262 g/kg to 431 g/kg. | [133] |
| Moist heat pressure | Canola meal | Heat at 127 °C with steam pressure of 117 kPa for 15, 30, 45, 60, and 90 min | Nylon bag, mobile nylon bag, in situ and in vitro techniques | Nitrogen disappearance declined in the rumen from 74.4% to 18.9% and increased in GI tract from 16.2 to 64.2% for control and CM heat treated for 45 min. | [134] |
| Heat treatment | Soybean meal and fish meal | Heat at 120 °C and 130 °C for 30 min | Rumen degradation and CP partial digestibility in the digestive tract | RUP increased in TSBM 120 and 130 from 36.6 to 67.8 and 71.7%, respectively. | [135] |
| Extrusion | Dehulled lupin (DL) and RM | Heat at 130 °C with 20% moisture for DL and 120 °C with 20% moisture | RUP increased in EDL by 8.9% and 37.35% in ERM. | [136] | |
| Fat coating | Soybean meal | Coat with 10 and 25% long-chain fatty acids (palmitic acid (PA) and stearic acid (SA)) | In situ rumen degradation using nylon bag | RUP increased 76.57% to 82.47% and 90.92% in 8 h rumen degradation for 10% and 25% fat-coated soybean meal, respectively. | [137] |
| Fat coating | Soybean meal | Coat with 400–500 g fat/kg at different ratios of PA and SA | In situ rumen incubation and in vitro intestinal protein digestibility | RUP increased from 262 g/kg to 308 and 364 g/kg for FL40 and FL50, respectively, at 100% PA coating. | [133] |
| Lignosulfonate | Canola meal and soybean meal | Added 7% LSO3 and heated at 95 °C for 1 h | In vitro and in situ digestibility | RUP increased from 41.9% to 65.3% and from 30.9% to 63.3% in treated soybean meal and canola meal, respectively. | [138] |
| Formaldehyde | Canola oil cake meal and sweet lupin seed | Added 40% (w/v) formaldehyde at concentration of 10 g/kg and 15 g/kg | In situ dry matter and crude protein digestibility | The CP effective degradation was decreased from 71.7% to 38.1% at formaldehyde concentration of 15 g/kg. | [139] |
| Fungal Additive | Concentration | Form of Addition | Experimental Approach and Subject Animals | Variables | Effects | Ref. |
|---|---|---|---|---|---|---|
| AO | 3 g/d | Added with 5.6% tallow | 28 Holstein cows | Neutral detergent fiber | Did not stimulate any variables. | [153] |
| AO and AN | 30 mg | Supplemented into 500 mg of TMR, corn silage, oat hay, and alfalfa hay | In vitro analysis | Gas production, DM, CP, ADF, NDF | Significantly increased in gas production, DM, CP, ADF, and NDF for all rations. | [22] |
| AO and AN | 30 mg | Supplemented into oat hay and alfalfa hay | In vitro analysis | Molar proportion of acetate and acetate to propionate ratio | The proportion and ratio of acetate:propionate was increased. | [22] |
| AO and AN | 30 mg | Supplemented into TMR, oat hay, and alfalfa hay | In vitro analysis | Bacterial community | Increased Prevotella and decreased Ruminococcus counts. | [22] |
| AO and SC | 10 g/d SC plus 3 g/d AO | Supplemented into diet (60% rolled barley and 40% timothy hay) | 8 steers | Ruminal fermentation and bacterial counts | Higher concentrations of acetate, propionate, and total VFAs tended to increase ruminal NH3-N concentration and decrease pH. | [25] |
| AO | 2 g/d | Supplemented into diet (grass hay and barley) | 4 sheep | Ruminal fermentation and bacterial counts | Reduction in lactate and propionate in rumen. | [154] |
| AO | 2 g/d | Supplemented into chopped barley straw (plus urea and minerals) | 8 sheep | Digestibility parameters and bacterial counts | Increased initial rate of feed degradation and total bacteria counts. | [151] |
| AO and/or monensin | 500 mg/day | Supplemented into the diet (hay, barley, molasses, fish meal, and mineral/vitamin mix) | In vitro analysis (Rusitec) | Fermentation parameters, microbial community | Increased propionate and reduced butyrate, increased bacterial count, and non-significant reduction in protozoal numbers. | [155] |
| AO extract with alpha-amylase activity | 450 FAU/kg DM | Supplemented into the basal diet | 24 multiparous Holstein cows | Total tract digestion, ruminal fermentation, nitrogen utilization | Increased CP and DM digestibility, isovalerate production, and live weight. | [156] |
| AO fermentation extract (Amaferm®) | 5 mg of AO/mL | Added to bacteria culture media | In vitro analysis | Ruminal bacteria interactions between antimicrobial compounds | Increased the growth rates of the fiber-digesting bacteria and the lactate-utilizing bacteria and diminished the negative effects of chlortetracycline or neomycin compounds. | [24] |
| AO fermentation extract (Amaferm®) | 1.2 g/L | Incubated with ground fibrous feedstuffs with rumen fluid and buffer inoculum | In vitro analysis | Fiber degradation | Increased NDF and ADF degradations. | [157] |
| AO extract with yeast culture | 90 g/d of AO and yeast culture | Supplemented into the basal diet | 4 non-lactating Holstein cows | Nutrient digestibility, ruminal fermentation parameters | Increased digestibility of CP, hemicellulose and DM increased acetate to propionate ratio. | [158] |
| AO fermentation extract (Amaferm®) | 5 g/day | Supplemented into the basal diet | 64 multiparous Chinese Holstein cows | Rumen microbial community and activity | Increased populations of rumen fungi, increased MCP and the activity of carboxymethylcellulase (CMCase). | [159] |
| AO extract with yeast culture | 6.0 and 26 g/kg of DM | Supplemented into a pelleted calf starter | 40 buffalo calves | Nutrient digestibility | Increased total tract digestibility, higher average daily gain, higher digestibility of fiber. | [160] |
| AO culture | 100 μL/50 mL incubation medium | Added to the cultures of ruminal microorganisms | In vitro | Ruminal fermentation | Decreased propionate, butyrate, and total volatile fatty acids. Increased the acetate:propionate ratio. | [161] |
| AO fermentation extract (Amaferm®) | 1.5 g/d | Supplemented into the TMR | 64 lactating cows | Digestibility of CP, NDF, and DM | Increased DMI. | [162] |
| AO culture | 900 mg/kg | Supplemented into the ration including corn, hay, steam bone meal, and wheat bran | 8 crossbreds wethers | Fiber digestion | Increased ADF degradation. | [163] |
| AO fermentation extract non-ionic surfactant | 100 g/d (prepartum) 150 g/d (postpartum) | Supplemented into the TMR | 40 Holstein dairy cows | Dry matter intake | Greater DMI in the transitional period, Increased milk fat content. | [164] |
| AO fermentation extract | 2 g/d | Top-dressed on texturized starter ration | 52 bull calves | Growth rate at weaning age and rumen development | Tended to increase ADG. | [165] |
| AO culture extract | 0.25 mg/mL | Added to the fermentation of a basal ration | In vitro (Rusitec) | Stoichiometry of the rumen fermentation, microbial community | Eliminated the transient fall in pH, increased the acetate:propionate ratio and butyrate proportion, increased ammonia concentration, doubled the number of total viable bacteria, reduced protozoal numbers. | [76] |
| AO culture | 3 g/d | Supplemented into the diet (70% concentrate and 30% forage) | 2 dry and 2 lactating cows | Nutrient utilization | Increased rumen and total tract digestibility of fiber fractions. | [77] |
| AO fermentation extract | 3 g/d | Top-dressing at the morning feeding plus 87 g of ground sorghum | 47 lactating cows (20 primiparous and 26 multiparous) | Feed digestibility | Increased digestibility of DM, CP, NDF, and ADF. | [166] |
| AO fermentation extract | 0.6 g/d | Supplemented into the TMR | 4 Holstein dairy cows | Rumen operation condition | Increased pH, decreased the changing time. | [167] |
| AO fermentation extract | 27 g/d | Added to a supplement based on soybean meal | 6 non-lactating beef cows | Ruminal fermentation | Higher total VFA, pH tended to be lower. | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uwineza, C.; Parchami, M.; Bouzarjomehr, M.; Taherzadeh, M.J.; Mahboubi, A. Recent Developments in the Application of Filamentous Fungus Aspergillus oryzae in Ruminant Feed. Animals 2024, 14, 2427. https://doi.org/10.3390/ani14162427
Uwineza C, Parchami M, Bouzarjomehr M, Taherzadeh MJ, Mahboubi A. Recent Developments in the Application of Filamentous Fungus Aspergillus oryzae in Ruminant Feed. Animals. 2024; 14(16):2427. https://doi.org/10.3390/ani14162427
Chicago/Turabian StyleUwineza, Clarisse, Milad Parchami, Mohammadali Bouzarjomehr, Mohammad J. Taherzadeh, and Amir Mahboubi. 2024. "Recent Developments in the Application of Filamentous Fungus Aspergillus oryzae in Ruminant Feed" Animals 14, no. 16: 2427. https://doi.org/10.3390/ani14162427
APA StyleUwineza, C., Parchami, M., Bouzarjomehr, M., Taherzadeh, M. J., & Mahboubi, A. (2024). Recent Developments in the Application of Filamentous Fungus Aspergillus oryzae in Ruminant Feed. Animals, 14(16), 2427. https://doi.org/10.3390/ani14162427








