Effects of Starve and Shelter Availability on the Group Behavior of Two Freshwater Fish Species (Chindongo demasoni and Spinibarbus sinensis)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Animal Ethics
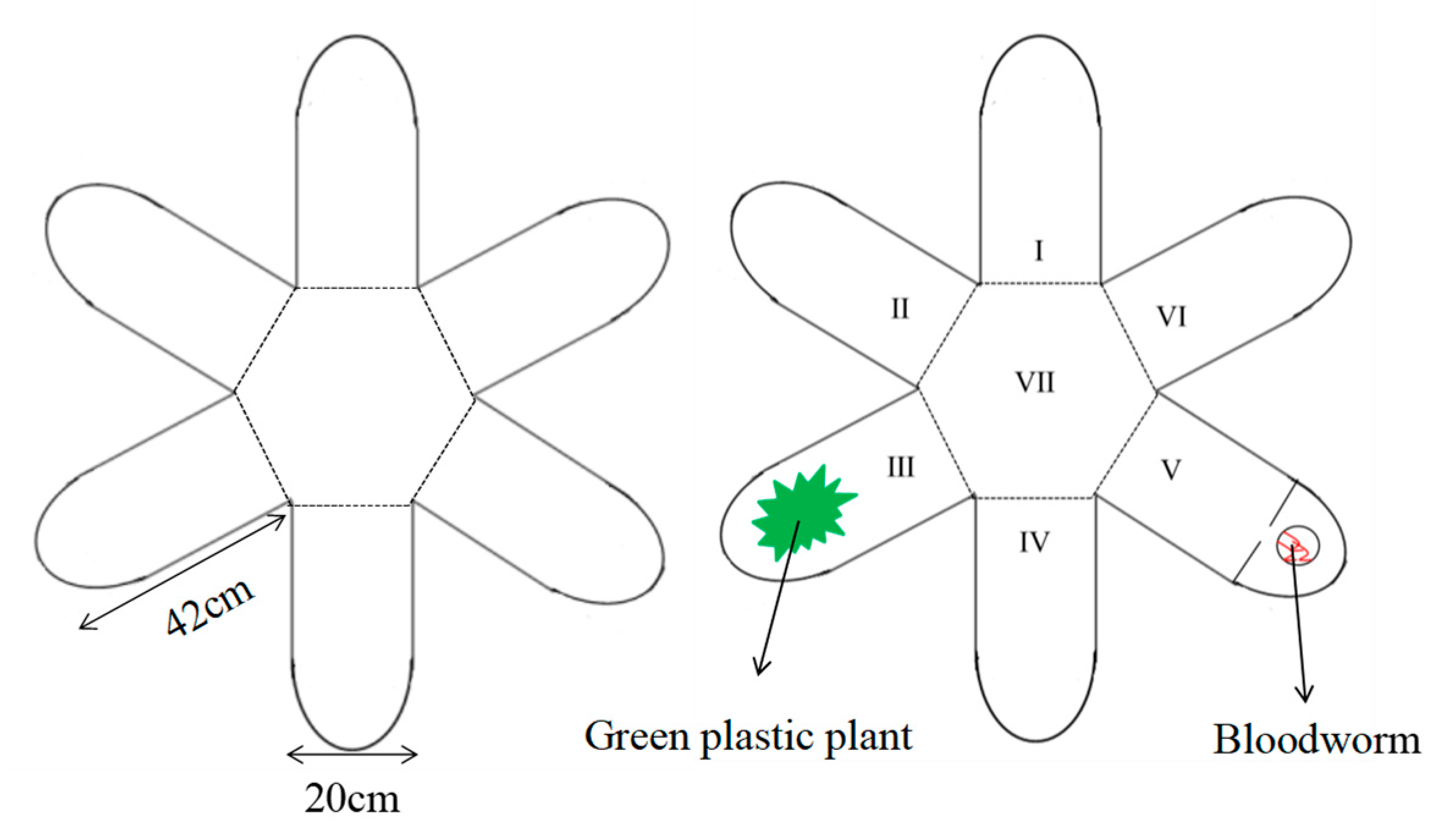
2.2. Experimental Overview
2.3. Experimental Devices and Measurement Methods
2.4. Data Collection and Calculations
2.5. Statistical Analysis
3. Results
3.1. Effects of Simulated Predation Attack, Fasting and Shelter on Dynamics
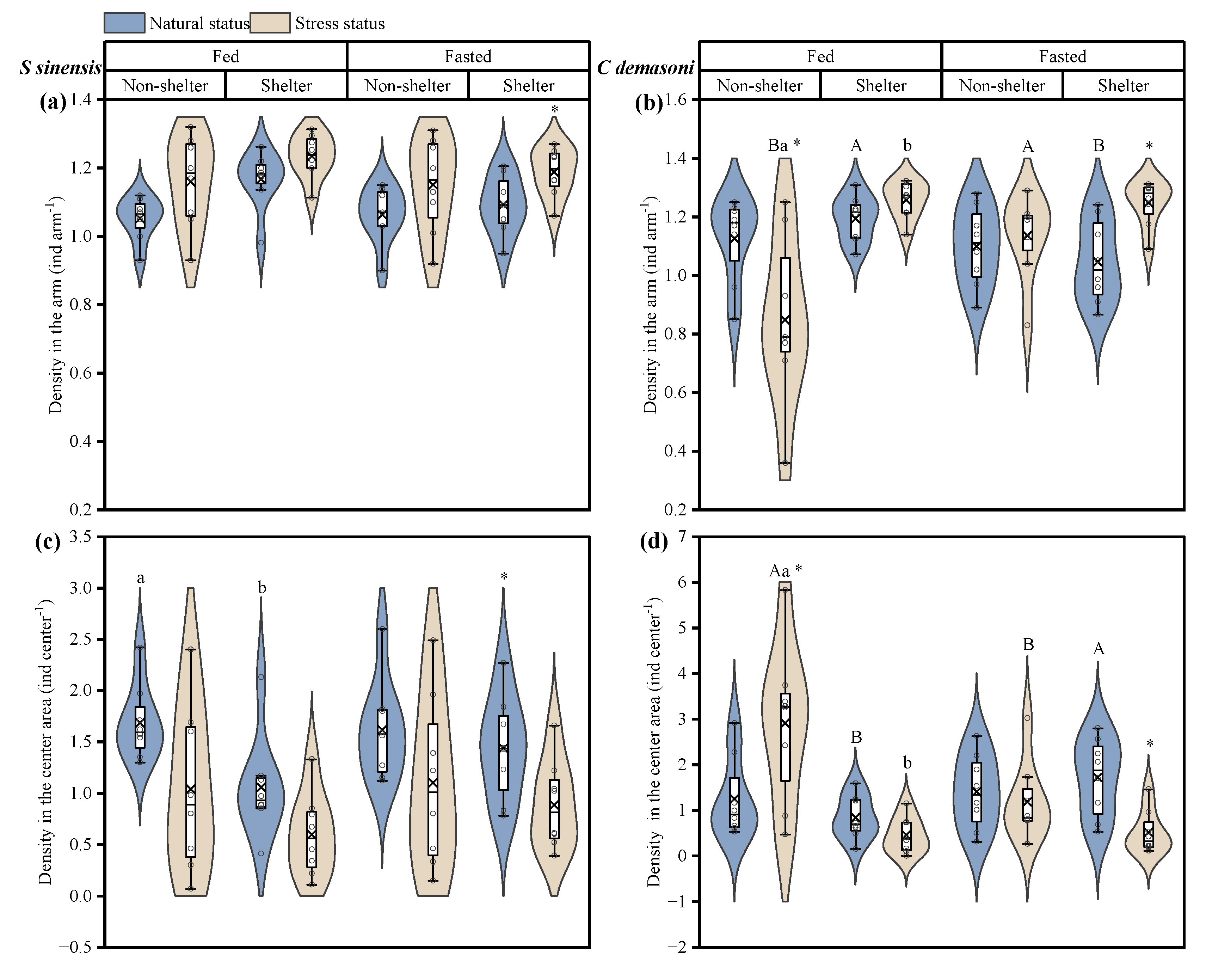
3.2. Effects of Simulated Predation Attack, Fasting and Shelter on Distribution
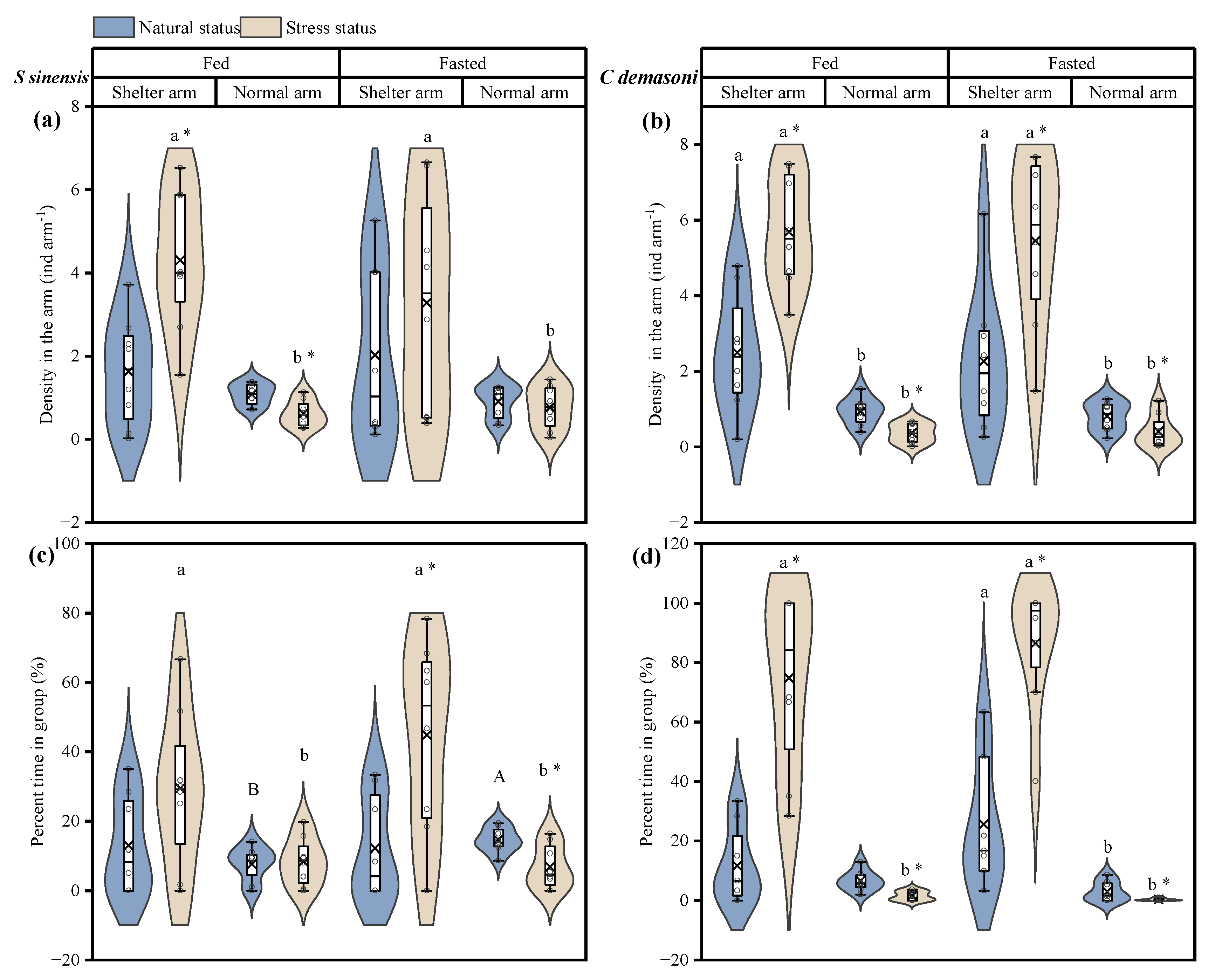
3.3. Effects of Simulated Predation Attack, Fasting and Shelter on Distribution and Dynamics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Creel, S.; Christianson, D. Relationships between direct predation and risk effects. Trends Ecol. Evol. 2008, 23, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.S.; Watts, P.C.; Pottinger, T.G.; Sneddon, L.U. Plasticity of boldness in rainbow trout, Oncorhynchus mykiss: Do hunger and predation influence risk-taking behavior? Horm. Behav. 2012, 615, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, D.; Nicieza, A.G. Predator avoidance behaviour in wild and hatchery-reared brown trout: The role of experience and domestication. J. Fish Biol. 2003, 63, 1565–1577. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Zhu, B.S.; Yu, L.Y.; Liu, D.P.; Wang, F.; Lu, Y.L. Selection of shelter shape by swimming crab (Portunus trituberculatus). Aquac. Rep. 2021, 21, 100908. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Zhu, B.S.; Yu, L.Y.; Wang, F. Shelter Color Selection of Juvenile Swimming Crabs (Portunus trituberculatus). Fishes 2022, 7, 296. [Google Scholar] [CrossRef]
- Blake, C.A.; Andersson, M.L.; Hulthén, K.; Nilsson, P.A.; Brönmark, C. Conspecific boldness and predator species determine predation-risk consequences of prey personality. Behav. Ecol. Sociobiol. 2018, 72, 133. [Google Scholar] [CrossRef]
- Miller, N.; Garnier, S.; Hartnett, A.T.; Couzin, I.D. Both information and social cohesion determine collective decisions in animal groups. Proc. Natl. Acad. Sci. USA 2013, 110, 5263–5268. [Google Scholar] [CrossRef]
- Herbert-Read, J.E. Understanding how animal groups achieve coordinated movement. J. Exp. Biol. 2016, 219, 2971–2983. [Google Scholar] [CrossRef]
- Jolles, J.W.; Boogert, N.J.; Sridhar, V.H.; Couzin, I.D.; Manica, A. Consistent individual differences drive collective behavior and group functioning of schooling fish. Curr. Biol. 2017, 27, 2862–2868. [Google Scholar] [CrossRef]
- Gimeno, E.; Quera, V.; Beltran, F.S.; Dolado, R. Differences in shoaling behavior in two species of freshwater fish (Danio rerio and Hyphessobrycon herbertaxelrodi). J. Comp. Psychol. 2016, 130, 358–368. [Google Scholar] [CrossRef]
- Suriyampola, P.S.; Shelton, D.S.; Shukla, R.; Roy, T.; Bhat, A.; Martins, E.P. Zebrafish social behavior in the wild. Zebrafish 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Godin, J.G.J.; Crossman, S.L. Hunger-dependent predator inspection and foraging behaviours in the threespine stickleback (Gasterosteus aculeatus) under predation risk. Behav. Ecol. Sociobiol. 1994, 34, 359–366. [Google Scholar] [CrossRef]
- McCue, M.D. Starvation physiology: Reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. A 2010, 156, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.P.; Su, X.P.; Wang, F.; Zhong, D.S.; Sun, Y.F. Starvation intensifies the impacts of interspecific interactions on foraging behavior of swimming crab (Portunus trituberculatus). Aquaculture 2019, 504, 22–29. [Google Scholar] [CrossRef]
- Hanson, K.A.; Mauland, B.A.; Shastri, A.; Wisenden, B.D. Yellowtail damselfish Chrysiptera parasema can associate predation risk with the acoustic call of a heterospecific damselfish following pairing with conspecific alarm cues. J. Fish Biol. 2024, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mehner, T.; Wieser, W. Energetics and metabolic correlates of starvation in juvenile perch (Perca fluviatilis). J. Fish Biol. 1994, 45, 325–333. [Google Scholar] [CrossRef]
- Viana, M.T.; D’Abramo, L.R.; Gonzalez, M.A.; García-Suárez, J.V.; Shimada, A.; Vásquez-Peláez, C. Energy and nutrient utilization of juvenile green abalone (Haliotis fulgens) during starvation. Aquaculture 2007, 264, 323–329. [Google Scholar] [CrossRef]
- Gao, L.J.; Chen, L.Q.; Song, B. Effect of starvation and compensatory growth on feeding growth and body biochemical composition in Acipenser schrenckii juveniles. J. Fish. China 2004, 28, 279–284. [Google Scholar]
- Redpath, T.D.; Cooke, S.J.; Suski, C.D.; Arlinghaus, R.; Couture, P.; Wahl, D.H.; Philipp, D.P. The metabolic and biochemical basis of vulnerability to recreational angling after three generations of angling-induced selec tion in a teleost fish. Can. J. Fish. Aquat. Sci. 2010, 67, 1983–1992. [Google Scholar] [CrossRef]
- Ariyomo, T.O.; Watt, P.J. Effect of hunger level and time of day on boldness and aggression in the zebra fish Danio rerio. J. Fish Biol. 2015, 86, 1852–1859. [Google Scholar] [CrossRef]
- Dar, S.A.; Srivastava, P.P.; Varghese, T.; Gupta, S.; Gireesh-Babu, P.; Krishna, G. Effects of starvation and refeeding on expression of ghrelin and leptin gene with variations in metabolic parameters in Labeo rohita fingerlings. Aquaculture 2018, 484, 219–227. [Google Scholar] [CrossRef]
- Navarro, I.; Gutierrez, J. Fasting and starvation. Biochem. Mol. Biol. Fishes 1995, 4, 393–434. [Google Scholar]
- MacNutt, M.J.; Hinch, S.G.; Farrell, A.P.; Topp, S. The effect of temperature and acclimation period on repeat swimming performance in cutthroat trout. J. Fish Biol. 2004, 65, 342–353. [Google Scholar] [CrossRef]
- Smart, I.E.; Cuthill, I.C.; Scott-Samuel, N.E. In the corner of the eye: Camouflaging motion in the peripheral visual field. Proc. R. Soc. B 2020, 287, 20192537. [Google Scholar] [CrossRef]
- Ding, R.H. Sichuan Ichthyography; Sichuan Science and Technology Publishing House: Chengdu, China, 1994; pp. 139–319. [Google Scholar]
- Li, S.; Konings, A.F.; Stauffer, J.R. A revision of the Pseudotropheus elongatus species group (Teleostei: Cichlidae) with description of a new genus and seven new species. Zootaxa 2016, 4168, 353–381. [Google Scholar] [CrossRef]
- Barluenga, M.; Stolting, K.N.; Salzburger, W.; Muschick, M.; Meyer, A. Sympatric speciation in Nicaraguan crater lake cichlid fish. Nature 2006, 439, 719–723. [Google Scholar] [CrossRef]
- Xia, J.G.; Liu, X.; Huang, Y. The link between chemical alarm cue-induced behavioral responses and personality in Rhodeus ocellatus. Acta Ecol. Sin. 2019, 39, 6425–6432. [Google Scholar]
- Delcourt, J.; Miller, N.Y.; Couzin, I.D.; Garnier, S. Methods for the effective study of collective behavior in a radial arm maze. Behav. Res. Methods 2018, 50, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Krause, J. Differential fitness returns in relation to spatial position in groups. Biol. Rev. Camb. Philos. Soc. 1994, 69, 187–206. [Google Scholar] [CrossRef]
- Krause, S.; Wilson, A.D.M.; Ramnarine, I.W.; Herber-Read, J.E.; Clément, R.J.G.; Krause, J. Guppies: Occupy consistent positions in social networks: Mechanisms andconsequences. Behav. Ecol. 2017, 28, 429–438. [Google Scholar] [CrossRef]
- Yang, Y.; Ling, H.; Fu, S.J.; Zeng, L.Q. Effects of ecological context and metabolic phenotype on collective behaviour of qingbo Spinibarbus sinensis. Acta Ecol. Sin. 2021, 41, 4447–4459. [Google Scholar]
- Martínez, M.; Guderley, H.; Dutil, J.D.; Winger, P.D.; He, P.; Walsh, S.J. Condition, prolonged swimming performance and muscle metabolic capacities of cod Gadus morhua. J. Exp. Biol. 2003, 206, 503–511. [Google Scholar] [CrossRef]
- Cai, L.; Fang, M.; Johnson, D.; Lin, S.M.; Tu, Z.Y.; Liu, G.Y.; Huang, Y.P. Interrelationships between feeding, food deprivation and swimming performance in juvenile grass carp. Aquat. Biol. 2014, 20, 69–76. [Google Scholar] [CrossRef]
- Qiu, H.X.; Liu, W.M.; Yan, Y.L.; Long, J.; Xie, X.J. Effects of waterborne cadmium exposure on Spinibarbus sinensis hepatopancreas and kidney: Mitochondrial cadmium accumulation and respiratory metabolism. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 248, 109115. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Fu, S.J.; Cao, Z.D. Habitat-specific anti-predator behavior variation among pale chub (Zacco platypus) along a river. Mar. Freshw. Behav. Physiol. 2015, 48, 267–278. [Google Scholar] [CrossRef]
- Tang, Z.H.; Wu, Q.; Fu, S.J. Inspection behaviour and interindividual cooperation in juvenile qingbo: The effects of prior predator exposure and food deprivation. J. Ethol. 2018, 36, 181–190. [Google Scholar] [CrossRef]
- Zhao, M.G.; Feng, G.P.; Wang, H.H.; Shen, C.C.; Fu, Y.L.; Zhang, Y.P.; Zhang, H.X.; Yao, Y.; Chen, J.H.; Xu, W.K. The Influence of Shelter Type and Coverage on Crayfish (Procambarus clarkii) Predation by Catfish (Silurus asotus): A Controlled Environment Study. Animals 2024, 14, 1147. [Google Scholar] [CrossRef]
- Pohlmann, K.; Grasso, F.W.; Breithaupt, T. Tracking wakes: The nocturnal predatory strategy of piscivorous catfish. Proc. Natl. Acad. Sci. USA 2001, 98, 7371–7374. [Google Scholar] [CrossRef]
- Millidine, K.J.; Armstrong, J.D.; Metcalfe, N.B. Presence of shelter reduces maintenance metabolism of juvenile salmon. Funct. Ecol. 2006, 20, 839–845. [Google Scholar] [CrossRef]
- Fan, J. The Relationships between Personality and Cognitive Ability and Evasion Ability of Freshwater Fish Species; Chongqing Normal University: Chongqing, China, 2022; pp. 1–56. [Google Scholar]
- Bartolini, T.; Butail, S.; Porfiri, M. Temperature influences sociality and activity of freshwater fish. Environ. Fishes 2015, 98, 825–832. [Google Scholar] [CrossRef]
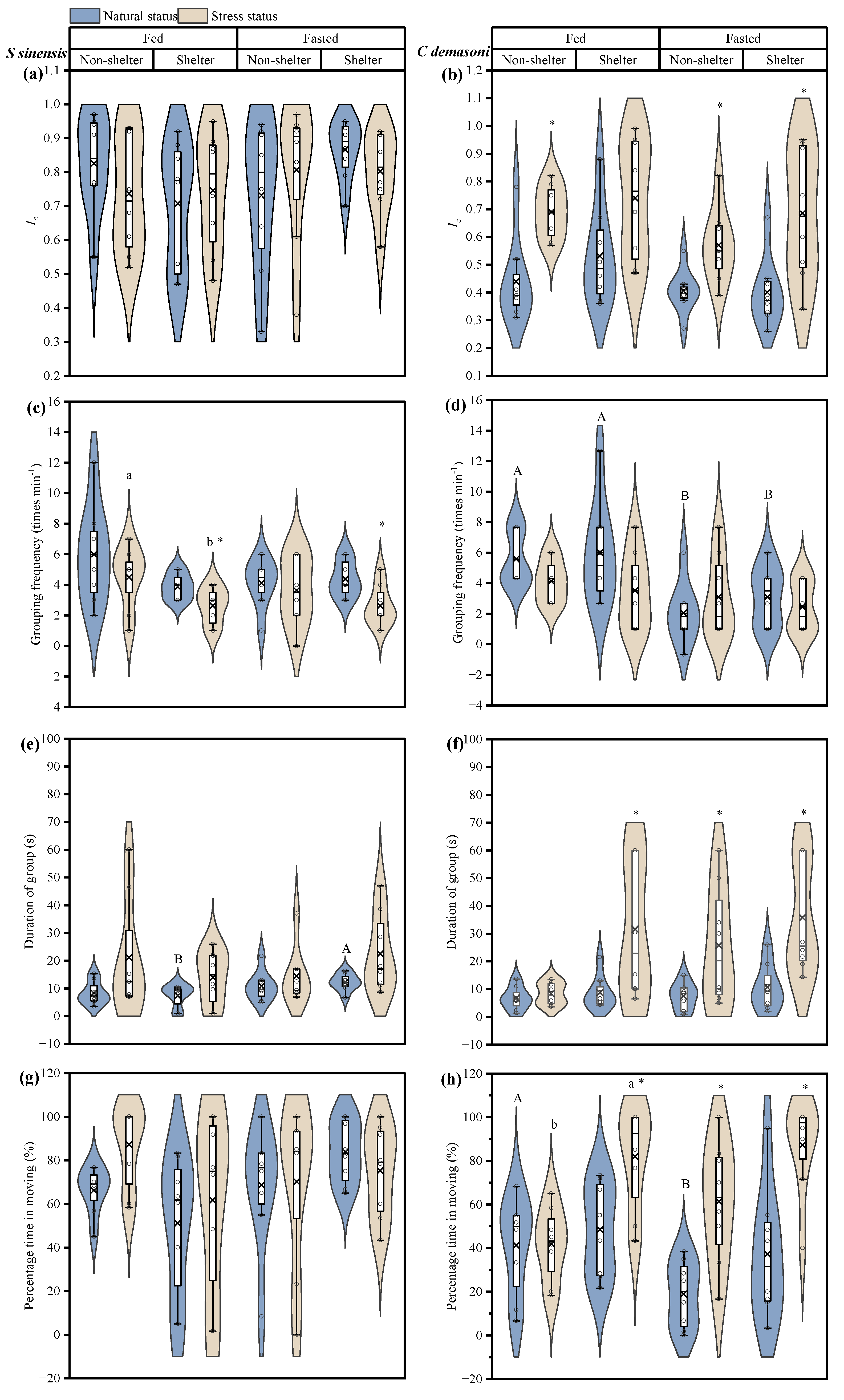
| Ic | Grouping Frequency (times/min−1) | Duration of Group (s) | Percentage of Time in Group (%) | |||||
|---|---|---|---|---|---|---|---|---|
| S. sinensis | C. demasoni | S. sinensis | C. demasoni | S. sinensis | C. demasoni | S. sinensis | C. demasoni | |
| Predation, P | F1,28 = 0.118 p = 0.734 | F1,56 = 32.191 p < 0.001 * | F1,28 = 11.024 p = 0.003 * | F1,28 = 1.954 p = 0.173 | F1,28 = 11.188 p = 0.003 * | F1,28 = 22.737 p < 0.001 * | F1,28 = 0.624 p = 0.436 | F1,28 = 36.843 p < 0.001 * |
| Fasting, F | F1,28 = 0.818 p = 0.374 | F1,56 = 4.494 p = 0.038 * | F1,28 = 1.127 p = 0.297 | F1,28 = 14.386 p < 0.001 * | F1,28 = 0.420 p = 0.522 | F1,28 = 2.660 p = 0.114 | F1,28 = 0.511 p = 0.480 | F1,28 = 0.144 p = 0.707 |
| Shelter, S | F1,28 = 0.010 p = 0.921 | F1,56 = 2.478 p = 0.121 | F1,28 = 5.024 p = 0.033 * | F1,28 = 0.030 p = 0.864 | F1,28 = 0.080 p = 0.779 | F1,28 = 7.545 p = 0.010 * | F1,28 = 0.624 p = 0.436 | F1,28 = 14.208 p < 0.001 * |
| P × F | F1,28 = 0.320 p = 0.576 | F1,56 = 0.004 p = 0.951 | F1,28 = 0.110 p = 0.742 | F1,28 = 3.231 p = 0.083 | F1,28 = 0.544 p = 0.467 | F1,28 = 1.927 p = 0.176 | F1,28 = 3.109 p = 0.089 | F1,28 = 7.808 p = 0.009 |
| P × S | F1,28 = 0.010 p = 0.921 | F1,56 = 0.233 p = 0.631 | F1,28 = 0.441 p = 0.512 | F1,28 = 2.553 p = 0.121 | F1,28 = 0.053 p = 0.819 | F1,28 = 3.642 p = 0.067 | F1,28 = 1.032 p = 0.318 | F1,28 = 3.749 p = 0.063 |
| F × S | F1,28 = 1.274 p = 0.269 | F1,56 = 0.041 p = 0.840 | F1,28 = 2.352 p = 0.136 | F1,28 = 0.268 p = 0.609 | F1,28 = 2.954 p = 0.097 | F1,28 = 0.591 p = 0.448 | F1,28 = 3.335 p = 0.078 | F1,28 = 0.014 p = 0.905 |
| P × F × S | F1,28 = 5.688 p = 0.024 * | F1,56 = 1.026 p = 0.351 | F1,28 = 0.992 p = 0.328 | F1,28 = 0.000 p = 1.000 | F1,28 = 2.232 p = 0.146 | F1,27 = 1.166 p = 0.289 | F1,28 = 0.000 p = 0.992 | F1,28 = 1.482 p = 0.234 |
| Density in the Arm (ind arm−1) | Density in the Center Area (ind center−1) | |||
|---|---|---|---|---|
| S. sinensis | C. demasoni | S. sinensis | C. demasoni | |
| Predation, P | F1,28 = 15.088 p = 0.001 * | F1,28 = 0.029 p = 0.865 | F1,28 = 15.151 p = 0.001 * | F1,28 = 0.037 p = 0.849 |
| Fasting, F | F1,28 = 1.540 p = 0.225 | F1,28 = 0.420 p = 0.552 | F1,28 = 1.374 p = 0.251 | F1,28 = 0.413 p = 0.526 |
| Shelter, S | F1,28 = 7.076 p = 0.013 * | F1,28 = 11.000 p = 0.003 * | F1,28 = 6.781 p = 0.015 * | F1,28 = 10.874 p = 0.003 * |
| P × F | F1,28 = 0.010 p = 0.921 | F1,28 = 11.633 p = 0.002 * | F1,28 = 0.005 p = 0.945 | F1,28 = 11.627 p = 0.002 * |
| P × S | F1,28 = 0.125 p = 0.921 | F1,28 = 14.595 p = 0.001 * | F1,28 = 0.071 p = 0.792 | F1,28 = 14.514 p = 0.001 * |
| F × S | F1,28 = 1.672 p = 0.207 | F1,28 = 6.669 p = 0.015 * | F1,28 = 1.448 p = 0.239 | F1,28 = 6.581 p = 0.016 * |
| P × F × S | F1,28 = 0.285 p = 0.597 | F1,28 = 1.781 p = 0.193 | F1,28 = 0.168 p = 0.685 | F1,28 = 1.782 p = 0.193 |
| Density in Arm (ind arm−1) | Percentage of Time in Group (%) | |||
|---|---|---|---|---|
| S. sinensis | C. demasoni | S. sinensis | C. demasoni | |
| Predation, P | F1,28 = 6.257 p = 0.018 * | F1,28 = 28.172 p < 0.001 * | F1,28 = 10.018 p = 0.004 * | F1,27.55 = 87.856 p < 0.001 * |
| Fasting, F | F1,28 = 0.185 p = 0.670 | F1,28 = 0.129 p = 0.490 | F1,28 = 1.418 p = 0.244 | F1,27.98 = 1.281 p = 0.267 |
| Arm type, A | F1,28 = 26.508 p < 0.001 * | F1,28 = 73.862 p < 0.001 * | F1,28 = 13.698 p = 0.001 * | F1,27.98 = 98.440 p < 0.001 * |
| P × F | F1,28 = 0.677 p = 0.418 | F1,28 = 0.025 p = 0.875 | F1,28 = 0.346 p = 0.561 | F1,27.55 = 0.002 p = 0.967 |
| P × A | F1,28 = 11.502 p = 0.002 * | F1,28 = 51.944 p < 0.001 * | F1,28 = 17.805 p < 0.001 * | F1,27.55 = 113.310 p < 0.001 * |
| F × A | F1,28 = 166 p = 0.687 | F1,28 = 0.063 p = 0.803 | F1,28 = 0.306 p = 0.584 | F1,27.98 = 2.697 p = 0.112 |
| P × F × A | F1,28 = 1.683 p = 0.205 | F1,28 = 0.035 p = 0.852 | F1,28 = 3.431 p = 0.075 | F1,27.55 = 0.189 p = 0.667 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Li, J.; Fu, S. Effects of Starve and Shelter Availability on the Group Behavior of Two Freshwater Fish Species (Chindongo demasoni and Spinibarbus sinensis). Animals 2024, 14, 2429. https://doi.org/10.3390/ani14162429
Li W, Li J, Fu S. Effects of Starve and Shelter Availability on the Group Behavior of Two Freshwater Fish Species (Chindongo demasoni and Spinibarbus sinensis). Animals. 2024; 14(16):2429. https://doi.org/10.3390/ani14162429
Chicago/Turabian StyleLi, Wuxin, Jiaqian Li, and Shijian Fu. 2024. "Effects of Starve and Shelter Availability on the Group Behavior of Two Freshwater Fish Species (Chindongo demasoni and Spinibarbus sinensis)" Animals 14, no. 16: 2429. https://doi.org/10.3390/ani14162429
APA StyleLi, W., Li, J., & Fu, S. (2024). Effects of Starve and Shelter Availability on the Group Behavior of Two Freshwater Fish Species (Chindongo demasoni and Spinibarbus sinensis). Animals, 14(16), 2429. https://doi.org/10.3390/ani14162429







