Identification of Aeromonas veronii as the Pathogen Associated with Massive Mortality in Bronze Gudgeon (Coreius heterodon)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Bronze Gudgeon
2.2. Histological Analysis
2.3. Bacterial Isolation
2.4. Identification of the Bacteria
2.5. WH10 Challenge Experiment
2.6. Susceptibility to Antibiotics Assay
2.7. Screening of Virulence-Related Genes
2.8. Blood Parameters
2.9. Statistical Analysis
3. Results
3.1. Clinical Signs of the Disease
3.2. Pathological Features
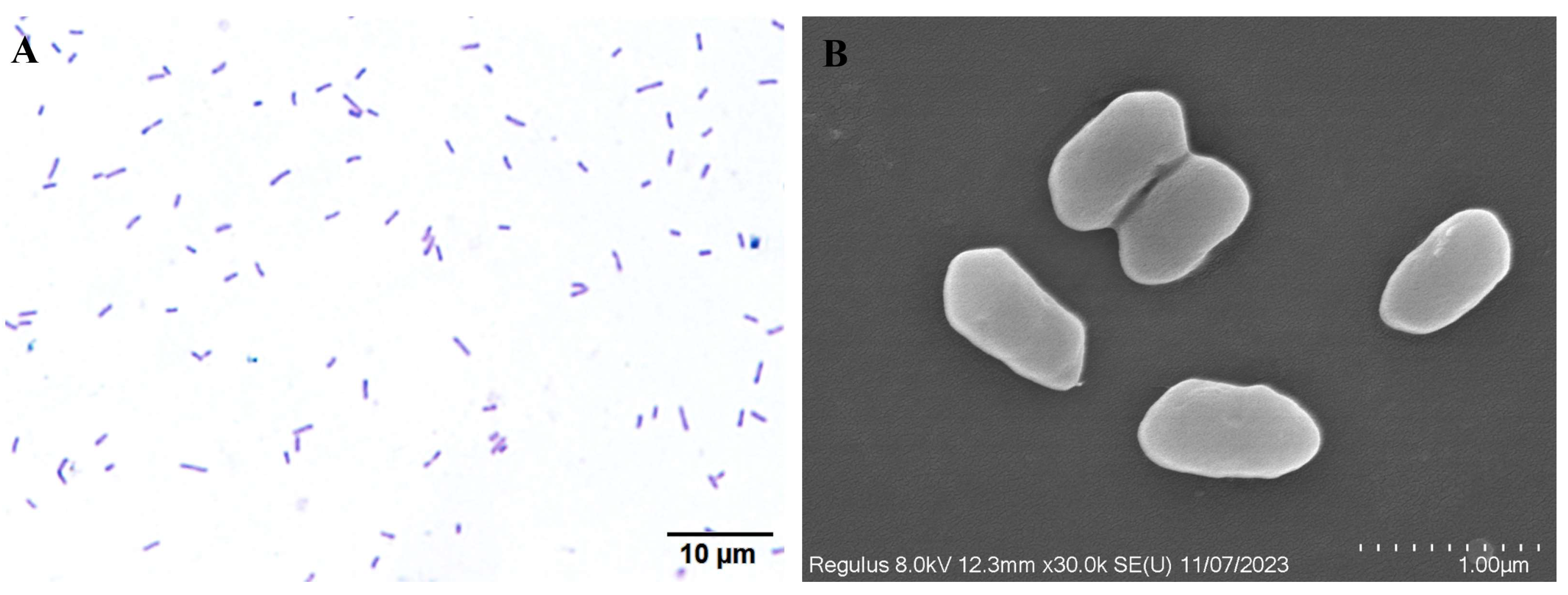
3.3. Morphological Characterization of WH10
3.4. Sequence Analysis of the 16S rRNA Gene
3.5. Virulence Gene Detection
3.6. Bacterial Biochemical Identification
3.7. Antibiotic Susceptibility Analysis
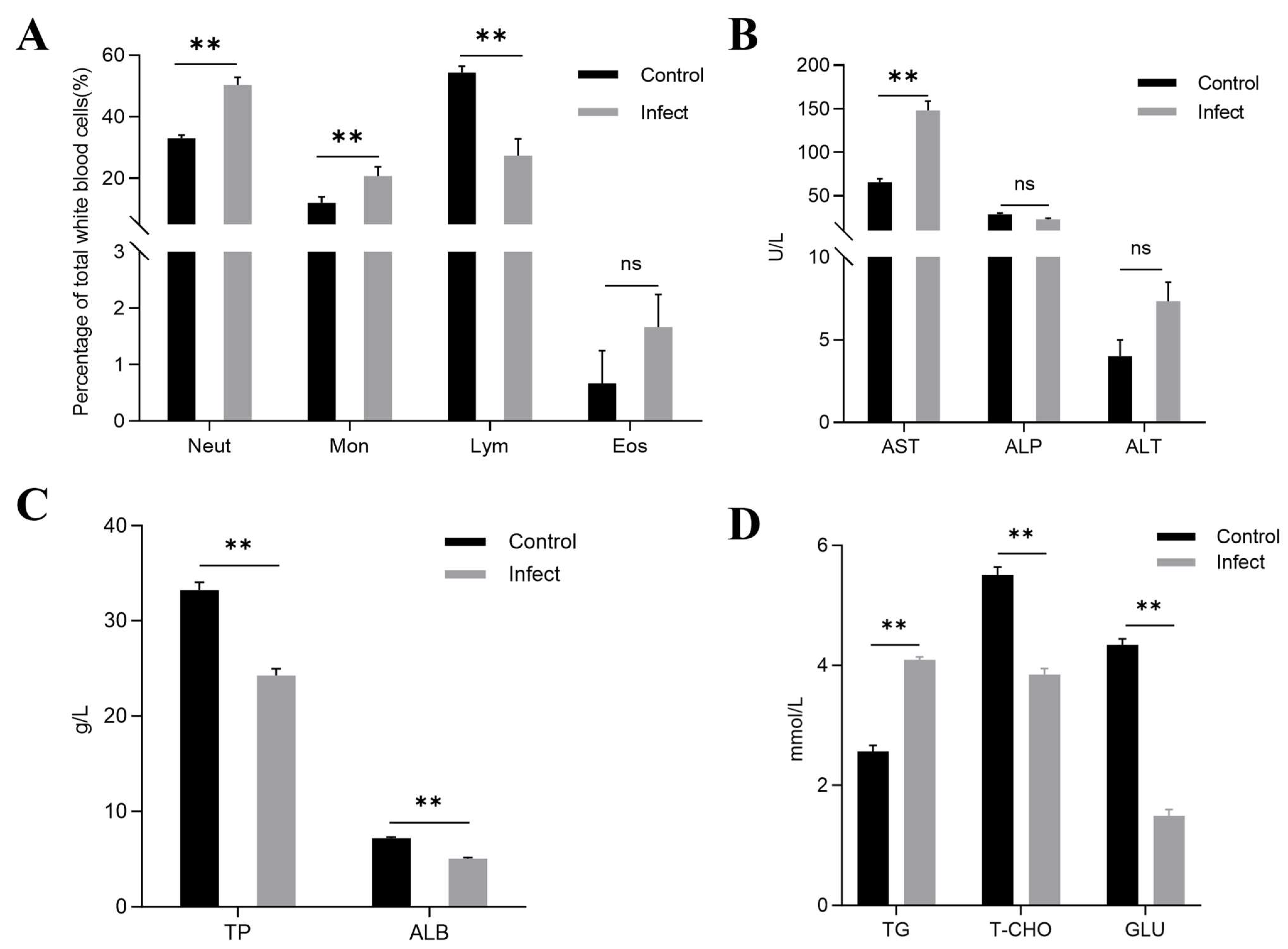
3.8. Differential Leukocyte Counts (DLC)
3.9. Serum Biochemical Analysis
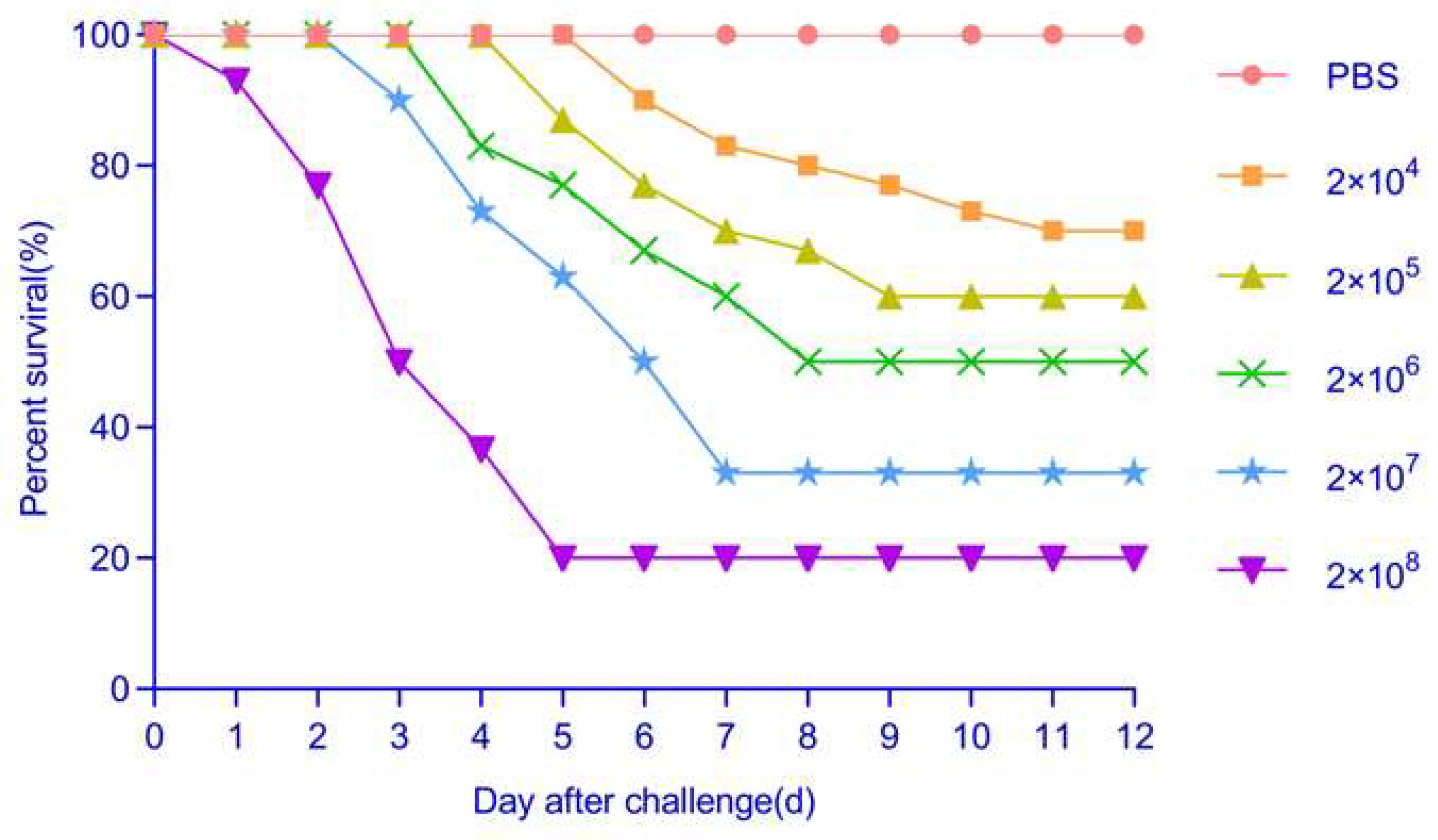
3.10. Pathogenicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, S.R.; Gao, X.; Li, M.Z.; Ma, B.S.; Liu, H.Z. Interannual variations of the fish assemblage in the transitional zone of the Three Gorges Reservoir: Persistence and stability. Environ. Biol. Fishes 2012, 93, 295–304. [Google Scholar] [CrossRef]
- Liu, H.Y.; Xiong, F.; Duan, X.B.; Liu, S.P.; Chen, D.Q. Estimating population parameters and abundance of Coreius heterodon in Jiangjin section of the upper Yangtze River. J. Nat. Resour. 2016, 31, 1420–1428. (In Chinese) [Google Scholar]
- Chen, B.Y.; Luo, H.H.; Yang, Q.R.; Huang, W.; Han, H.L. The eco-hydrological demand research on Coreius heterodon reproduction in the upper Yangtze River reserve. Appl. Mech. Mater. 2014, 641, 226–231. [Google Scholar] [CrossRef]
- Li, T.; Mo, K.L.; Wang, J.; Chen, Q.W.; Zhang, J.Y.; Zeng, C.J.; Zhang, H.; Yang, P.S. Mismatch between critical and accumulated temperature following river damming impacts fish spawning. Sci. Total Environ. 2021, 756, 144052. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Wang, G.Q.; Wu, H.L.; Chen, P.; Li, D.F.; Jin, Z.W.; Guo, C.; Ren, S.; Gao, Y. Changes in flow and sediment transport caused by cascade hydropower in the upper reaches of Yangtze River and their influence on spawning of Coreius heterodon. Catena 2024, 235, 107622. [Google Scholar] [CrossRef]
- Xu, D.D.; Li, P.; Zhang, Y.G.; Peng, Z.G. Comparative study of the complete mitochondrial genomes of the bronze gudgeon (Coreius heterodon) and largemouth bronze gudgeon (Coreius guichenoti). Mitochondrial DNA 2013, 24, 189–190. [Google Scholar] [CrossRef]
- Cai, S.W.; Ni, Z.H.; Li, Y.F.; Shen, Z.W.; Xiong, Z.T.; Zhang, Y.; Zhou, Y.T. Metals in the tissues of two fish species from the rare and endemic fish nature reserve in the upper reaches of the Yangtze River, China. Bull. Environ. Contam. Toxicol. 2012, 88, 922–927. [Google Scholar] [CrossRef]
- Luo, Y.P.; Huang, Q.D.; Zhang, Y.R.; Liu, S.T.; Wang, W. Comparison of the body proximate compositions of juvenile bronze gudgeon (Coreius heterodon) and largemouth bronze gudgeon (C. guichenoti) in the upstream region of the Yangtze River. SpringerPlus 2013, 2, 75. [Google Scholar] [CrossRef]
- Hickman-Brenner, F.W.; Macdonald, K.L.; Steigerwalt, A.G.; Fanning, G.R.; Brenner, D.J.; Farmer, J.J. Aeromonas veronii, a new ornithine decarboxylase-positive species that may cause diarrhea. J. Clin. Microbiol. 1987, 25, 900–906. [Google Scholar] [CrossRef]
- Liu, W.Z.; Zhang, Y.C.; Ma, J.; Jiang, N.; Fan, Y.D.; Zhou, Y.; Cain, K.; Yi, M.S.; Jia, K.T.; Wen, H.; et al. Determination of a novel parvovirus pathogen associated with massive mortality in adult tilapia. PLoS Pathog. 2020, 16, e1008765. [Google Scholar] [CrossRef]
- Arroyo, E.; Enríquez, L.; Sánchez, A.; Ovalle, M.; Olivas, A. Scanning electron microscopy of bacteria Tetrasphaera duodecadis. Scanning 2014, 36, 547–550. [Google Scholar] [CrossRef]
- Hu, X.W.; Xiao, Z.D.; Li, B.; Xue, M.Y.; Jiang, N.; Fan, Y.D.; Chen, P.; Qi, F.; Kong, X.H.; Zhou, Y. Isolation, identification, and characterization of Aeromonas veronii from Chinese soft-shelled turtle (Trionyx sinensis). Microorganisms 2023, 11, 1304. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Zhang, H.P.; Xu, G.Y.; Muhamamda, I.; Dong, W.L.; Wang, Y.M.; Kong, L.C.; Ma, H.X. Virulence and drug resistance of Aeromonas veronii isolated from shellfish. Thai J Vet Med. 2021, 51, 21–27. [Google Scholar] [CrossRef]
- Yuan, G.L.; Zhu, L.; Jiang, X.Y.; Zhang, J.; Pei, C.; Zhao, X.L.; Li, L.; Kong, X.H. Diagnosis of co-infection with white spot syndrome virus and Aeromonas veronii in red swamp crayfish Procambarus clarkii. Aquaculture 2021, 532, 736010. [Google Scholar] [CrossRef]
- Li, T.; Raza, S.H.A.; Yang, B.T.; Sun, Y.F.; Wang, G.Q.; Sun, W.W.; Qian, A.D.; Wang, C.F.; Kang, Y.H.; Shan, X.F. Aeromonas veronii infection in commercial freshwater fish: A potential threat to public health. Animals 2020, 10, 608. [Google Scholar] [CrossRef]
- Wang, B.T.; Mao, C.; Feng, J.; Li, Y.; Hu, J.M.; Jiang, B.; Gu, Q.H.; Su, Y.L. A First report of Aeromonas veronii infection of the sea bass, Lateolabrax maculatus in China. Front. Vet Sci. 2021, 7, 600587. [Google Scholar] [CrossRef]
- Reed, L.J. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Rahman, M.; Colque-Navarro, P.; Kuhn, I.; Huys, G.; Swings, J.; Mollby, R. Identification and characterization of pathogenic Aeromonas veronii biovar sobria associated with epizootic ulcerative syndrome in fish in Bangladesh. Appl. Environ. Microb. 2002, 68, 650–655. [Google Scholar] [CrossRef]
- Gui, M.; Wu, R.; Liu, L.; Wang, S.; Zhang, L.; Li, P. Effects of quorum quenching by AHL lactonase on AHLs, protease, motility and proteome patterns in Aeromonas veronii LP-11. Int. J. Food Microbiol. 2017, 252, 61–68. [Google Scholar] [CrossRef]
- Fernández-Bravo, A.; Figueras, M.J. An Update on the genus Aeromonas: Taxonomy, epidemiology, and pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef]
- Abd-El-Malek, A.M. Incidence and virulence characteristics of Aeromonas spp. in fish. Vet World. 2017, 10, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, S.B.; Alarcon, M.F.; Ballaben, A.S.; Harakava, R.; Galetti, R.; Guimarães, M.C.; Natori, M.M.; Takahashi, L.S.; Ildefonso, R.; Rozas-Serri, M. First report of Aeromonas veronii as an emerging bacterial pathogen of farmed Nile Tilapia (Oreochromis niloticus) in Brazil. Pathogens 2023, 8, 1020. [Google Scholar] [CrossRef]
- Haitham, H.M.; Eric, P. Winter kill in intensively stocked channel catfish (Ictalurus punctatus): Coinfection with Aeromonas veronii, Streptococcus parauberis and Shewanella putrefaciens. J. Fish Dis. 2018, 9, 1339–1347. [Google Scholar]
- Chen, F.; Sun, J.F.; Han, Z.R.; Yang, X.J.; Xian, J.A.; Lv, A.J.; Hu, X.C.; Shi, H.Y. Isolation, identification and characteristics of Aeromonas veronii from diseased crucian carp (Carassius auratus gibelio). Front. Microbiol. 2019, 10, 2742. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.K.; Parida, S.N.; Kumar, V.; Swain, H.S.; Parida, P.K.; Bisai, K.; Dhar, S.; Das, B.K. Aeromonas veronii is a lethal pathogen isolated from gut of infected Labeo rohita: Molecular insight to understand the bacterial virulence and its induced host immunity. Pathogens 2023, 12, 598. [Google Scholar] [CrossRef]
- Reyes-Rodríguez, N.E.; Salgado-Miranda, C.; Flores-Valle, I.T.; González-Gómez, M.; Soriano-Vargas, E.; Peláez-Acero, A.; Vega-Sánchez, V. Molecular identification and virulence potential of the genus Aeromonas isolated from wild rainbow trout (Oncorhynchus mykiss) in Mexico. J. Food Prot. 2019, 10, 1706–1713. [Google Scholar] [CrossRef]
- Cannon, M.S.; Mollenhauer, H.H.; Eurell, T.E.; Lewis, D.H.; Cannon, A.M.; Tompkins, C. An ultrastructural study of the leucocytes of the channel fish, Ictalurus punctatus. J. Morphol. 1980, 164, 1–23. [Google Scholar] [CrossRef]
- Zarejabad, A.M.; Jalali, M.A.; Sudagar, M.; Pouralimotlagh, S. Hematology of great sturgeon (Huso huso Linnaeus, 1758) juvenile exposed to brackish water environment. Fish Physiol. Biochem. 2010, 36, 655–659. [Google Scholar] [CrossRef]
- Dalmo, R.A.; Ingebrigtsen, K.; Bøgwald, J. Non-specific defence mechanisms in fish, with particular reference to the reticuloendothelial system (RES). J. Fish Dis. 1997, 20, 241–273. [Google Scholar] [CrossRef]
- Kühlwein, H.; Merrifield, D.L.; Rawling, M.D.; Foey, A.D.; Davies, S.J. Effects of dietary β-(1,3) (1,6)-D-glucan supplementation on growth performance, intestinal morphology and haemato-immunological profile of mirror carp (Cyprinus carpio L.). J. Anim. Physiol. Anim. Nutr. 2014, 98, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Rattanachan, S.; Bunnajirakul, S.; Punyadarsaniya, D. Effect of pre-supplementation with Pleurotus sajor-caju crude extracts on body weight and consequence responses of leukocytes and immune organs in fancy carp following inoculation with Aeromonas veronii. Vet. World 2020, 13, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.D.; Li, X.D.; Xue, M.Y.; Zhang, M.W.; Liu, W.; Fan, Y.D.; Chen, X.H.; Chu, Z.P.; Gong, F.L.; Zeng, L.B.; et al. Vibrio metschnikovii, a potential pathogen in freshwater-cultured hybrid sturgeon. Animals 2022, 12, 1101. [Google Scholar] [CrossRef]
- Charlie-Silva, I.; Klein, A.; Gomes, J.M.M.; Prado, E.J.R.; Moraes, A.C.; Eto, S.F.; Fernandes, D.C.; Fagliari, J.J.; Junior, J.D.C.; Lima, C.; et al. Acute-phase proteins during inflammatory reaction by bacterial infection: Fish-model. Sci. Rep. 2019, 9, 4776. [Google Scholar] [CrossRef]
- Javed, M.; Ahmad, M.I.; Usmani, N.; Ahmad, M. Multiple biomarker responses (serum biochemistry, oxidative stress, genotoxicity and histopathology) in Channa punctatus exposed to heavy metal loaded waste. Sci. Rep. 2017, 7, 1675. [Google Scholar] [CrossRef] [PubMed]
- van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Phar. 2003, 13, 57–149. [Google Scholar] [CrossRef] [PubMed]
- Bukha, K.K.; Mahgiubi, S.A.M.; Elbahi, A.M.; Sharif, E.A.; Showehdi, M.L.; Ahmed, M.O.; Kammon, A.M.; Abouzeed, Y.M. Pathological lesions associated with Vibrio infection in Atlantic horse mackerel (Trachurus trachurus L.; 1758) from the western coast of Tripoli, Libya. Open Vet. J. 2023, 13, 327–336. [Google Scholar]
- Smith, P. Accuracy, precision and meaning of antimicrobial agent susceptibility testing of bacteria associated with fish diseases. Aquaculture 2001, 196, 253–266. [Google Scholar] [CrossRef]
- Shameena, S.S.; Kumar, K.; Kumar, S.; Rathore, G. Characteristics of Aeromonas veronii biovars isolated from infected freshwater goldfish (Carassius auratus). Aquaculture 2020, 518, 734819. [Google Scholar] [CrossRef]
- Jagoda, S.S.; Wijewardana, T.G.; Arulkanthan, A.; Igarashi, Y.; Tan, E.; Kinoshita, S.; Watabe, S.; Asakawa, S. Characterization and antimicrobial susceptibility of motile aeromonads isolated from freshwater ornamental fish showing signs of septicaemia. Dis. Aquat.Org. 2014, 109, 127–137. [Google Scholar] [CrossRef]
- Majeed, S.; De Silva, L.A.D.S.; Kumarage, P.M.; Heo, G.J. Occurrence of potential virulence determinants in Aeromonas spp. isolated from different aquatic environments. J. Appl. Microbiol. 2023, 134, lxad031. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, X.R.; Li, J.; Li, G.Y.; Liu, Z.P.; Mo, Z.L. Identification and virulence properties of Aeromonas veronii Bv. Sobria isolates causing an ulcerative syndrome of loach Misgurnus anguillicaudatus. J. Fish Dis. 2016, 39, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Kozlova, E.V.; Chopra, A. Role of various enterotoxins in Aeromonas hydrophila-induced gastroenteritis: Generation of enterotoxin gene-deficient mutants and evaluation of their enterotoxic activity. Infect. Immun. 2002, 70, 1924–1935. [Google Scholar] [CrossRef]
- Chen, X.; Schauder, S.; Potier, N.; Van Dorsselaer, A.; Pelczer, I.; Bassler, B.L.; Hughson, F.M. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 2002, 415, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Chandrathna, H.P.S.U.; Nikapitiya, C.; Dananjaya, S.H.S.; Wijerathne, C.U.B.; Wimalasena, S.H.M.P.; Kwun, H.J.; Heo, G.J.; Lee, J.; De Zoysa, M. Outcome of co-infection with opportunistic and multidrug resistant Aeromonas hydrophila and A. veronii in zebrafish: Identification, characterization, pathogenicity and immune responses. Fish Shellfish Immunol. 2018, 80, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.O.; Park, Y.; Lamont, R.J.; McNab, R.; Barbieri, B.; Demuth, D.R. Signaling system in Porphyromonas gingivalis based on a LuxS protein. J. Bacteriol. 2001, 183, 3903–3909. [Google Scholar] [CrossRef]
- Frias, J.; Olle, E.; Alsina, M. Periodontal pathogens produce quorum sensing signal molecules. Infect. Immun. 2001, 69, 3431–3434. [Google Scholar] [CrossRef]



| Gene Name | Primer Name | Primers (5′-3″) | References |
|---|---|---|---|
| 16S rRNA | 16S rRNA-F | AGAGTTTGATCATGGCTCAG | [12] |
| 16S rRNA-R | TACGGTTACCTTGTTACGACTT | ||
| ascV | ascV-F | AGCAGATGAGTATCGACGG | [13] |
| ascV-R | AGGCATTCTCCTGTACCAG | ||
| hlyA | hlyA-F | GGCCGGTGGCCCGAAGATACGGG | [14] |
| hlyA-R | GGCGGCGCCGGACGAGACGGGG | ||
| aerA | aerA-F | CAAGAACAAGTTCAAGTGGCCA | [13] |
| aerA-R | ACGAAGGTGTGGTTCCAGT | ||
| LuxS | LuxS-F | GATCCTCTCCGAGGCGTGG | [14] |
| LuxS-R | AGGCTTTTCAGCTTCTCTTCC | ||
| act | act-F | TCTCCATGCTTCCCTTCCACT | [13] |
| act-R | AACTGACATCGGCCTTGAACTC | ||
| alt | alt-F | TGACCCAGTCCTGGCACGGC | [13] |
| alt-R | GGTGATCGATCACCACCAGC | ||
| ast | ast-F | TCTCCATGCTTCCCTTCCACT | [13] |
| ast-R | GTGTAGGGATTGAAGAAGCCG | ||
| lip | lip-F | CACCTGGT(T/G)CCGCTCAAG | [15] |
| lip-R | GTACCGAACCAGTCGGAGAA | ||
| exu | exu-F | AGACATGCACAACCTCTTCC | [16] |
| exu-R | GATTGGTATTGCCTTGCAAG | ||
| aha | aha-F | GGCTATTGCTATCCCGGCTCTGTT | [15] |
| aha-R | CGGTCCACTCGTCGTCCATCTTG |
| Reaction Item | Result * | Reaction Item | Result * | ||
|---|---|---|---|---|---|
| A1 | Negative control | N | E1 | Gelatin | B |
| A2 | Dextrin | P | E2 | Glycyl-L-Proline | B |
| A3 | D-Maltose | B | E3 | L-Alanine | B |
| A4 | D-Trehalose | B | E4 | L-Arginine | B |
| A5 | D-Cellobiose | B | E5 | L-Aspartic Acid | B |
| A6 | Gentiobiose | B | E6 | L-Glutamic Acid | B |
| A7 | Sucrose | P | E7 | L-Histidine | B |
| A8 | D-Turanose | B | E8 | L-Pyroglutamic Acid | B |
| A9 | Stachyose | B | E9 | L-Serine | B |
| A10 | Positive control | P | E10 | Lincomycin | N |
| A11 | Acidic PH PH6 | P | E11 | Guanidine HCl | B |
| A12 | Acidic PH PH5 | N | E12 | Niaproof 4 | P |
| B1 | D-Raffinose | B | F1 | Pectin | B |
| B2 | α-D-Lactose | B | F2 | D-Galacturonic Acid | B |
| B3 | D-Melibiose | B | F3 | L-Galactonic Acid Lactone | B |
| B4 | β-Methyl-D-Glucoside | B | F4 | D-Galactonic Acid | P |
| B5 | D-Salicin | B | F5 | D-Glucuronic Acid | N |
| B6 | N-Acetyl-D-Glucosamine | P | F6 | Glucuronamide | N |
| B7 | N-Acetyl-β-Mannosamine | B | F7 | Mucic Acid | B |
| B8 | N-Acetyl-D-Galactosamine | N | F8 | Quinic Acid | B |
| B9 | N-Acetyl Neuraminic | B | F9 | D-Saccharic Acid | B |
| B10 | 1% NaCl | P | F10 | Vancomycin | P |
| B11 | 4% NaCl | N | F11 | Tetrazolium Violet | N |
| B12 | 8% NaCl | N | F12 | Tetrazolium Blue | P |
| C1 | α-D-Glucose | B | G1 | P-Hydroxy-Phenylacetic Acid | N |
| C2 | D-Mannose | B | G2 | Methyl Pyruvate | B |
| C3 | D-Fructose | B | G3 | D-Lactic Acid Methyl Ester | B |
| C4 | D-Galactose | B | G4 | Lactic Acid | B |
| C5 | 3-Methyl Glucose | N | G5 | Citric Acid | B |
| C6 | D-Ducrose | N | G6 | α-Keto-Glutaric Acid | B |
| C7 | L-Fucose | B | G7 | D-Malic Acid | N |
| C8 | L-Rhamnose | B | G8 | L-Malic Acid | P |
| C9 | Inosine | N | G9 | Bromo-Succinic-Acid | B |
| C10 | 1% Sodium Lactate | B | G10 | Nalidixic Acid | P |
| C11 | Fusidic Acid | N | G11 | Lithium Chloride | B |
| C12 | D-Serine | B | G12 | Potassium Tellurite | N |
| D1 | D-Sorbitol | B | H1 | Tween 40 | P |
| D2 | D-Mannitol | B | H2 | γ-Amino-Butyric-Acid | B |
| D3 | D-Arabitol | N | H3 | α-Hydroxy- Butyric-Acid | B |
| D4 | Myo-Inositol | B | H4 | β--Hydroxy-D, L Butyric-Acid | B |
| D5 | Glycerol | B | H5 | α-Keto-Butyric Acid | N |
| D6 | D-Glucose-6-po4 | P | H6 | Acetoacetic Acid | B |
| D7 | D-Fructose-6-po4 | P | H7 | Propionic Acid | N |
| D8 | D-Aspartic acid | N | H8 | Acetic Acid | B |
| D9 | D-Serine | B | H9 | Formic Acid | N |
| D10 | Troleandomycin | N | H10 | Aztreonam | B |
| D11 | Rifamycin SV | P | H11 | Sodium Butyrate | N |
| D12 | Minocycline | N | H12 | Sodium Bromate | N |
| Drugs | Content (μg/Piece) | Inhibition Zone (mm) | Sensitivity |
|---|---|---|---|
| Compound Sulfamethoxazoles | 23.75 | 29 | S |
| Cefothiophene | 30 | 24 | S |
| Doxycycline | 30 | 19 | S |
| Sulfamethoxazole | 300 | 16 | S |
| Gentamicin | 10 | 13 | I |
| Amikacin | 30 | 14 | I |
| Neomycin | 30 | 14 | I |
| Florfenicol | 30 | 15 | I |
| Enrofloxacin | 10 | 13 | I |
| Ciprofloxacin | 5 | 8 | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Li, M.; Xue, M.; Zhou, Y.; Jiang, N.; Meng, Y.; Liu, Y.; Jiang, J.; Liao, X.; Fan, Y. Identification of Aeromonas veronii as the Pathogen Associated with Massive Mortality in Bronze Gudgeon (Coreius heterodon). Animals 2024, 14, 2440. https://doi.org/10.3390/ani14162440
Liu W, Li M, Xue M, Zhou Y, Jiang N, Meng Y, Liu Y, Jiang J, Liao X, Fan Y. Identification of Aeromonas veronii as the Pathogen Associated with Massive Mortality in Bronze Gudgeon (Coreius heterodon). Animals. 2024; 14(16):2440. https://doi.org/10.3390/ani14162440
Chicago/Turabian StyleLiu, Wenzhi, Mengmeng Li, Mingyang Xue, Yong Zhou, Nan Jiang, Yan Meng, Yisha Liu, Jingwen Jiang, Xiaolin Liao, and Yuding Fan. 2024. "Identification of Aeromonas veronii as the Pathogen Associated with Massive Mortality in Bronze Gudgeon (Coreius heterodon)" Animals 14, no. 16: 2440. https://doi.org/10.3390/ani14162440





