The Impact of Genetic and Non-Genetic Factors on Lamb Loin Shear Force
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experiment Design
2.2. Animal Harvest and Chilling
2.3. Sample Collection and Measurement
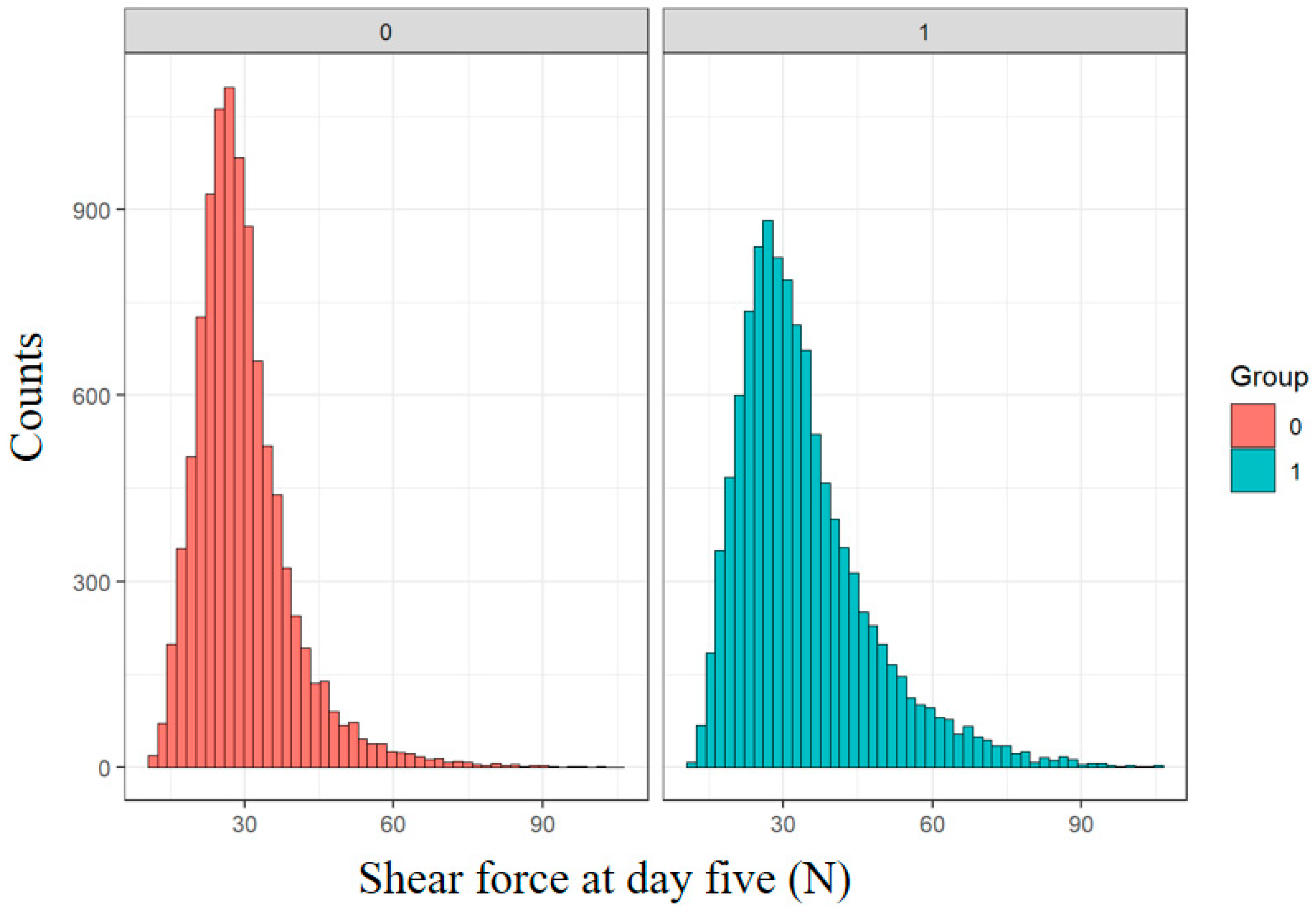
2.4. Classification of Cold Shortening
2.5. Statistical Analysis
3. Results
3.1. Animals
3.2. The Impact of Fixed Effects on Shear Force (SF5) at Day Five
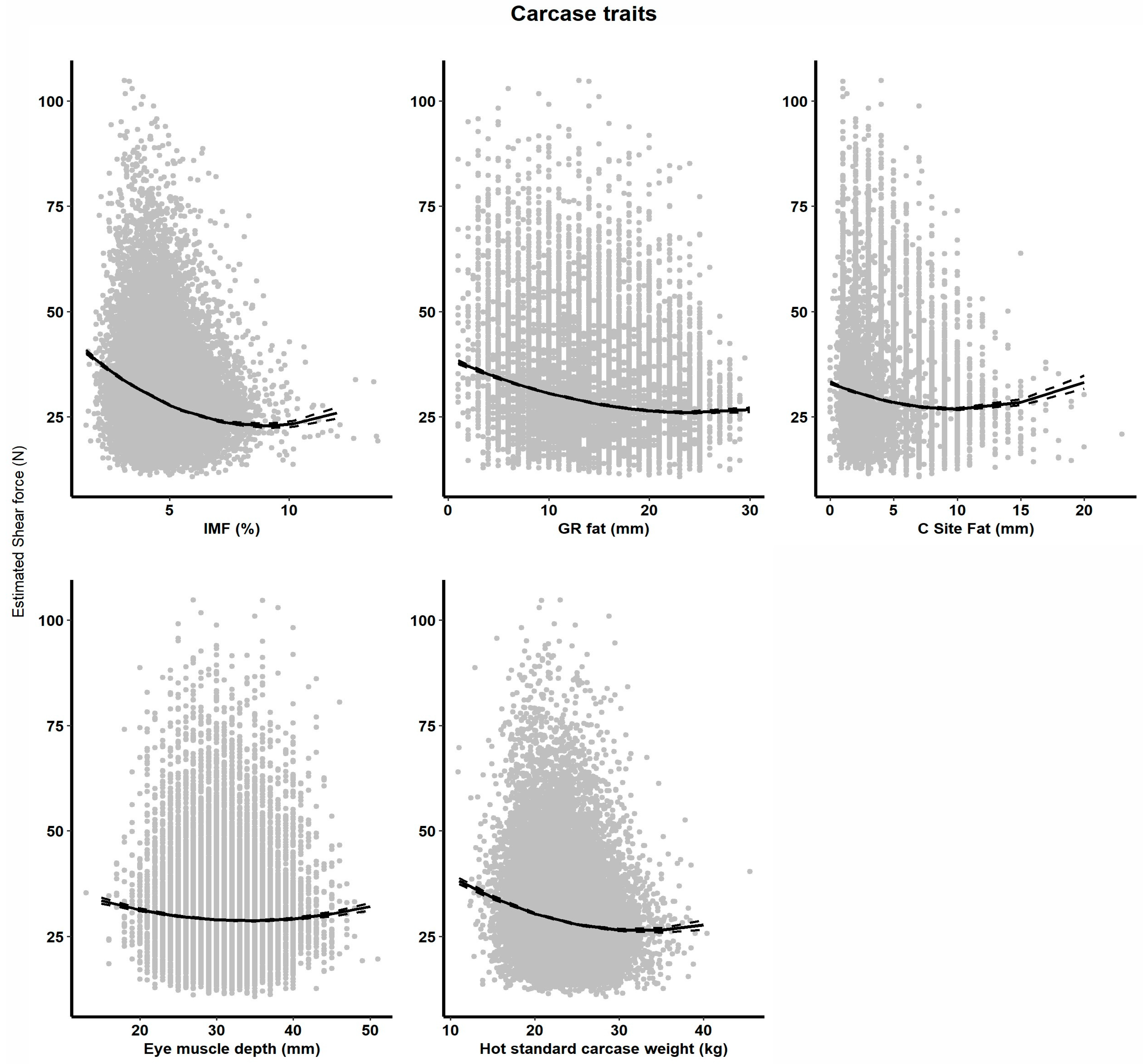
3.3. Phenotypic Effects on Shear Force (SF) at Day Five
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pannier, L.; Gardner, G.; Pearce, K.; McDonagh, M.; Ball, A.; Jacob, R.; Pethick, D. Associations of sire estimated breeding values and objective meat quality measurements with sensory scores in Australian lamb. Meat Sci. 2014, 96, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Hocquette, J.-F.; Van Wezemael, L.; Chriki, S.; Legrand, I.; Verbeke, W.; Farmer, L.; Scollan, N.D.; Polkinghorne, R.; Rødbotten, R.; Allen, P. Modelling of beef sensory quality for a better prediction of palatability. Meat Sci. 2014, 97, 316–322. [Google Scholar] [CrossRef]
- Lawrie, R.A.; Ledward, D. Lawrie’s Meat Science; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Pethick, D.W.; Mcgilchrist, P.; Polkinghorne, R.; Warner, R.; Tarr, G.; Garmyn, A.; Thompson, J.; Hocquette, J.-F. Travaux de recherche internationaux sur la qualité sensorielle de la viande ovine et bovine. Rev. Fr. Rech. Viandes Prod. Carnés 2018, 34, 1–2. [Google Scholar]
- O’Reilly, R.; Pannier, L.; Gardner, G.; Garmyn, A.; Luo, H.; Meng, Q.; Miller, M.; Pethick, D. Minor differences in perceived sheepmeat eating quality scores of Australian, Chinese and American consumers. Meat Sci. 2020, 164, 108060. [Google Scholar] [CrossRef] [PubMed]
- Rødbotten, M.; Kubberød, E.; Lea, P.; Ueland, Ø. A sensory map of the meat universe. Sensory profile of meat from 15 species. Meat Sci. 2004, 68, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Holman, B.W.; Collins, D.; Kilgannon, A.K.; Hopkins, D. Using shear force, sarcomere length, particle size, collagen content, and protein solubility metrics to predict consumer acceptance of aged beef tenderness. J. Texture Stud. 2020, 51, 559–566. [Google Scholar] [CrossRef]
- Bonny, S.P.; O’Reilly, R.A.; Pethick, D.W.; Gardner, G.E.; Hocquette, J.-F.; Pannier, L. Update of Meat Standards Australia and the cuts based grading scheme for beef and sheepmeat. J. Integr. Agric. 2018, 17, 1641–1654. [Google Scholar] [CrossRef]
- Lyford, C.P.; Thompson, J.M.; Polkinghorne, R.; Miller, M.F.; Nishimura, T.; Neath, K.; Allen, P.; Belasco, E.J. Is willingness to pay (WTP) for beef quality grades affected by consumer demographics and meat consumption preferences? Aust. Agribus. Rev. 2010, 18, 1–17. [Google Scholar]
- Watson, R.; Gee, A.; Polkinghorne, R.; Porter, M. Consumer assessment of eating quality–development of protocols for Meat Standards Australia (MSA) testing. Aust. J. Exp. Agric. 2008, 48, 1360–1367. [Google Scholar] [CrossRef]
- Watson, R.; Polkinghorne, R.; Thompson, J.M. Development of the Meat Standards Australia (MSA) prediction model for beef palatability. Aust. J. Exp. Agric. 2008, 48, 1368–1379. [Google Scholar] [CrossRef]
- Dransfield, E.; Jones, R.; MacFie, H. Tenderising in M. longissimus dorsi of beef, veal, rabbit, lamb and pork. Meat Sci. 1981, 5, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Harper, G. Trends in skeletal muscle biology and the understanding of toughness in beef. Aust. J. Agric. Res. 1999, 50, 1105–1129. [Google Scholar] [CrossRef]
- Koohmaraie, M.; Geesink, G. Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Sci. 2006, 74, 34–43. [Google Scholar] [CrossRef]
- Thompson, J.; Gee, A.; Hopkins, D.; Pethick, D.; Baud, S.; O’Halloran, W. Development of a sensory protocol for testing palatability of sheep meats. Aust. J. Exp. Agric. 2005, 45, 469–476. [Google Scholar] [CrossRef]
- Hopkins, D.; Ponnampalam, E.; Van De Ven, R.; Warner, R. The effect of pH decline rate on the meat and eating quality of beef carcasses. Anim. Prod. Sci. 2014, 54, 407–413. [Google Scholar] [CrossRef]
- Hocquette, J.-F.; Gondret, F.; Baéza, E.; Médale, F.; Jurie, C.; Pethick, D. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal 2010, 4, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.; Greenwood, P.; Pethick, D.; Ferguson, D. Genetic and environmental effects on meat quality. Meat Sci. 2010, 86, 171–183. [Google Scholar] [CrossRef]
- Perry, D.; Thompson, J.; Hwang, I.; Butchers, A.; Egan, A. Relationship between objective measurements and taste panel assessment of beef quality. Aust. J. Exp. Agric. 2001, 41, 981–989. [Google Scholar] [CrossRef]
- Hwang, I.; Polkinghorne, R.; Lee, J.; Thompson, J.M. Demographic and design effects on beef sensory scores given by Korean and Australian consumers. Aust. J. Exp. Agric. 2008, 48, 1387–1395. [Google Scholar] [CrossRef]
- Van Wezemael, L.; De Smet, S.; Ueland, Ø.; Verbeke, W. Relationships between sensory evaluations of beef tenderness, shear force measurements and consumer characteristics. Meat Sci. 2014, 97, 310–315. [Google Scholar] [CrossRef]
- Platter, W.; Tatum, J.; Belk, K.; Chapman, P.; Scanga, J.; Smith, G. Relationships of consumer sensory ratings, marbling score, and shear force value to consumer acceptance of beef strip loin steaks. J. Anim. Sci. 2003, 81, 2741–2750. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.; Shorthose, W.; Ferguson, D.; Thompson, J. Methods used in the CRC program for the determination of carcass yield and beef quality. Aust. J. Exp. Agric. 2001, 41, 953–957. [Google Scholar] [CrossRef]
- Caine, W.; Aalhus, J.; Best, D.; Dugan, M.; Jeremiah, L. Relationship of texture profile analysis and Warner-Bratzler shear force with sensory characteristics of beef rib steaks. Meat Sci. 2003, 64, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Destefanis, G.; Brugiapaglia, A.; Barge, M.T.; Dal Molin, E. Relationship between beef consumer tenderness perception and Warner–Bratzler shear force. Meat Sci. 2008, 78, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, D.; Hegarty, R.; Walker, P.; Pethick, D. Relationship between animal age, intramuscular fat, cooking loss, pH, shear force and eating quality of aged meat from sheep. Aust. J. Exp. Agric. 2006, 46, 879–884. [Google Scholar] [CrossRef]
- Mortimer, S.; Van der Werf, J.; Jacob, R.; Hopkins, D.; Pannier, L.; Pearce, K.; Gardner, G.; Warner, R.D.; Geesink, G.; Edwards, J.H. Genetic parameters for meat quality traits of Australian lamb meat. Meat Sci. 2014, 96, 1016–1024. [Google Scholar] [CrossRef]
- Hopkins, D.; Holman, B.W.; van de Ven, R.J. Modelling lamb carcase pH and temperature decline parameters: Relationship to shear force and abattoir variation. Meat Sci. 2015, 100, 85–90. [Google Scholar] [CrossRef]
- Fogarty, N.; Banks, R.; Van Der Werf, J.; Ball, A.; Gibson, J. The information nucleus–a new concept to enhance sheep industry genetic improvement. In Proceedings of the Association for the Advancement of Animal Breeding and Genetics, Armidale, NSW, Australia, 23–26 September 2007; pp. 29–32. [Google Scholar]
- Van der Werf, J.; Kinghorn, B.; Banks, R. Design and role of an information nucleus in sheep breeding programs. Anim. Prod. Sci. 2010, 50, 998–1003. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Butler, K.L.; Jacob, R.H.; Pethick, D.; Ball, A.J.; Edwards, J.E.H.; Geesink, G.; Hopkins, D. Health beneficial long chain omega-3 fatty acid levels in Australian lamb managed under extensive finishing systems. Meat Sci. 2014, 96, 1104–1110. [Google Scholar] [CrossRef]
- Pearce, K.; Van De Ven, R.; Mudford, C.; Warner, R.; Hocking-Edwards, J.; Jacob, R.; Pethick, D.; Hopkins, D. Case studies demonstrating the benefits on pH and temperature decline of optimising medium-voltage electrical stimulation of lamb carcasses. Anim. Prod. Sci. 2010, 50, 1107–1114. [Google Scholar] [CrossRef]
- AUS-Meat-H.A.M. Handbook 8th Edition (HAM); AUS-MEAT: Murarrie, Australia, 2022; Available online: https://shop.ausmeat.com.au/products/handbook-8th-edition-ham (accessed on 25 May 2023).
- Hopkins, D.L.; Thompson, J.M. The relationship between tenderness, proteolysis, muscle contraction and dissociation of actomyosin. Meat Sci. 2001, 57, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Nlme: Linear and Nonlinear Mixed Effects Models, R Package Version 3.1-137; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Jacob, R.; Rosenvold, K.; North, M.; Kemp, R.; Warner, R.; Geesink, G. Rapid tenderisation of lamb M. longissimus with very fast chilling depends on rapidly achieving sub-zero temperatures. Meat Sci. 2012, 92, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.; Thomson, K. The importance of chill rate when characterising colour change of lamb meat during retail display. Meat Sci. 2012, 90, 478–484. [Google Scholar] [CrossRef]
- Milan, D.; Jeon, J.-T.; Looft, C.; Amarger, V.; Robic, A.; Thelander, M.; Rogel-Gaillard, C.; Paul, S.; Iannuccelli, N.; Rask, L. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science 2000, 288, 1248–1251. [Google Scholar] [CrossRef]
- Devine, C.E.; Wahlgren, N.M.; Tornberg, E. Effect of rigor temperature on muscle shortening and tenderisation of restrained and unrestrained beef M. longissimus thoracicus et lumborum. Meat Sci. 1999, 51, 61–72. [Google Scholar] [CrossRef]
- Maltin, C.; Balcerzak, D.; Tilley, R.; Delday, M. Determinants of meat quality: Tenderness. Proc. Nutr. Soc. 2003, 62, 337–347. [Google Scholar] [CrossRef]
- Josell, Å.; Enfält, A.-C.; von Seth, G.; Lindahl, G.; Hedebro-Velander, I.; Andersson, L.; Lundström, K. The influence of RN genotype, including the new V199I allele, on the eating quality of pork loin. Meat Sci. 2003, 65, 1341–1351. [Google Scholar] [CrossRef]
- Pommier, S. Vitamin A, electrical stimulation, and chilling rate effects on lysosomal enzyme activity in aging bovine muscle. J. Food Sci. 1992, 57, 30–35. [Google Scholar] [CrossRef]
- Rhee, M.; Ryu, Y.; Imm, J.; Kim, B. Combination of low voltage electrical stimulation and early postmortem temperature conditioning on degradation of myofibrillar proteins in Korean native cattle (Hanwoo). Meat Sci. 2000, 55, 391–396. [Google Scholar] [CrossRef]
- Salm, C.; Forrest, J.; Aberle, E.; Mills, E.; Snyder, A.; Judge, M. Bovine muscle shortening and protein degradation after electrical stimulation, excision and chilling. Meat Sci. 1983, 8, 163–183. [Google Scholar] [CrossRef]
- Pannier, L.; Pethick, D.; Geesink, G.; Ball, A.; Jacob, R.; Gardner, G. Intramuscular fat in the longissimus muscle is reduced in lambs from sires selected for leanness. Meat Sci. 2014, 96, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Pethick, D.; Hopkins, D.; D’Souza, D.; Thompson, J.; Walker, P. Effects of animal age on the eating quality of sheep meat. Aust. J. Exp. Agric. 2005, 45, 491–498. [Google Scholar] [CrossRef]
- Hopkins, D.; Stanley, D.; Martin, L.; Toohey, E.; Gilmour, A.R. Genotype and age effects on sheep meat production 3. Meat quality. Aust. J. Exp. Agric. 2007, 47, 1155–1164. [Google Scholar] [CrossRef]
- Navajas, E.; Lambe, N.; Fisher, A.; Nute, G.; Bünger, L.; Simm, G. Muscularity and eating quality of lambs: Effects of breed, sex and selection of sires using muscularity measurements by computed tomography. Meat Sci. 2008, 79, 105–112. [Google Scholar] [CrossRef]
- Hopkins, D. The relationship between muscularity, muscle: Bone ratio and cut dimensions in male and female lamb carcasses and the measurement of muscularity using image analysis. Meat Sci. 1996, 44, 307–317. [Google Scholar] [CrossRef]
- Wolf, B.T.; Jones, D.; Owen, M. Carcass composition, conformation and muscularity in Texel lambs of different breeding history, sex and leg shape score. Anim. Sci. 2001, 72, 465–475. [Google Scholar] [CrossRef]
- Anderson, F.; Pannier, L.; Pethick, D.; Gardner, G. Intramuscular fat in lamb muscle and the impact of selection for improved carcass lean meat yield. Animal 2015, 9, 1081–1090. [Google Scholar] [CrossRef]
- Johnson, P.; Purchas, R.; McEwan, J.; Blair, H. Carcass composition and meat quality differences between pasture-reared ewe and ram lambs. Meat Sci. 2005, 71, 383–391. [Google Scholar] [CrossRef]
- Butler-Hogg, B.; Brown, A. Muscle weight distribution in lambs: A comparison of entire male and female. Anim. Sci. 1986, 42, 343–348. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M. Muscle fiber properties in cattle and their relationships with meat qualities: An overview. J. Agric. Food Chem. 2020, 68, 6021–6039. [Google Scholar] [CrossRef]
- Beline, M.; Gómez, J.F.M.; Antonelo, D.S.; Silva, J.; Buarque, V.L.M.; Cônsolo, N.R.B.; Leme, P.R.; Matarneh, S.K.; Gerrard, D.E.; Silva, S.L. Muscle fiber type, postmortem metabolism, and meat quality of Nellore cattle with different post-weaning growth potential. Livest. Sci. 2021, 244, 104348. [Google Scholar] [CrossRef]
- Schreurs, N.M.; Garcia, F.; Jurie, C.; Agabriel, J.; Micol, D.; Bauchart, D.; Listrat, A.; Picard, B. Meta-analysis of the effect of animal maturity on muscle characteristics in different muscles, breeds, and sexes of cattle. J. Anim. Sci. 2008, 86, 2872–2887. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.M.; Moody, W.; Boling, J.; Bradley, N. Influence of breed type, sex, feeding systems, and muscle bundle size on bovine fiber type characteristics. J. Food Sci. 1981, 46, 1760–1765. [Google Scholar] [CrossRef]
- Gardner, G.; Williams, A.; Siddell, J.; Ball, A.; Mortimer, S.; Jacob, R.; Pearce, K.; Edwards, J.H.; Rowe, J.; Pethick, D. Using Australian Sheep Breeding Values to increase lean meat yield percentage. Anim. Prod. Sci. 2010, 50, 1098–1106. [Google Scholar] [CrossRef]
- Pomponio, L.; Ertbjerg, P.; Karlsson, A.H.; Costa, L.N.; Lametsch, R. Influence of early pH decline on calpain activity in porcine muscle. Meat Sci. 2010, 85, 110–114. [Google Scholar] [CrossRef]
- Delgado, E.; Geesink, G.; Marchello, J.; Goll, D.; Koohmaraie, M. The calpain system in three muscles of normal and callipyge sheep. J. Anim. Sci. 2001, 79, 398–412. [Google Scholar] [CrossRef]
- Storr, S.J.; Carragher, N.O.; Frame, M.C.; Parr, T.; Martin, S.G. The calpain system and cancer. Nat. Rev. Cancer 2011, 11, 364–374. [Google Scholar] [CrossRef]
- Liu, J.; Ruusunen, M.; Puolanne, E.; Ertbjerg, P. Effect of pre-rigor temperature incubation on sarcoplasmic protein solubility, calpain activity and meat properties in porcine muscle. LWT-Food Sci. Technol. 2014, 55, 483–489. [Google Scholar] [CrossRef]
| Flocks * | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | IN01 | IN02 | IN03 | IN04 | IN05 | IN06 | IN07 | IN08 | Total/Year |
| 1 | 248 | 298 | 298 | 224 | 298 | 270 | 417 | 2053 | |
| 2 | 400 | 303 | 158 | 309 | 195 | 132 | 280 | 580 | 2357 |
| 3 | 493 | 282 | 328 | 389 | 218 | 264 | 318 | 409 | 2701 |
| 4 | 370 | 267 | 197 | 208 | 197 | 183 | 299 | 462 | 2514 |
| 5 | 399 | 127 | 264 | 286 | 232 | 188 | 292 | 401 | 2386 |
| 6 | 1326 | - | - | - | - | - | - | 821 | 2147 |
| 7 | 1128 | - | - | - | - | - | - | 674 | 1802 |
| 8 | 975 | - | - | - | - | - | - | 855 | 1830 |
| 9 | 877 | - | - | - | - | - | - | 858 | 1735 |
| 10 | 719 | - | - | - | - | - | - | 542 | 1563 |
| 11 | 890 | - | - | - | - | - | - | 918 | 2007 |
| 12 | 709 | - | - | - | - | - | - | 821 | 1678 |
| Total animals | 8534 | 979 | 1245 | 1490 | 1066 | 1065 | 1459 | 7758 | 23,696 |
| Base Model | Model Corrected for Hot Carcass Weight (kg) | Model Corrected for Eye Muscle Depth (mm) | Model Corrected for C-Site Fat (mm) | Model Corrected for gr Fat (mm) | Model Corrected for Intramuscular Fat % | Model Included All Carcass Traits | Model Included Carcass Traits and Quadratic Terms | ||
|---|---|---|---|---|---|---|---|---|---|
| EFFECT | DF | F-Value | F-Value | F-Value | F-Value | F-Value | F-Value | F-Value | F-Value |
| Cohort | 195 | 53 *** | 53 *** | 54 *** | 54 *** | 55 *** | 57 *** | 55 *** | 56 *** |
| Sire type | 2 | 24 *** | 24 *** | 23 *** | 23 *** | 25 *** | 25 *** | 24 *** | 24 *** |
| Gender | 1 | 139 *** | 144 *** | 133 *** | 134 *** | 143 *** | 147 *** | 146 *** | 148 *** |
| Age at slaughter | 1 | 8 ** | 8 ** | 8 ** | 7 ** | 8 ** | 8 ** | 8 ** | 8 ** |
| Cold shorten 18 | 1 | 433 *** | 430 *** | 432 *** | 436 *** | 444 *** | 454 *** | 453 *** | 457 *** |
| HCWT | 1 | - | 228 *** | - | - | - | - | 234 *** | 236 *** |
| HCWT2 | 1 | - | 40 *** | - | - | - | - | - | 48 *** |
| CEMD | 1 | - | - | 11 *** | - | - | - | 7 ** | 10 ** |
| CEMD2 | 1 | - | - | 28 *** | - | - | - | - | 6 * |
| CFAT | 1 | - | - | - | 210 | - | - | 101 *** | 97 *** |
| CFAT2 | 1 | - | - | - | 67 *** | - | - | - | 43 *** |
| GR FAT | 1 | - | - | - | - | 490 *** | - | 192 *** | 164 *** |
| GR FAT2 | 1 | - | - | - | - | 94 *** | - | - | 52 *** |
| IMF% | 1 | - | - | - | - | - | 1035 *** | 772 *** | 729 *** |
| IMF%2 | 1 | - | - | - | - | - | 129 *** | - | 88 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Moadhen, H.; Lees, J.C.; van der Werf, J.H.J.; McGilchrist, P. The Impact of Genetic and Non-Genetic Factors on Lamb Loin Shear Force. Animals 2024, 14, 2628. https://doi.org/10.3390/ani14182628
Al-Moadhen H, Lees JC, van der Werf JHJ, McGilchrist P. The Impact of Genetic and Non-Genetic Factors on Lamb Loin Shear Force. Animals. 2024; 14(18):2628. https://doi.org/10.3390/ani14182628
Chicago/Turabian StyleAl-Moadhen, Hussein, Jarrod C. Lees, Julius H. J. van der Werf, and Peter McGilchrist. 2024. "The Impact of Genetic and Non-Genetic Factors on Lamb Loin Shear Force" Animals 14, no. 18: 2628. https://doi.org/10.3390/ani14182628






