Antimicrobial Susceptibility of Commensal Escherichia coli from Pig Fecal Samples and Enhanced Sensitivity for Direct Detection of the blaCTX-M Gene by Nested PCR
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Farm Selection
2.2. Collection of Fecal Samples
2.3. Bacterial Culture, Identification, and Antibiotic Susceptibility Testing
2.4. DNA Extraction
2.5. Multiplex PCR for Detecting CTX-M, TEM, OXA, and SHV Genes and Characterization of ESBL Variants
2.6. Direct Detection of the blaCTX-M Gene by sPCR, nPCR, and mPCR from Pigs’ Fecal Samples
2.7. Specificity of nPCR
2.8. Limit of Detection of nPCR at Various Incubation Times of Fecal Samples
2.9. Validation of nPCR for the Direct Detection of the blaCTX-M Gene from Pig Feces
3. Results
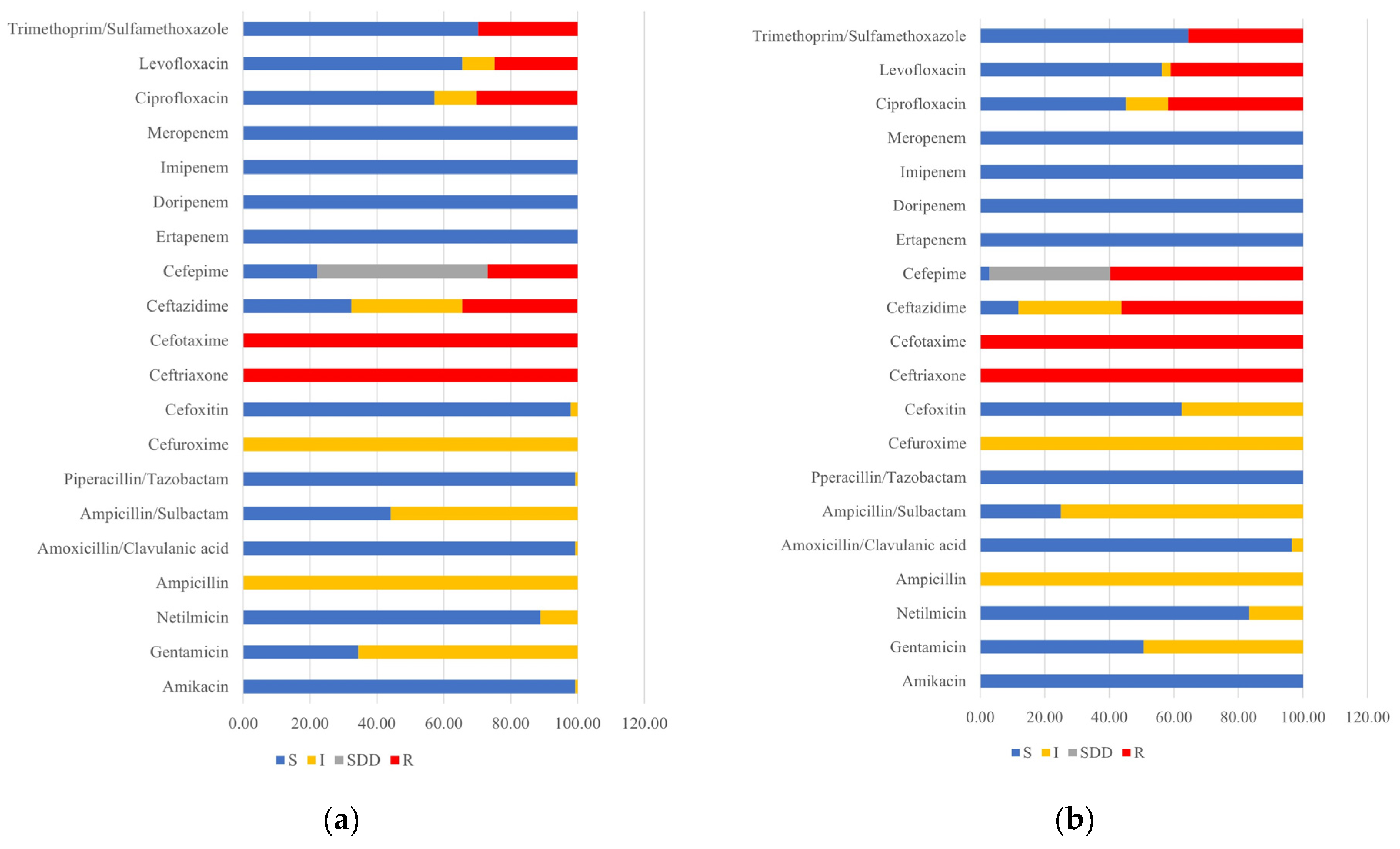
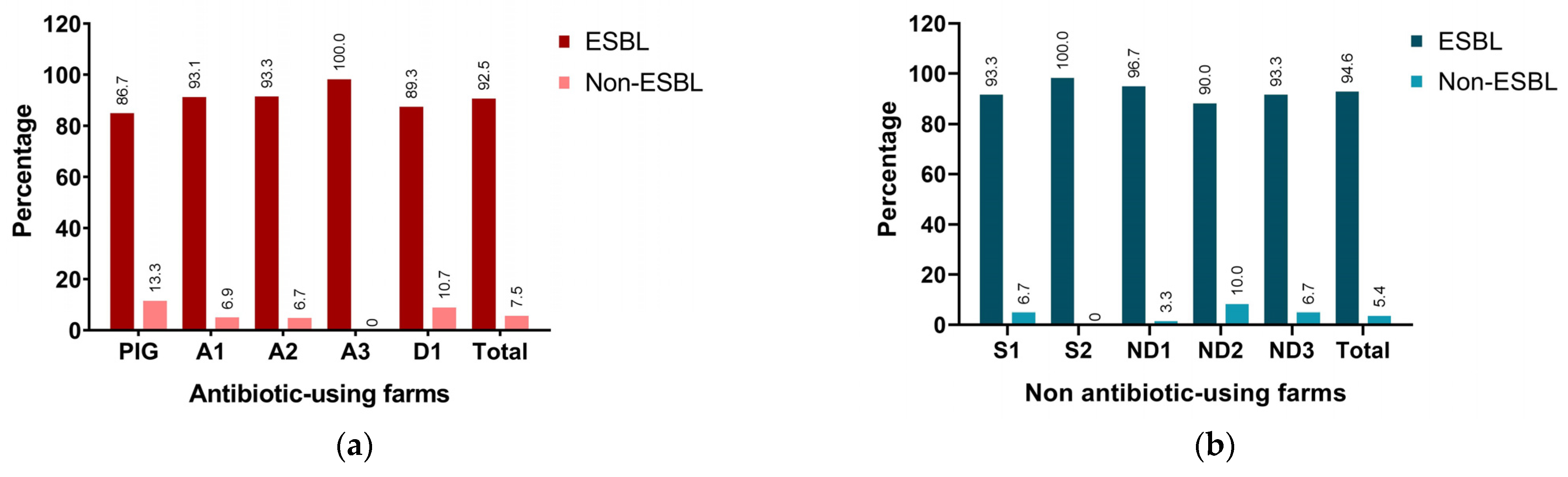
3.1. Antimicrobial Susceptibility Pattern and ESBL Detection
3.2. Characterization of ESBL Variants
3.3. Specificity of nPCR for the Direct Fecal Detection of blaCTX-M
3.4. Limit of Detection of nPCR for the Direct Fecal Detection of blaCTX-M
3.5. Comparison of the Direct Detection of the blaCTX-M Gene from Pig Feces Using mPCR, sPCR, and nPCR Techniques
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, D.; Wang, B.; Zhang, X.; Zhang, J.; Zhang, H.; Liu, X.; Gao, Z.; Yu, Z. The spread of antibiotic resistance to humans and potential protection strategies. Ecotoxicol. Environ. Saf. 2023, 254, 114734. [Google Scholar] [CrossRef] [PubMed]
- Husna, A.; Rahman, M.M.; Badruzzaman, A.T.M.; Sikder, M.H.; Islam, M.R.; Rahman, M.T.; Alam, J.; Ashour, H.M. Extended-Spectrum β-Lactamases (ESBL): Challenges and Opportunities. Biomedicines 2023, 11, 2937. [Google Scholar] [CrossRef] [PubMed]
- Kanokudom, S.; Assawakongkarat, T.; Akeda, Y.; Ratthawongjirakul, P.; Chuanchuen, R.; Chaichanawongsaroj, N. Rapid detection of extended spectrum β-lactamase producing Escherichia coli isolated from fresh pork meat and pig cecum samples using multiplex recombinase polymerase amplification and lateral flow strip analysis. PLoS ONE 2021, 16, e0248536. [Google Scholar] [CrossRef] [PubMed]
- Hammerum, A.M.; Larsen, J.; Andersen, V.D.; Lester, C.H.; Skovgaard Skytte, T.S.; Hansen, F.; Olsen, S.S.; Mordhorst, H.; Skov, R.L.; Aarestrup, F.M.; et al. Characterization of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli obtained from Danish pigs, pig farmers and their families from farms with high or no consumption of third- or fourth-generation cephalosporins. J. Antimicrob. Chemother. 2014, 69, 2650–2657. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Mir, R.A.; Park, S.H.; Kim, D.; Kim, H.Y.; Boughton, R.K.; Morris, J.G., Jr.; Jeong, K.C. Prevalence of extended-spectrum β-lactamases in the local farm environment and livestock: Challenges to mitigate antimicrobial resistance. Crit. Rev. Microbiol. 2020, 46, 1–14. [Google Scholar] [CrossRef]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef]
- Schrader, C.; Schielke, A.; Ellerbroek, L.; Johne, R. PCR inhibitors—Occurrence, properties and removal. J. Appl. Microbiol. 2012, 113, 1014–1026. [Google Scholar] [CrossRef]
- Qiu, E.; Li, Z.; Han, S. Methods for detection of Helicobacter pylori from stool sample: Current options and developments. Braz. J. Microbiol. 2021, 52, 2057–2062. [Google Scholar] [CrossRef]
- Arjona, A.; Barquero, N.; Doménech, A.; Tejerizo, G.; Collado, V.M.; Toural, C.; Martín, D.; Gomez-Lucia, E. Evaluation of a novel nested PCR for the routine diagnosis of feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV). J. Feline Med. Surg. 2007, 9, 14–22. [Google Scholar] [CrossRef]
- Sroka-Oleksiak, A.; Ufir, K.; Salamon, D.; Bulanda, M.; Gosiewski, T. Nested-PCR real time as alternative molecular tool for detection of Borrelia burgdorferi compared to the classical serological diagnosis of the blood. Med. Dosw. Mikrobiol. 2016, 68, 47–56. [Google Scholar] [PubMed]
- Fuehrer, H.P.; Fally, M.A.; Habler, V.E.; Starzengruber, P.; Swoboda, P.; Noedl, H. Novel nested direct PCR technique for malaria diagnosis using filter paper samples. J. Clin. Microbiol. 2011, 49, 1628–1630. [Google Scholar] [CrossRef] [PubMed]
- M100-S31; Performance Standards for Antimicrobial Susceptibility Testing. Thirty-One Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021.
- Hasman, H.; Mevius, D.; Veldman, K.; Olesen, I.; Aarestrup, F.M. beta-Lactamases among extended-spectrum beta-lactamase (ESBL)-resistant Salmonella from poultry, poultry products and human patients in The Netherlands. J. Antimicrob. Chemother. 2005, 56, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeon, S.; Rhie, H.; Lee, B.; Park, M.; Lee, H.; Lee, J.; Kim, S. Rapid Detection of Extended Spectrum β-Lactamase (ESBL) for Enterobacteriaceae by use of a Multiplex PCR-based Method. Infect. Chemother. 2009, 41, 181–184. [Google Scholar] [CrossRef]
- Alvarez, L.; Carhuaricra, D.; Palomino-Farfan, J.; Calle, S.; Maturrano, L.; Siuce, J. Genomic Profiling of Multidrug-Resistant Swine Escherichia coli and Clonal Relationship to Human Isolates in Peru. Antibiotics 2023, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Beltrán, J.; Sørum, V.; Toll-Riera, M.; de la Vega, C.; Peña-Miller, R.; San Millán, Á. Genetic dominance governs the evolution and spread of mobile genetic elements in bacteria. Proc. Natl. Acad. Sci. USA 2020, 117, 15755–15762. [Google Scholar] [CrossRef]
- Pires, J.; Huber, L.; Hickman, R.A.; Dellicour, S.; Lunha, K.; Leangapichart, T.; Jiwakanon, J.; Magnusson, U.; Sunde, M.; Järhult, J.D.; et al. Genome-associations of extended-spectrum ß-lactamase producing (ESBL) or AmpC producing E. coli in small and medium pig farms from Khon Kaen province, Thailand. BMC Microbiol. 2022, 22, 253. [Google Scholar] [CrossRef] [PubMed]
- Sudatip, D.; Mostacci, N.; Tiengrim, S.; Thamlikitkul, V.; Chasiri, K.; Kritiyakan, A.; Phanprasit, W.; Thinphovong, C.; Abdallah, R.; Baron, S.A.; et al. The risk of pig and chicken farming for carriage and transmission of Escherichia coli containing extended-spectrum beta-lactamase (ESBL) and mobile colistin resistance (mcr) genes in Thailand. Microb. Genom. 2023, 9, 951. [Google Scholar] [CrossRef]
- Lay, K.K.; Jeamsripong, S.; Sunn, K.P.; Angkititrakul, S.; Prathan, R.; Srisanga, S.; Chuanchuen, R. Colistin Resistance and ESBL Production in Salmonella and Escherichia coli from Pigs and Pork in the Thailand, Cambodia, Lao PDR, and Myanmar Border Area. Antibiotics 2021, 10, 657. [Google Scholar] [CrossRef]
- Hussain, H.I.; Aqib, A.I.; Seleem, M.N.; Shabbir, M.A.; Hao, H.; Iqbal, Z.; Kulyar, M.F.-E.-A.; Zaheer, T.; Li, K. Genetic basis of molecular mechanisms in β-lactam resistant gram-negative bacteria. Microb. Pathog. 2021, 158, 105040. [Google Scholar] [CrossRef] [PubMed]
- Meissner, K.; Sauter-Louis, C.; Heiden, S.E.; Schaufler, K.; Tomaso, H.; Conraths, F.J.; Homeier-Bachmann, T. Extended-Spectrum ß-Lactamase-Producing Escherichia coli in Conventional and Organic Pig Fattening Farms. Microorganisms 2022, 10, 603. [Google Scholar] [CrossRef]
- Storey, N.; Cawthraw, S.; Turner, O.; Rambaldi, M.; Lemma, F.; Horton, R.; Randall, L.; Duggett, N.A.; AbuOun, M.; Martelli, F.; et al. Use of genomics to explore AMR persistence in an outdoor pig farm with low antimicrobial usage. Microb. Genom. 2022, 8, 782. [Google Scholar] [CrossRef] [PubMed]
- Van Hoek, A.H.A.M.; Veenman, C.; Florijn, A.; Huijbers, P.M.C.; Graat, E.A.M.; de Greeff, S.; Dierikx, C.M.; van Duijkeren, E. Longitudinal study of ESBL Escherichia coli carriage on an organic broiler farm. J. Antimicrob. Chemother. 2018, 73, 3298–3304. [Google Scholar] [CrossRef] [PubMed]
- Pollock, J.; Muwonge, A.; Hutchings, M.R.; Mainda, G.; Bronsvoort, B.M.; Gally, D.L.; Corbishley, A. Resistance to change: AMR gene dynamics on a commercial pig farm with high antimicrobial usage. Sci. Rep. 2020, 10, 1708. [Google Scholar] [CrossRef] [PubMed]
- Fidler, G.; Tolnai, E.; Stagel, A.; Remenyik, J.; Stundl, L.; Gal, F.; Biro, S.; Paholcsek, M. Tendentious effects of automated and manual metagenomic DNA purification protocols on broiler gut microbiome taxonomic profiling. Sci. Rep. 2020, 10, 3419. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulou, M.-E.; Stavrou, V.; Kotsalou, C.; Vantarakis, A. Boiling Extraction Method VS Commercial Kits for Bacterial DNA Isolation from Food Samples. J. Food Sci. Nutr. Res. 2020, 3, 311–319. [Google Scholar] [CrossRef]
- Yamagishi, J.; Sato, Y.; Shinozaki, N.; Ye, B.; Tsuboi, A.; Nagasaki, M.; Yamashita, R. Comparison of Boiling and Robotics Automation Method in DNA Extraction for Metagenomic Sequencing of Human Oral Microbes. PLoS ONE 2016, 11, e0154389. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; De Dios Colmenero, J.; Macias, M.; Bravo, M.J.; Morata, P. Preparation of bacterial DNA template by boiling and effect of immunoglobulin G as an inhibitor in real-time PCR for serum samples from patients with brucellosis. Clin. Vaccine Immunol. 2008, 15, 293–296. [Google Scholar] [CrossRef]
- Peng, X.; Yu, K.Q.; Deng, G.H.; Jiang, Y.X.; Wang, Y.; Zhang, G.X.; Zhou, H.W. Comparison of direct boiling method with commercial kits for extracting fecal microbiome DNA by Illumina sequencing of 16S rRNA tags. J. Microbiol. Methods 2013, 95, 455–462. [Google Scholar] [CrossRef]
- Shin, S.K.; Lee, Y.; Kwon, H.; Rhee, J.S.; Kim, J.K. Validation of Direct Boiling Method for Simple and Efficient Genomic DNA Extraction and PCR-based Macroalgal Species Determination. J. Phycol. 2021, 57, 1368–1372. [Google Scholar] [CrossRef]
- Ribeiro de Souza da Cunha, M.d.L. Molecular Biology in Microbiological Analysis. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Green, M.R.; Sambrook, J. Nested Polymerase Chain Reaction (PCR). Cold Spring Harb. Protoc. 2019, 2019, pdb-prot095182. [Google Scholar] [CrossRef]
- Dutta, S.; Chatterjee, A.; Dutta, P.; Rajendran, K.; Roy, S.; Pramanik, K.C.; Bhattacharya, S.K. Sensitivity and performance characteristics of a direct PCR with stool samples in comparison to conventional techniques for diagnosis of Shigella and enteroinvasive Escherichia coli infection in children with acute diarrhoea in Calcutta, India. J. Med. Microbiol. 2001, 50, 667–674. [Google Scholar] [CrossRef] [PubMed]

| Target Genes | Primer Sequence (5′-3′) | Amplicon Size (bp) | References |
|---|---|---|---|
| blaCTX-M | F-ATGTGCAGYACCAGTAARGTKATGGC | 593 | [13] |
| R-TGGGTRAARTARGTSACCAGAAYCAGCGG | |||
| blaTEM | F-AGTGCTGCCATAACCATGAGTG | 431 | [14] |
| R-CTGACTCCCC GTCGTGTAGATA | |||
| blaOXA | F-ATTATCTACAGCAGCGCCAGTG | 296 | |
| R-TGCATCCACGTCTTTGGTG | |||
| blaSHV | F-GATGAACGCTTTCCCATGATG | 214 | |
| R-CGCTGTTATCGCTCATGGTAA | |||
| uspA | F-AATGCAGGCTACCCAATCAC | 162 | [3] |
| R-GGTGTTGATCAGCTGACGTG |
| ESBL Variants | Antibiotic-Using Farms | Nonantibiotic-Using Farms | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No (%) | No (%) | |||||||||||||
| PIG (n = 28) | A1 (n = 30) | A2 (n = 29) | A3 (n = 32) | D1 (n = 26) | Total (n = 145) | p-Value * | S1 (n = 28) | S2 (n = 29) | ND1 (n = 32) | ND2 (n = 27) | ND3 (n = 28) | Total (n = 144) | p-Value * | |
| CTX-M-55, TEM-1b | 12 (42.9) | 9 (30) | - | 20 (62.5) | 11 (42.3) | 52 (35.9) | 0.406 | 28 (100) | 29 (100) | 12 (37.5) | 12 (44.4) | 27 (96.4) | 108 (75) | 0.406 |
| CTX-M-55 | 13 (46.4) | 6 (20) | 20 (69) | 10 (31.3) | 15 (57.7) | 64 (44.1) | 0.406 | - | - | 19 (59.4) | 9 (33.3) | - | 28 (19.4) | 0.406 |
| CTX-M-55, TEM-176 | - | - | 8 (27.6) | - | - | 8 (5.5) | 0.406 | - | - | - | - | - | - | 1.000 |
| CTX-M-55, CTX-M-14 | - | - | - | - | - | - | 1.000 | - | - | - | - | 1 (3.6) | 1 (0.7) | 0.406 |
| CTX-M-14, TEM-1b | 1 (3.6) | 11 (36.7) | 1 (3.4) | 2 (6.3) | - | 15 (10.3) | 0.406 | - | - | - | - | - | - | 1.000 |
| CTX-M-14 | 2 (7.1) | 2 (6.7) | - | - | - | 4 (2.8) | 0.406 | - | - | 1 (3.1) | 1 (3.7) | - | 2 (1.4) | 0.406 |
| CTX-M-15 | - | - | - | - | - | - | 1.000 | - | - | - | 5 (18.5) | - | 5 (3.5) | 0.406 |
| TEM-1b | - | 2 (6.7) | - | - | - | 2 (1.4) | 0.406 | - | - | - | - | - | - | 1.000 |
| CFU/mL | Methods | Incubation Time | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | 4 h | 6 h | 8 h | ||
| 108 | sPCR | + | + | + | + | + | + | + |
| nPCR | + | + | + | + | + | + | + | |
| 107 | sPCR | +w | + | + | + | + | + | + |
| nPCR | + | + | + | + | + | + | + | |
| 106 | sPCR | − | + | + | + | + | + | + |
| nPCR | + | + | + | + | + | + | + | |
| 105 | sPCR | − | +w | + | + | + | + | + |
| nPCR | +w | + | + | + | + | + | + | |
| 104 | sPCR | − | − | +w | +w | + | + | + |
| nPCR | − | +w | + | + | + | + | + | |
| 103 | sPCR | − | − | − | − | +w | + | + |
| nPCR | − | − | +w | + | + | + | + | |
| 102 | sPCR | − | − | − | − | − | +w | +w |
| nPCR | − | − | +w | +w | + | + | + | |
| 101 | sPCR | − | − | − | − | − | − | − |
| nPCR | − | − | − | − | +w | +w | + | |
| 100 | sPCR | − | − | − | − | − | − | − |
| nPCR | − | − | − | − | − | − | +w | |
| PCR from E. coli Isolates | Direct Detection from Pig Fecal Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| mPCR | sPCR | nPCR | |||||||
| + | − | Total | + | − | Total | + | − | Total | |
| blaCTX-M + | 41 | 0 | 41 | 95 | 0 | 95 | 125 | 0 | 125 |
| blaCTX-M − | 95 | 0 | 95 | 41 | 0 | 41 | 11 | 0 | 11 |
| Total | 136 | 0 | 136 | 136 | 0 | 136 | 136 | 0 | 136 |
| Sensitivity: 30.15% CI 95% [22.58–38.60%] PPV: 100% CI 95% [91.40–100.00%] | Sensitivity: 69.85% CI 95% [61.40–77.42%] PPV: 100% CI 95% [96.19–100.00%] | Sensitivity: 91.91% CI 95% [85.99–95.89%] PPV: 100% CI 95% [97.09–100.00%] | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchanta, N.; Ullah, N.; Santanirand, P.; Am-In, N.; Chaichanawongsaroj, N. Antimicrobial Susceptibility of Commensal Escherichia coli from Pig Fecal Samples and Enhanced Sensitivity for Direct Detection of the blaCTX-M Gene by Nested PCR. Animals 2024, 14, 2630. https://doi.org/10.3390/ani14182630
Suchanta N, Ullah N, Santanirand P, Am-In N, Chaichanawongsaroj N. Antimicrobial Susceptibility of Commensal Escherichia coli from Pig Fecal Samples and Enhanced Sensitivity for Direct Detection of the blaCTX-M Gene by Nested PCR. Animals. 2024; 14(18):2630. https://doi.org/10.3390/ani14182630
Chicago/Turabian StyleSuchanta, Nutchaba, Naeem Ullah, Pitak Santanirand, Nutthee Am-In, and Nuntaree Chaichanawongsaroj. 2024. "Antimicrobial Susceptibility of Commensal Escherichia coli from Pig Fecal Samples and Enhanced Sensitivity for Direct Detection of the blaCTX-M Gene by Nested PCR" Animals 14, no. 18: 2630. https://doi.org/10.3390/ani14182630





