Evaluating the Physiologic Effects of Alfaxalone, Dexmedetomidine, and Midazolam Combinations in Common Blue-Tongued Skinks (Tiliqua scincoides)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Pilot Sedation Trials
2.3. Primary Sedation Trials
2.4. Statistical Analysis
3. Results
3.1. Pilot Sedation Trials
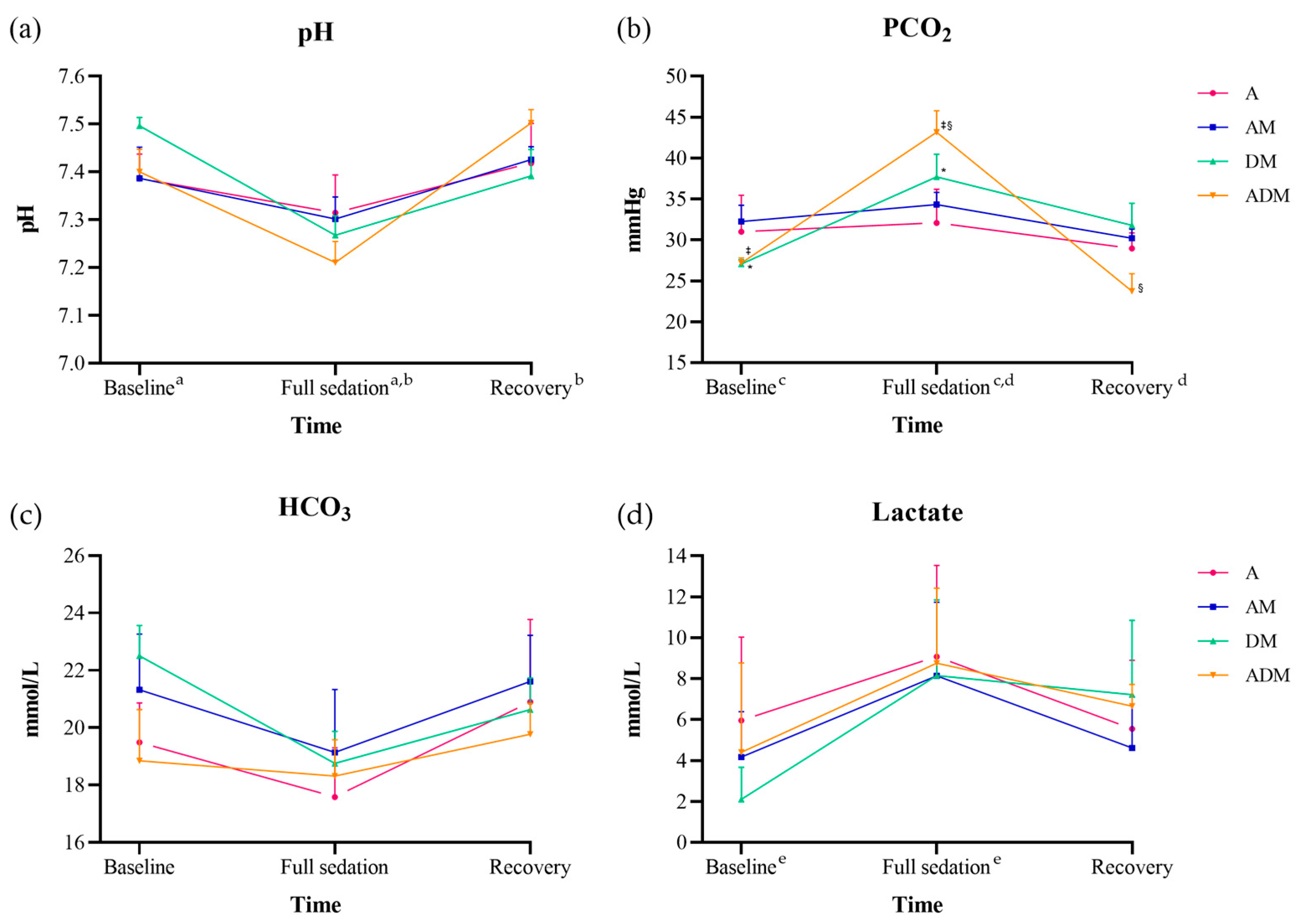
3.2. Primary Sedation Trials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheelings, T.F. Surgical management of maxillary and mandibular fractures in an eastern bluetongue skink, Tiliqua scincoides scincoides. J. Herpetol. Med. Surg. 2007, 17, 136–140. [Google Scholar] [CrossRef]
- Köchli, B.; Schmid, N.; Hatt, J.-M.; Dennler, M.; Steinmetz, H.W. Nonsurgical treatment of a bilateral mandibular fracture in a blue-tongued skink. Exot. DVM 2008, 10, 25–28. [Google Scholar]
- Scheelings, T.F.; Baker, R.T.; Hammersley, G.; Hollis, K.; Elton, I.; Holz, P. A preliminary investigation into the chemical restraint with alfaxalone of selected Australian squamate species. J. Herpetol. Med. Surg. 2011, 21, 63–67. [Google Scholar] [CrossRef]
- Kehoe, S.P.; Guzman, D.S.-M.; Sokoloff, A.M.; Grosset, C.; Weber, E.S., III; Murphy, B.; Culp, W.T.N. Partial glossectomy in a blue-tongued skink (Tiliqua scincoides) with lingual squamous cell carcinoma. J. Herpetol. Med. Surg. 2016, 26, 36–41. [Google Scholar] [CrossRef]
- Vergneau-Grosset, C.; Carmel, É.N.; Raulic, J.; Tucoulet, J.; Summa, N.; Langlois, I.; Benoit, J.-M. Vitamin d toxicosis in a blue-tongued skink (Tiliqua scincoides) presented with epistaxis and tongue discoloration. J. Herpetol. Med. Surg. 2021, 30, 224–231. [Google Scholar] [CrossRef]
- McKenzie, A.; Li, T.; Doneley, B. A comparison of two techniques to identify the sex of the eastern blue-tongue skink (Tiliqua scincoides scincoides). Aust. Vet. J. 2022, 100, 407–413. [Google Scholar] [CrossRef]
- Vetere, A.; Di Girolamo, N.; Porter, I.; Tollefson, C.; Di Ianni, F.; Nardini, G. Sex identification in juvenile and adult Indonesian blue-tongued skinks (Tiliqua gigas) through cystoscopy and accuracy of contrast radiography. J. Am. Vet. Med. Assoc. 2023, 261, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, M.F.; Sauer, C.D. Alfaxalone anaesthesia in the green iguana (Iguana iguana). Vet. Anaesth. Analg. 2011, 38, 461–466. [Google Scholar] [CrossRef]
- Knotek, Z.; Hrda, A.; Kley, N.; Knotkova, Z. Alfaxalone anaesthesia in veiled chameleon (Chamaeleo calyptratus). In Proceedings of the 18th Annual Conference ARAV, Seattle, WA, USA, 10–12 April 2011; pp. 179–181. [Google Scholar]
- Wenger, S.; Wyss, F.; Peterson, S.; Gull, J.; Hatt, J.M. The use of alfaxalone for induction of anaesthesia in selected reptile species: A preliminary clinical investigation. In Proceedings of the Diseases of Zoo and Wild Animals, Vienna, Austria, 8–11 May 2013; p. 24. [Google Scholar]
- Hansen, L.L.; Bertelsen, M.F. Assessment of the effects of intramuscular administration of alfaxalone with and without medetomidine in horsfield’s tortoises (Agrionemys horsfieldii). Vet. Anaesth. Analg. 2013, 40, e68–e75. [Google Scholar] [CrossRef]
- Kischinovsky, M.; Duse, A.; Wang, T.; Bertelsen, M.F. Intramuscular administration of alfaxalone in red-eared sliders (Trachemys scripta elegans)—Effects of dose and body temperature. Vet. Anaesth. Analg. 2013, 40, 13–20. [Google Scholar] [CrossRef]
- Knotek, Z.; Hrdá, A.; Knotková, Z.; Trnková, Š.; Babák, V. Alfaxalone anaesthesia in the green iguana (Iguana iguana). Acta Vet. Brno 2013, 82, 109–114. [Google Scholar] [CrossRef]
- Olsson, A.; Phalen, D.; Dart, C. Preliminary studies of alfaxalone for intravenous immobilization of juvenile captive estuarine crocodiles (Crocodylus porosus) and Australian freshwater crocodiles (Crocodylus johnstoni) at optimal and selected sub–optimal thermal zones. Vet. Anaesth. Analg. 2013, 40, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Scheelings, T.F. Use of intravenous and intramuscular alfaxalone in macquarie river turtles (Emydura macquarii). J. Herpetol. Med. Surg. 2013, 23, 91–94. [Google Scholar] [CrossRef]
- Shepard, M.K.; Divers, S.; Braun, C.; Hofmeister, E.H. Pharmacodynamics of alfaxalone after single-dose intramuscular administration in red-eared sliders (Trachemys scripta elegans): A comparison of two different doses at two different ambient temperatures. Vet. Anaesth. Analg. 2013, 40, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Knotek, Z. Alfaxalone as an induction agent for anaesthesia in terrapins and tortoises. Vet. Rec. 2014, 175, 327. [Google Scholar] [CrossRef]
- Doss, G.A.; Fink, D.M.; Sladky, K.K.; Mans, C. Comparison of subcutaneous dexmedetomidine–midazolam versus alfaxalone–midazolam sedation in leopard geckos (Eublepharis macularius). Vet. Anaesth. Analg. 2017, 44, 1175–1183. [Google Scholar] [CrossRef]
- Knotek, Z. Induction to inhalation anaesthesia in agamid lizards with alfaxalone. Veterinární Medicína 2017, 62, 41–43. [Google Scholar] [CrossRef]
- Morici, M. Intravenous Alfaxalone Anaesthesia in Two Squamate Species: Eublepharis macularius and Morelia spilota cheynei. Ph.D. Thesis, University of Messina, Messina, Italy, 2017. [Google Scholar]
- Perrin, K.L.; Bertelsen, M.F. Intravenous alfaxalone and propofol anesthesia in the bearded dragon (Pogona vitticeps). J. Herpetol. Med. Surg. 2017, 27, 123–126. [Google Scholar] [CrossRef]
- Phillips, B.E.; Posner, L.P.; Lewbart, G.A.; Christiansen, E.F.; Harms, C.A. Effects of alfaxalone administered intravenously to healthy yearling loggerhead sea turtles (Caretta caretta) at three different doses. J. Am. Vet. Med. Assoc. 2017, 250, 909–917. [Google Scholar] [CrossRef]
- Heuvel, M.v.d. Repeated Measurement Comparison of Different Protocols for Anesthesia and Analgesia Consisting of Alfaxalone, Meloxicam, and Butorphanol or Tramadol IM in Leopard Geckos (Eublepharis macularius). Master’s Thesis, University Utrecht, Utrecht, The Netherlands, 2017. [Google Scholar]
- Kleinschmidt, L.M.; Hanley, C.S.; Sahrmann, J.M.; Padilla, L.R. Randomized controlled trial comparing the effects of alfaxalone and ketamine hydrochloride in the Haitian giant galliwasp (Celestus warreni). J. Zoo Wildl. Med. 2018, 49, 283–290. [Google Scholar] [CrossRef]
- James, L.E.; Williams, C.J.A.; Bertelsen, M.F.; Wang, T. Anaesthetic induction with alfaxalone in the ball python (Python regius): Dose response and effect of injection site. Vet. Anaesth. Analg. 2018, 45, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Morici, M.; Di Giuseppe, M.; Spadola, F.; Oliveri, M.; Knotkova, Z.; Knotek, Z. Intravenous alfaxalone anaesthesia in leopard geckos (Eublepharis macularius). J. Exot. Pet Med. 2018, 27, 11–14. [Google Scholar] [CrossRef]
- Sadar, M.J.; Ambros, B. Use of alfaxalone or midazolam–dexmedetomidine–ketamine for implantation of radiotransmitters in bullsnakes (Pituophis catenifer sayi). J. Herpetol. Med. Surg. 2018, 28, 93–98. [Google Scholar] [CrossRef]
- Yaw, T.J.; Mans, C.; Johnson, S.M.; Doss, G.A.; Sladky, K.K. Effect of injection site on alfaxalone-induced sedation in ball pythons (python regius). J. Small Anim. Pract. 2018, 59, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Bardi, E.; Di Cesare, F.; D’Urso, E.; Gioeni, D.; Rabbogliatti, V.; Romussi, S. Total intramuscular multimodal anesthesia in pond sliders (Trachemys scripta) undergoing endoscopic gonadectomy. In Proceedings of the 4th International Conference on Avian Herpetological and Exotic Mammal Medicine, London, UK, 28 April–2 May 2019. [Google Scholar]
- Ferreira, T.H.; Mans, C.; Di Girolamo, N. Evaluation of the sedative and physiological effects of intramuscular lidocaine in bearded dragons (Pogona vitticeps) sedated with alfaxalone. Vet. Anaesth. Analg. 2019, 46, 496–500. [Google Scholar] [CrossRef]
- Strahl-Heldreth, D.E.; Clark-Price, S.C.; Keating, S.C.J.; Escalante, G.C.; Graham, L.F.; Chinnadurai, S.K.; Schaeffer, D.J. Effect of intracoelomic administration of alfaxalone on the righting reflex and tactile stimulus response of common garter snakes (Thamnophis sirtalis). Am. J. Vet. Res. 2019, 80, 144–151. [Google Scholar] [CrossRef]
- Chen, K.; Keating, S.; Strahl-Heldreth, D.; Clark-Price, S. Effects of intracoelomic alfaxalone–dexmedetomidine on righting reflex in common garter snakes (Thamnophis sirtalis): Preliminary data. Vet. Anaesth. Analg. 2020, 47, 793–796. [Google Scholar] [CrossRef]
- Nara, T.; Kondoh, D.; Yanagawa, M. Comparison of anesthetic protocols indicates Japanese grass lizards (Takydromus tachydromoides) are insensitive to medetomidine. Res. Bull. Obihiro Univ. 2020, 41, 1–5. [Google Scholar]
- Rasys, A.M.; Divers, S.J.; Lauderdale, J.D.; Menke, D.B. A systematic study of injectable anesthetic agents in the brown anole lizard (Anolis sagrei). Lab. Anim. 2020, 54, 281–294. [Google Scholar] [CrossRef]
- Yaw, T.J.; Mans, C.; Johnson, S.; Bunke, L.; Doss, G.A.; Sladky, K.K. Evaluation of subcutaneous administration of alfaxalone-midazolam and dexmedetomidine-midazolam for sedation of ball pythons (Python regius). J. Am. Vet. Med. Assoc. 2020, 256, 573–579. [Google Scholar] [CrossRef]
- Aymen, J.; Queiroz-Williams, P.; Hampton, C.C.E.; Cremer, J.; Liu, C.-C.; Nevarez, J.G. Comparison of ketamine–dexmedetomidine–midazolam versus alfaxalone–dexmedetomidine–midazolam administered intravenously to American alligators (Alligator mississippiensis). J. Herpetol. Med. Surg. 2021, 31, 132–140. [Google Scholar] [CrossRef]
- Bertelsen, M.F.; Buchanan, R.; Jensen, H.M.; Leite, C.A.C.; Abe, A.S.; Wang, T. Pharmacodynamics of propofol and alfaxalone in rattlesnakes (Crotalus durissus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 256, 110935. [Google Scholar] [CrossRef] [PubMed]
- Morici, M.; Lubian, E.; Costa, G.L.; Spadola, F. Difference between cranial and caudal intravenous alfaxalone administration in yellow-bellied sliders (Trachemys scripta scripta). Acta Vet. Eurasia 2021, 47, 88–92. [Google Scholar] [CrossRef]
- Prinz, C. Intramuscular vs Intravenous Administration of Alfaxalon for Induction in General Anaesthesia in Geochelone platynota and Astrochelys radiata. University of Veterinary Medicine Vienna, Wien, Austria, 2021. Available online: https://phaidra.vetmeduni.ac.at/open/o:1490 (accessed on 8 September 2024).
- Rockwell, K.; Boykin, K.; Padlo, J.; Ford, C.; Aschebrock, S.; Mitchell, M. Evaluating the efficacy of alfaxalone in corn snakes (Pantherophis guttatus). Vet. Anaesth. Analg. 2021, 48, 364–371. [Google Scholar] [CrossRef]
- Webb, J.K.; Keller, K.A.; Chinnadurai, S.K.; Kadotani, S.; Allender, M.C.; Fries, R. Optimizing the pharmacodynamics and evaluating cardiogenic effects of the injectable anesthetic alfaxalone in prairie rattlesnakes (Crotalus viridis). J. Zoo Wildl. Med. 2021, 52, 1105–1112. [Google Scholar] [CrossRef]
- Cardinali, M. Immobilization of a juvenile Komodo dragon using alfaxalone subcutaneously. Vet. Anaesth. Analg. 2022, 49, 521–523. [Google Scholar] [CrossRef]
- Freitag, F.A.V.; Barboza, T.K.; Dutton, C.; Buck, R.K. Alfaxalone for anesthesia of a giant snake. Vet. Anaesth. Analg. 2022, 49, 147–148. [Google Scholar] [CrossRef]
- Gantner, L.; Portier, K.; Quintard, B. Comparison of intramuscular alfaxalone with medetomidine-ketamine for inducing anaesthesia in Trachemys scripta spp. Undergoing sterilization. Vet. Anaesth. Analg. 2023, 50, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Morici, M.; Spadola, F.; Oliveri, M.; Lubian, E.; Knotek, Z. Anaesthetic induction with alfaxalone in jungle carpet python (Morelia spilota cheynei). Vet. Arh. 2023, 93, 129–136. [Google Scholar] [CrossRef]
- Shippy, S.; Allgood, H.; Messenger, K.; Hernandez, J.A.; Gatson, B.; Martin de Bustamante, M.G.; Alexander, A.B.; Wellehan, J.F.X.; Johnson, A. Pharmacokinetics and pharmacodynamics of intramuscular alfaxalone in central bearded dragons (Pogona vitticeps): Effect of injection site. Vet. Anaesth. Analg. 2023, 50, 280–288. [Google Scholar] [CrossRef]
- Webb, J.K.; Keller, K.A.; Chinnadurai, S.K.; Kadotani, S.; Allender, M.C.; Fries, R. Use of alfaxalone in bearded dragons (Pogona vitticeps): Optimizing pharmacodynamics and evaluating cardiogenic effects via echocardiography. J. Am. Vet. Med. Assoc. 2023, 261, 126–131. [Google Scholar] [CrossRef]
- Suganthan, H.; Stefano, D.D.; Buck, L.T. Alfaxalone is an effective anesthetic for the electrophysiological study of anoxia-tolerance mechanisms in western painted turtle pyramidal neurons. PLoS ONE 2024, 19, e0298065. [Google Scholar] [CrossRef] [PubMed]
- Zec, S.; Mitchell, M.A.; Rockwell, K.; Lindemann, D. Evaluating the anesthetic and physiologic effects of intramuscular and intravenous alfaxalone in eastern mud turtles (Kinosternon subrubrum). Animals 2024, 14, 460. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.J.; Belelli, D.; Peden, D.R.; Vardy, A.W.; Peters, J.A. Neurosteroid modulation of GABAa receptors. Prog. Neurobiol. 2003, 71, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L. Therapeutic review: Alfaxalone. J. Exot. Pet Med. 2012, 21, 347–353. [Google Scholar] [CrossRef]
- Lock, B.A.; Heard, D.J.; Dennis, P. Preliminary evaluation of medetomidine/ketamine combinations for immobilization and reversal with atipamezole in three tortoise species. Bull. Assoc. Reptil. Amphib. Vet. 1998, 8, 6–11. [Google Scholar] [CrossRef]
- Sleeman, J.M.; Gaynor, J. Sedative and cardiopulmonary effects of medetomidine and reversal with atipamezole in desert tortoises (Gopherus agassizii). J. Zoo Wildl. Med. 2000, 31, 28–35. [Google Scholar]
- Greer, L.L.; Jenne, K.J.; Diggs, H.E. Medetomidine-ketamine anesthesia in red-eared slider turtles (Trachemys scripta elegans). J. Am. Assoc. Lab. Anim. Sci. 2001, 40, 8–11. [Google Scholar]
- Chittick, E.J.; Stamper, M.A.; Beasley, J.F.; Lewbart, G.A.; Horne, W.A. Medetomidine, ketamine, and sevoflurane for anesthesia of injured loggerhead sea turtles: 13 cases (1996–2000). J. Am. Vet. Med. Assoc. 2002, 221, 1019–1025. [Google Scholar] [CrossRef]
- Dennis, P.M.; Heard, D.J. Cardiopulmonary effects of a medetomidine-ketamine combination administered intravenously in gopher tortoises. J. Am. Vet. Med. Assoc. 2002, 220, 1516–1519. [Google Scholar] [CrossRef]
- Terrell, G.H.-J.; Jeff, C.H.K.; Heaton-Jones, D.L. Evaluation of medetomidine–ketamine anesthesia with atipamezole reversal in American alligators (Alligator mississippiensis). J. Zoo Wildl. Med. 2002, 33, 36–44. [Google Scholar] [CrossRef]
- Young, J.S.; Min-Su, K.; Young, K.S.; Kang-Moon, S.; Tchi-Chou, N. Anesthetic effects of medetomidine-tiletamine/zolazepam combination in green iguanas (Iguana iguana). J. Vet. Clin. 2005, 22, 194–197. [Google Scholar]
- Tchi-Chou, N. The reverse effects of atipamezole on medetomidine-tiletamine/zolazepam combination anesthesia in the green iguana (Iguana iguana). J. Vet. Clin. 2006, 23, 18–21. [Google Scholar]
- Harms, C.A.; Eckert, S.A.; Kubis, S.A.; Campbell, M.; Levenson, D.H.; Crognale, M.A. Field anaesthesia of leatherback sea turtles (Dermochelys coriacea). Vet. Rec. 2007, 161, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.; Phalen, D. Preliminary studies of chemical immobilization of captive juvenile estuarine (Crocodylus porosus) and Australian freshwater (C. Johnstoni) crocodiles with medetomidine and reversal with atipamezole. Vet. Anaesth. Analg. 2012, 39, 345–356. [Google Scholar] [CrossRef]
- Schnellbacher, R.W.; Hernandez, S.M.; Tuberville, T.D.; Mayer, J.; Alhamhoom, Y.; Arnold, R.D. The efficacy of intranasal administration of dexmedetomidine and ketamine to yellow-bellied sliders (Trachemys scripta scripta). J. Herpetol. Med. Surg. 2012, 22, 91–98. [Google Scholar] [CrossRef]
- Olsson, A.; Phalen, D. The effects of decreased body temperature on the onset, duration and action of medetomidine and its antagonist atipamezole in juvenile farmed estuarine crocodiles (Crocodylus porosus). Vet. Anaesth. Analg. 2013, 40, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Emery, L.; Parsons, G.; Gerhardt, L.; Schumacher, J.; Souza, M. Sedative effects of intranasal midazolam and dexmedetomidine in 2 species of tortoises (Chelonoidis carbonaria and Geochelone platynota). J. Exot. Pet Med. 2014, 23, 380–383. [Google Scholar] [CrossRef]
- McGuire, J.L.; Hernandez, S.M.; Smith, L.L.; Yabsley, M.J. Safety and utility of an anesthetic protocol for the collection of biological samples from gopher tortoises. Wildl. Soc. Bull. 2014, 38, 43–50. [Google Scholar] [CrossRef]
- Nardini, G.; Silvetti, S.; Magnelli, I.; Girolamo, N.d.; Bielli, M. Medetomidine-ketamine-midazolam and buthorphanol (MKMB) as intramuscolar injectable combination for anesthesia in loggerhead sea turtles (Caretta caretta). Veterinaria 2014, 28, 27–31. [Google Scholar]
- Morici, M.; Interlandi, C.; Costa, G.L.; Di Giuseppe, M.; Spadola, F. Sedation with intracloacal administration of dexmedetomidine and ketamine in yellow-bellied sliders (Trachemys scripta scripta). J. Exot. Pet Med. 2017, 26, 188–191. [Google Scholar] [CrossRef]
- Stegmann, G.F.; Franklin, C.; Wang, T.; Axelsson, M.; Williams, C.J.A. Long-term surgical anaesthesia with isoflurane in human habituated Nile crocodiles. J. S. Afr. Vet. Assoc. 2017, 88, 1–6. [Google Scholar] [CrossRef]
- Barrillot, B.; Roux, J.; Arthaud, S.; Averty, L.; Clair, A.; Herrel, A.; Libourel, P.-A. Intramuscular administration of ketamine-medetomidine assures stable anaesthesia needed for long-term surgery in the Argentine tegu Salvator merianae. J. Zoo Wildl. Med. 2018, 49, 291–296. [Google Scholar] [CrossRef]
- Bisetto, S.P.; Melo, C.F.; Carregaro, A.B. Evaluation of sedative and antinociceptive effects of dexmedetomidine, midazolam and dexmedetomidine–midazolam in tegus (Salvator merianae). Vet. Anaesth. Analg. 2018, 45, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Bochmann, M.; Wenger, S.; Hatt, J.-M. Preliminary clinical comparison of anesthesia with ketamine/medetomidine and s-ketamine/medetomidine in Testudo spp. J. Herpetol. Med. Surg. 2018, 28, 40–46. [Google Scholar] [CrossRef]
- Budden, L.; Doss, G.A.; Clyde, V.L.; Mans, C. Retrospective evaluation of sedation in 16 lizard species with dexmedetomidine-midazolam with or without ketamine. J. Herpetol. Med. Surg. 2018, 28, 47–50. [Google Scholar] [CrossRef]
- Bunke, L.G.; Sladky, K.K.; Johnson, S.M. Antinociceptive efficacy and respiratory effects of dexmedetomidine in ball pythons (Python regius). Am. J. Vet. Res. 2018, 79, 718–726. [Google Scholar] [CrossRef]
- Cermakova, E.; Ceplecha, V.; Knotek, Z. Efficacy of two methods of intranasal administration of anaesthetic drugs in red-eared terrapins (Trachemys scripta elegans). Veterinární Medicína 2018, 63, 87–93. [Google Scholar] [CrossRef]
- Fink, D.M.; Doss, G.A.; Sladky, K.K.; Mans, C. Effect of injection site on dexmedetomidine-ketamine induced sedation in leopard geckos (Eublepharis macularius). J. Am. Vet. Med. Assoc. 2018, 253, 1146–1150. [Google Scholar] [CrossRef]
- Monticelli, P.; Ronaldson, H.L.; Hutchinson, J.R.; Cuff, A.R.; d’Ovidio, D.; Adami, C. Medetomidine–ketamine–sevoflurane anaesthesia in juvenile Nile crocodiles (Crocodylus niloticus) undergoing experimental surgery. Vet. Anaesth. Analg. 2019, 46, 84–89. [Google Scholar] [CrossRef]
- Turcu, M.R.; Pavel, R.; Degan, A.; GÎRdan, G.; Micsa, C.; Ovidiu, R.; IoniȚĂ, L. The use of two different anesthetic protocols for ovariectomy in Trachemys scripta elegans. Sci. Work. Ser. C Vet. Med. 2020, 66, 57–61. [Google Scholar]
- Scheelings, T.F.; Gatto, C.; Reina, R.D. Anaesthesia of hatchling green sea turtles (Chelonia mydas) with intramuscular ketamine-medetomidine-tramadol. Aust. Vet. J. 2020, 98, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Yaw, T.J.; Mans, C.; Martinelli, L.; Sladky, K.K. Comparison of subcutaneous administration of alfaxalone–midazolam–dexmedetomidine with ketamine–midazolam–dexmedetomidine for chemical restraint in juvenile blue poison dart frogs (Dendrobates tinctorius azureus). J. Zoo Wildl. Med. 2020, 50, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Eshar, D.; Rooney, T.A.; Gardhouse, S.; Beaufrère, H. Evaluation of the effects of a dexmedetomidine-midazolam-ketamine combination administered intramuscularly to captive red-footed tortoises (Chelonoidis carbonaria). Am. J. Vet. Res. 2021, 82, 858–864. [Google Scholar] [CrossRef]
- Karklus, A.A.; Sladky, K.K.; Johnson, S.M. Respiratory and antinociceptive effects of dexmedetomidine and doxapram in ball pythons (Python regius). Am. J. Vet. Res. 2021, 82, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Rooney, T.A.; Eshar, D.; Gardhouse, S.; Beaufrère, H. Evaluation of a dexmedetomidine–midazolam–ketamine combination administered intramuscularly in captive ornate box turtles (Terrapene ornata ornata). Vet. Anaesth. Analg. 2021, 48, 914–921. [Google Scholar] [CrossRef]
- Turner, R.C.; Gatson, B.J.; Hernandez, J.A.; Alexander, A.B.; Aitken-Palmer, C.; Vigani, A.; Heard, D.J. Sedation and anesthesia of Galapagos (Chelonoidis nigra), Aldabra (Aldabrachelys gigantea), and African spurred tortoises (Centrochelys sulcata): A retrospective review (2009–2019). Animals 2021, 11, 2920. [Google Scholar] [CrossRef]
- Emmel, E.S.; Rivera, S.; Cabrera, F.; Blake, S.; Deem, S.L. Field anesthesia and gonadal morphology of immature western Santa Cruz tortoises (Chelonoidis porteri). J. Zoo Wildl. Med. 2021, 51, 848–855. [Google Scholar]
- Heniff, A.C.; Petritz, O.A.; Carpenter, R.G.; Lewbart, G.A.; Balko, J.A. Anesthetic efficacy of dexmedetomidine-ketamine in eastern box turtles (Terrapene carolina carolina) is enhanced with the addition of midazolam and when administered in the forelimb versus the hindlimb. Am. J. Vet. Res. 2023, 1, 1–9. [Google Scholar] [CrossRef]
- Masi, M.; Vetere, A.; Casalini, J.; Corsi, F.; Di Ianni, F.; Nardini, G. Comparison of subcutaneous versus intramuscular dexmedetomidine–midazolam–ketamine–morphine (DMKM) mixture as chemical restraint for endoscopic sex determination in Aldabra giant tortoises (Aldabrachelys gigantea). Animals 2023, 13, 3626. [Google Scholar] [CrossRef]
- LutvİKadİC, I.; MaksimoviĆ, A. A comparison of anesthesia induction by two different administration routes and doses of ketamine and medetomidine in red-eared sliders (Trachemys scripta elegans). Ank. Üniversitesi Vet. Fakültesi Derg. 2024, 71, 231–237. [Google Scholar] [CrossRef]
- Schwartz, M.; Muñana, K.R.; Nettifee-Osborne, J.A.; Messenger, K.M.; Papich, M.G. The pharmacokinetics of midazolam after intravenous, intramuscular, and rectal administration in healthy dogs. J. Vet. Pharmacol. Ther. 2013, 36, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Le Chevallier, D.; Slingsby, L.; Murrell, J. Use of midazolam in combination with medetomidine for premedication in healthy dogs. Vet. Anaesth. Analg. 2019, 46, 74–78. [Google Scholar] [CrossRef]
- Bienzle, D.; Boyd, C.J. Sedative effects of ketamine and midazolam in snapping turtles (Chelydra serpentina). J. Zoo Wildl. Med. 1992, 23, 201–204. [Google Scholar]
- Harvey-Clark, C. Midazolam fails to sedate painted turtles, Chrysemys picta. Bull. Assoc. Reptil. Amphib. Vet. 1993, 3, 7–8. [Google Scholar] [CrossRef]
- Holz, P.; Holz, R.M. Evaluation of ketamine, ketamine/xylazine, and ketamine/midazolam anesthesia in red-eared sliders (Trachemys scripta elegans). J. Zoo Wildl. Med. 1994, 25, 531–537. [Google Scholar]
- Oppenheim, Y.C.; Moon, P.F. Sedative effects of midazolam in red-eared slider turtles (Trachemys scripta elegans). J. Zoo Wildl. Med. 1995, 26, 409–413. [Google Scholar]
- Santos, A.L.Q.; Gomes, D.O.; Lima, C.A.d.P.; Nascimento, L.R.; Menezes, L.T.; Kaminishi, Á.P.S. Anesthesia of turtle Trachemys dorbigni (duméril e bibron, 1835)-testudine: Emydidae with the combination of midazolam and propofol. Pubvet 2011, 5, 15. [Google Scholar] [CrossRef]
- Simone, S.B.S.d.; Santos, A.L.Q. Effects of the combination midazolam, fentanyl and ketamine in boas Boa constrictor linnaeus, 1758 (squamata boidae). Pubvet 2011, 5, ref. 35. [Google Scholar]
- Alves-Júnior, J.R.F.; Bosso, A.C.S.; Andrade, M.B.; Werther, K.; Santos, A.L.Q. Association of midazolam with ketamine in giant Amazon river turtles podocnemis expansa breed in captivity. Acta Cir. Bras. 2012, 27, 144–147. [Google Scholar] [CrossRef]
- Santos, A.L.Q.; Oliveira, S.R.P.d.; Kaminishi, Á.P.S.; Andrade, M.B.; Menezes, L.T.; Souza, R.R.d.; Ferreira, C.H.; Nascimento, L.R.; Moraes, F.M.d. Evaluation of the use of the combination of propofol and midazolam in the chemical restraint and anesthesia of the turtle-de-beard Phrynops geoffroanus schweigger, 1812 (testudines, chelidae). Pubvet 2012, 6, ref. 21. [Google Scholar]
- Olsson, A.; Phalen, D. Comparison of biochemical stress indicators in juvenile captive estuarine crocodiles (Crocodylus porosus) following physical restraint or chemical restraint by midazolam injection. J. Wildl. Dis. 2013, 49, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Arnett-Chinn, E.R.; Hadfield, C.A.; Clayton, L.A. Review of intramuscular midazolam for sedation in reptiles at the national aquarium, Baltimore. J. Herpetol. Med. Surg. 2016, 26, 59–63. [Google Scholar] [CrossRef]
- Lopes, I.G.; Armelin, V.A.; da Silva Braga, V.H.; Florindo, L.H. The influence of midazolam on heart rate arises from cardiac autonomic tones alterations in Burmese pythons, Python molurus. Auton. Neurosci. 2017, 208, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Simone, S.B.S.D.; Hirano, L.Q.L.; Santos, A.L.Q. Effects of midazolam in different doses in redtail boa Boa constrictor linnaeus, 1758 (squamata: Boidae). Ciência Anim. Bras. 2017, 18, e22230. [Google Scholar]
- Miller, L.J.; Fetterer, D.P.; Garza, N.L.; Lackemeyer, M.G.; Donnelly, G.C.; Steffens, J.T.; Van Tongeren, S.A.; Fiallos, J.O.; Moore, J.L.; Marko, S.T. A fixed moderate-dose combination of tiletamine+ zolazepam outperforms midazolam in induction of short-term immobilization of ball pythons (Python regius). PLoS ONE 2018, 13, e0199339. [Google Scholar] [CrossRef]
- B Larouche, C. The Use of Midazolam, Isoflurane, and Nitrous oxide for Sedation and Anesthesia of Ball Pythons (Python regius). Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2019. [Google Scholar]
- Bressan, T.F.; Sobreira, T.; Carregaro, A.B. Use of rodent sedation tests to evaluate midazolam and flumazenil in green iguanas (Iguana iguana). J. Am. Assoc. Lab. Anim. Sci. 2019, 58, 810–816. [Google Scholar] [CrossRef]
- Larouche, C.B.; Beaufrère, H.; Mosley, C.; Nemeth, N.M.; Dutton, C. Evaluation of the effects of midazolam and flumazenil in the ball python (Python regius). J. Zoo Wildl. Med. 2019, 50, 579–588. [Google Scholar] [CrossRef]
- Larouche, C.B.; Johnson, R.; Beaudry, F.; Mosley, C.; Gu, Y.; Zaman, K.A.; Beaufrère, H.; Dutton, C. Pharmacokinetics of midazolam and its major metabolite 1-hydroxymidazolam in the ball python (Python regius) after intracardiac and intramuscular administrations. J. Vet. Pharmacol. Ther. 2019, 42, 722–731. [Google Scholar] [CrossRef]
- Larouche, C.B.; Mosley, C.; Beaufrère, H.; Dutton, C. Effects of midazolam and nitrous oxide on the minimum anesthetic concentration of isoflurane in the ball python (Python regius). Vet. Anaesth. Analg. 2019, 46, 807–814. [Google Scholar] [CrossRef]
- González, V.R.; Britez, V.C.; Bazán, Y.; Maldonado, A.E.; Vetter, R.; Fiore, F. Effect of ketamine-midazolam/tiletamine-zolazepam protocols on physiological parameters in Chelonoidis carbonaria turtles subjected to routine exploratory procedures. Rev. Investig. Vet. Peru (RIVEP) 2022, 33, ref. 19. [Google Scholar]
- Hirano, L.Q.L.; de Oliveira, A.L.R.; de Barros, R.F.; Veloso, D.F.M.C.; Lima, E.M.; Santos, A.L.Q.; Moreno, J.C.D. Pharmacokinetics and pharmacodynamics of dextroketamine alone or combined with midazolam in Caiman crocodilus. J. Vet. Pharmacol. Ther. 2024, 47, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Kim, H.-y.; Matsunaga, S.; Hayashi, K.; Sasaki, N.; Tamura, H.; Takeuchi, A. Sedative effect induced by a combination of medetomidine and midazolam in pigs. J. Vet. Med. Sci. 1993, 55, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Boehm, C.A.; Carney, E.L.; Tallarida, R.J.; Wilson, R.P. Midazolam enhances the analgesic properties of dexmedetomidine in the rat. Vet. Anaesth. Analg. 2010, 37, 550–556. [Google Scholar] [CrossRef]
- Sánchez, A.; Belda, E.; Escobar, M.; Agut, A.; Soler, M.; Laredo, F.G. Effects of altering the sequence of midazolam and propofol during co-induction of anaesthesia. Vet. Anaesth. Analg. 2013, 40, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Tranquilli, W.J.; Grimm, K.A. Introduction: Use, definitions, history, concepts, classification, and considerations for anesthesia and analgesia. In Veterinary Anesthesia and Analgesia, 5th ed.; Grimm, K.A., Lamont, L.A., Tranquilli, W.J., Greene, S.A., Robertson, S.A., Eds.; WILEY Blackwell: Hoboken, NJ, USA, 2015; pp. 1–10. [Google Scholar]
- Kristensen, L.; Malte, C.L.; Malte, H.; Wang, T.; Williams, C.J.A. Obesity prolongs induction times in reptiles. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2022, 271, 111255. [Google Scholar] [CrossRef]
- Valverde, A.; Skelding, A.M. Alternatives to opioid analgesia in small animal anesthesia: Alpha-2 agonists. Vet. Clin. Small Anim. Pract. 2019, 49, 1013–1027. [Google Scholar] [CrossRef]
- Yaksh, T.L. Pharmacology of spinal adrenergic systems which modulate spinal nociceptive processing. Pharmacol. Biochem. Behav. 1985, 22, 845–858. [Google Scholar] [CrossRef]
- Gil, D.W.; Cheevers, C.V.; Kedzie, K.M.; Manlapaz, C.A.; Rao, S.; Tang, E.; Donello, J.E. A-1-adrenergic receptor agonist activity of clinical α-adrenergic receptor agonists interferes with α-2-mediated analgesia. J. Am. Soc. Anesthesiol. 2009, 110, 401–407. [Google Scholar] [CrossRef]
- Virtanen, R.; Savola, J.-M.; Saano, V.; Nyman, L. Characterization of the selectivity, specificity and potency of medetomidine as an α2-adrenoceptor agonist. Eur. J. Pharmacol. 1988, 150, 9–14. [Google Scholar]
- Lovell, S.; Simon, B.; Boudreau, E.C.; Mankin, J.; Jeffery, N. Randomized clinical trial comparing outcomes after fentanyl or ketamine-dexmedetomidine analgesia in thoracolumbar spinal surgery in dogs. J. Vet. Intern. Med. 2022, 36, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Cabanac, A.; Cabanac, M. Heart rate response to gentle handling of frog and lizard. Behav. Process. 2000, 52, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Klide, A.M.; Calderwood, H.W.; Soma, L.R. Cardiopulmonary effects of xylazine in dogs. Am. J. Vet. Res. 1975, 36, 931–935. [Google Scholar]
- Giovannitti, J.A., Jr.; Thoms, S.M.; Crawford, J.J. Alpha-2 adrenergic receptor agonists: A review of current clinical applications. Anesth. Prog. 2015, 62, 31–39. [Google Scholar] [CrossRef]
- Cardoso, C.G.; Marques, D.R.C.; da Silva, T.H.M.; de Mattos-Junior, E. Cardiorespiratory, sedative and antinociceptive effects of dexmedetomidine alone or in combination with methadone, morphine or tramadol in dogs. Vet. Anaesth. Analg. 2014, 41, 636–643. [Google Scholar] [CrossRef]
- Cerreta, A.J.; Cannizzo, S.A.; Smith, D.C.; Minter, L.J. Venous hematology, biochemistry, and blood gas analysis of free-ranging eastern copperheads (Agkistrodon contortrix) and eastern ratsnakes (Pantherophis alleghaniensis). PLoS ONE 2020, 15, e0229102. [Google Scholar] [CrossRef] [PubMed]
- Congdon, J.M.; Marquez, M.; Niyom, S.; Boscan, P. Cardiovascular, respiratory, electrolyte and acid–base balance during continuous dexmedetomidine infusion in anesthetized dogs. Vet. Anaesth. Analg. 2013, 40, 464–471. [Google Scholar] [CrossRef]
- Bertelsen, M.F.; Buchanan, R.; Jensen, H.M.; Leite, C.A.C.; Abe, A.S.; Nielsen, S.S.; Wang, T. Assessing the influence of mechanical ventilation on blood gases and blood pressure in rattlesnakes. Vet. Anaesth. Analg. 2015, 42, 386–393. [Google Scholar] [CrossRef]
- Allen, S.E.; Holm, J.L. Lactate: Physiology and clinical utility. J. Vet. Emerg. Crit. Care 2008, 18, 123–132. [Google Scholar] [CrossRef]
- Franklin, C.E.; Davis, B.M.; Peucker, S.K.J.; Stephenson, H.; Mayer, R.; Whittier, J.; Lever, J.; Grigg, G.C. Comparison of stress induced by manual restraint and immobilisation in the estuarine crocodile, Crocodylus porosus. J. Exp. Zool. Part A Comp. Exp. Biol. 2003, 298A, 86–92. [Google Scholar] [CrossRef]
- Goessling, J.M.; Mendonça, M.T. Physiological responses of gopher tortoises (Gopherus polyphemus) to trapping. Conserv. Physiol. 2021, 9, coab003. [Google Scholar] [CrossRef] [PubMed]


| Drugs | Pilot | Primary | Alfaxalone | Dexmedetomidine | Midazolam |
|---|---|---|---|---|---|
| N of Skinks | |||||
| A | |||||
| 2 | 10 mg/kg | ||||
| 2 | 15 mg/kg | ||||
| 2 | 17 mg/kg | ||||
| 2 | 11 | 20 mg/kg | |||
| AM | |||||
| 2 | 11 | 10 mg/kg | 1 mg/kg | ||
| 2 | 15 mg/kg | 1 mg/kg | |||
| DM | |||||
| 2 | 0.05 mg/kg | 1 mg/kg | |||
| 2 | 11 | 0.1 mg/kg | 1 mg/kg | ||
| ADM | |||||
| 2 | 11 | 5 mg/kg | 0.05 mg/kg | 0.5 mg/kg | |
| Variables | A | AM | DM | ADM |
|---|---|---|---|---|
| Time to loss of righting reflex | 8.18 ± 3.37 | 8.18 ± 4.05 | 14.09 ± 6.64 | 9.09 ± 4.37 |
| Duration of loss of righting reflex * | 38.18 ± 8.86 a | 41.82 ± 9.83 | 40.91 ± 18.07 b | 55.45 ± 4.98 ab |
| Duration of loss of escape reflex * | 20 (0–35) c | 30 (15–35) d | 35 (0–45) | 55 (45–60) cd |
| Time to intubation * | 10 (5–20) e | 5 (5–10) f | 10 (10–30) efg | 5 (5–15) g |
| Duration of intubation * | 23.18 ± 9.11 h | 31.36 ± 10.46 i | 28.18 ± 21.24 j | 48.18 ± 10.06 hij |
| Time to recovery † | 55 (45–60) | 60 (50–60) | 60 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhim, H.; Godke, A.M.; Aguilar, M.G.; Mitchell, M.A. Evaluating the Physiologic Effects of Alfaxalone, Dexmedetomidine, and Midazolam Combinations in Common Blue-Tongued Skinks (Tiliqua scincoides). Animals 2024, 14, 2636. https://doi.org/10.3390/ani14182636
Rhim H, Godke AM, Aguilar MG, Mitchell MA. Evaluating the Physiologic Effects of Alfaxalone, Dexmedetomidine, and Midazolam Combinations in Common Blue-Tongued Skinks (Tiliqua scincoides). Animals. 2024; 14(18):2636. https://doi.org/10.3390/ani14182636
Chicago/Turabian StyleRhim, Haerin, Ashleigh M. Godke, M. Graciela Aguilar, and Mark A. Mitchell. 2024. "Evaluating the Physiologic Effects of Alfaxalone, Dexmedetomidine, and Midazolam Combinations in Common Blue-Tongued Skinks (Tiliqua scincoides)" Animals 14, no. 18: 2636. https://doi.org/10.3390/ani14182636





