Seasonal Analysis of Spatial Distribution Patterns and Characteristics of Sepiella maindroni and Sepia kobiensis in the East China Sea Region
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
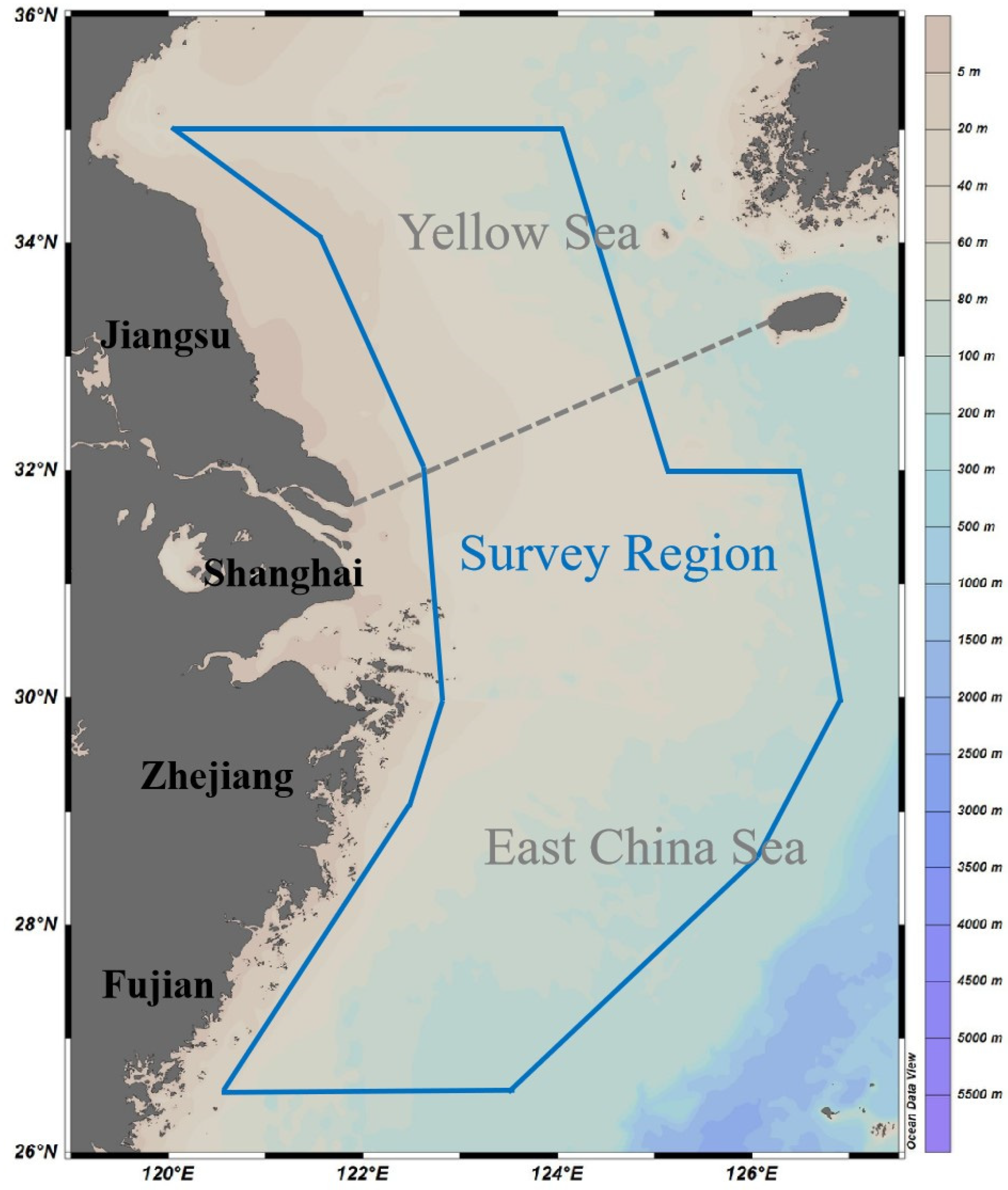
2.1. Survey Area and Procedures
2.2. Modeling
2.3. Predictions for the Future
3. Results and Discussion
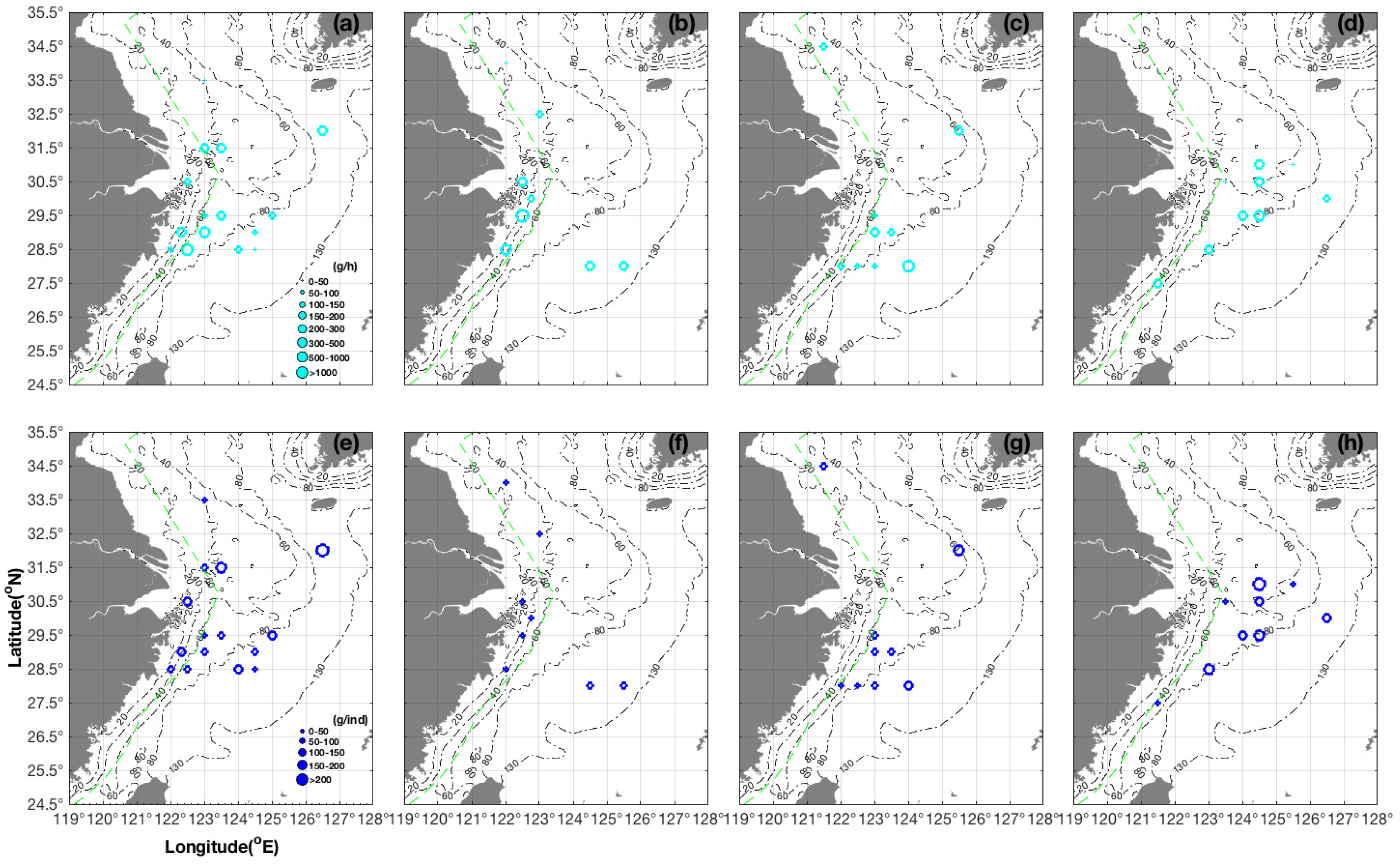
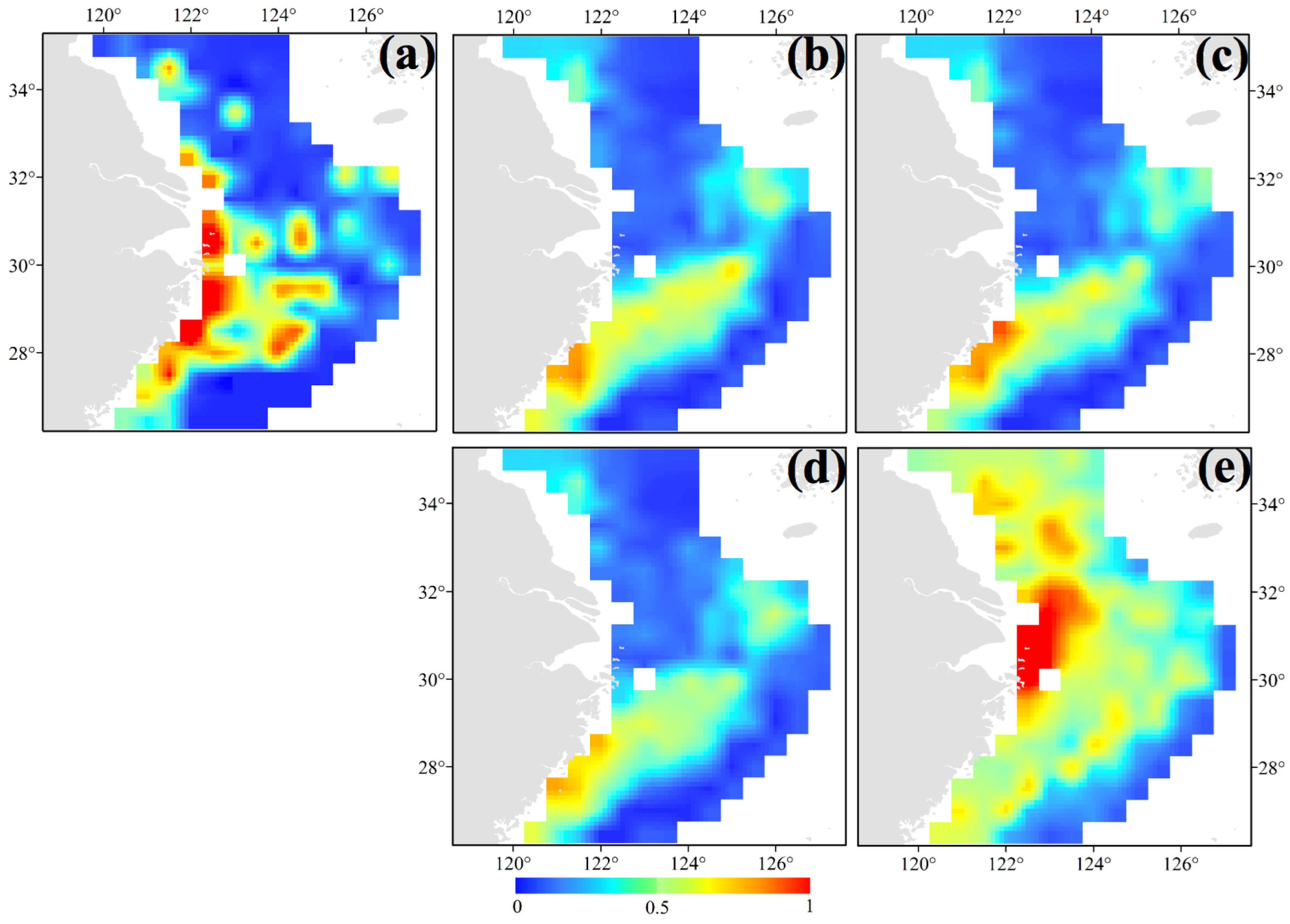
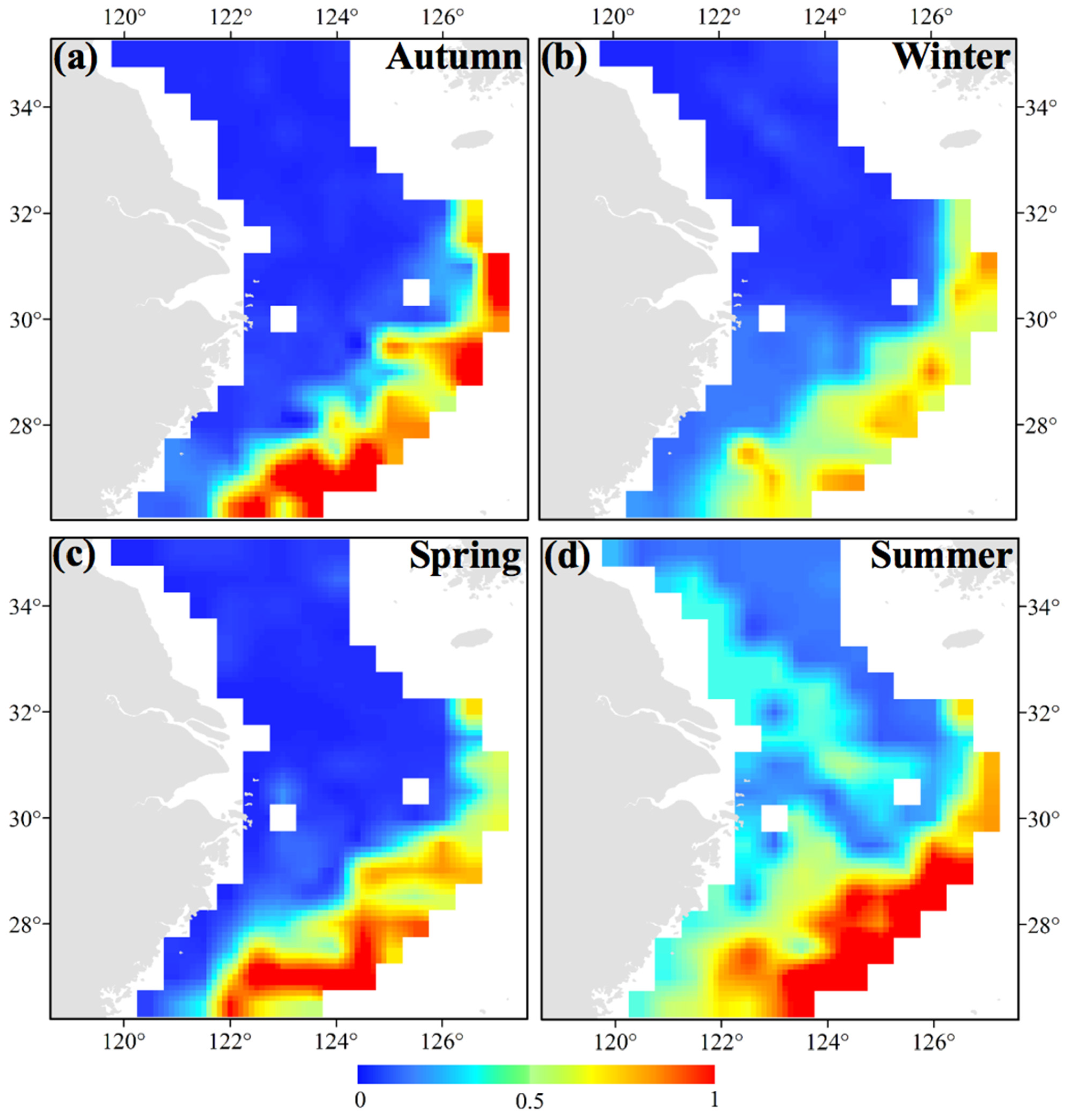
3.1. Seasonal and Spatial Distribution Characteristics of S. maindroni
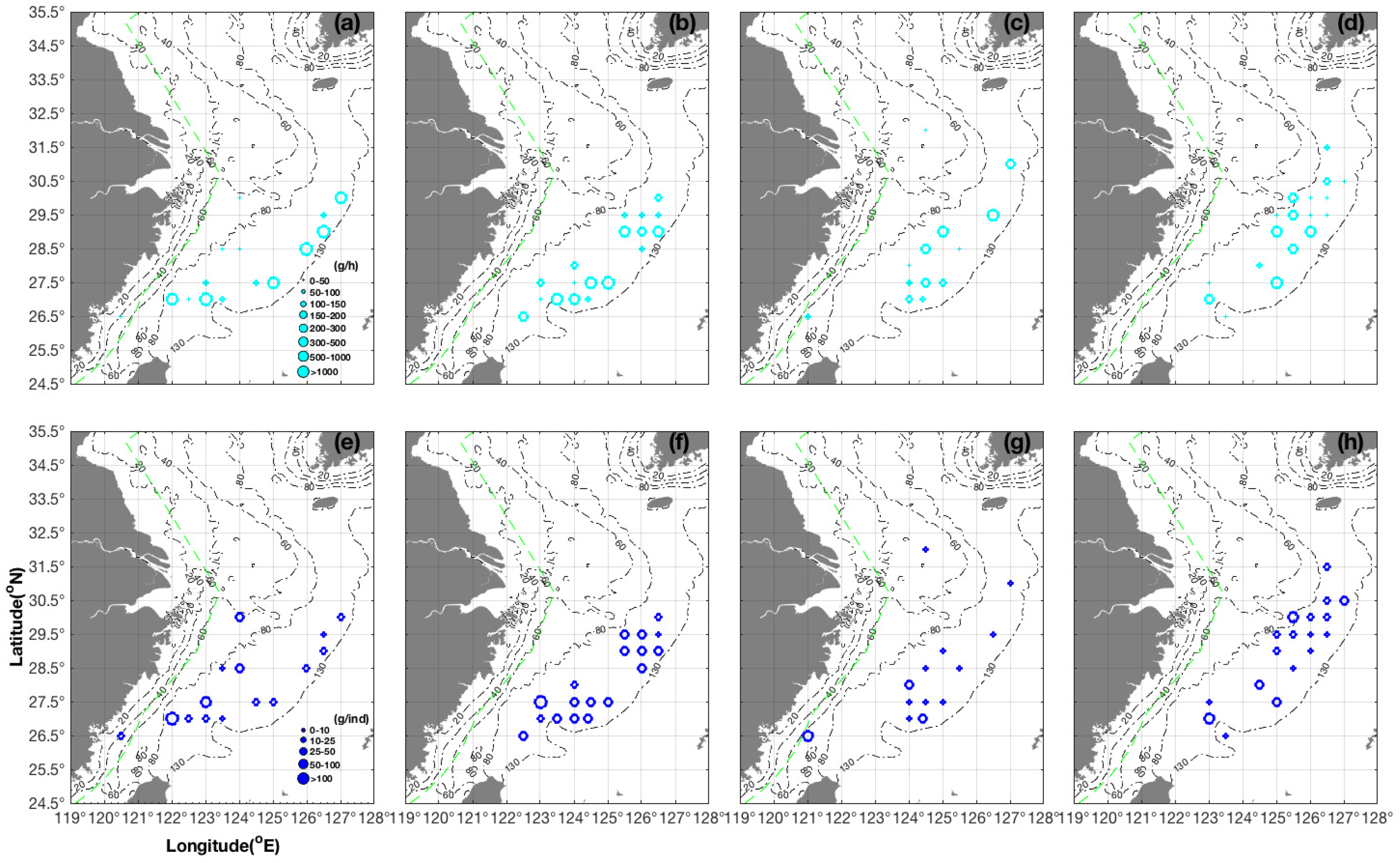
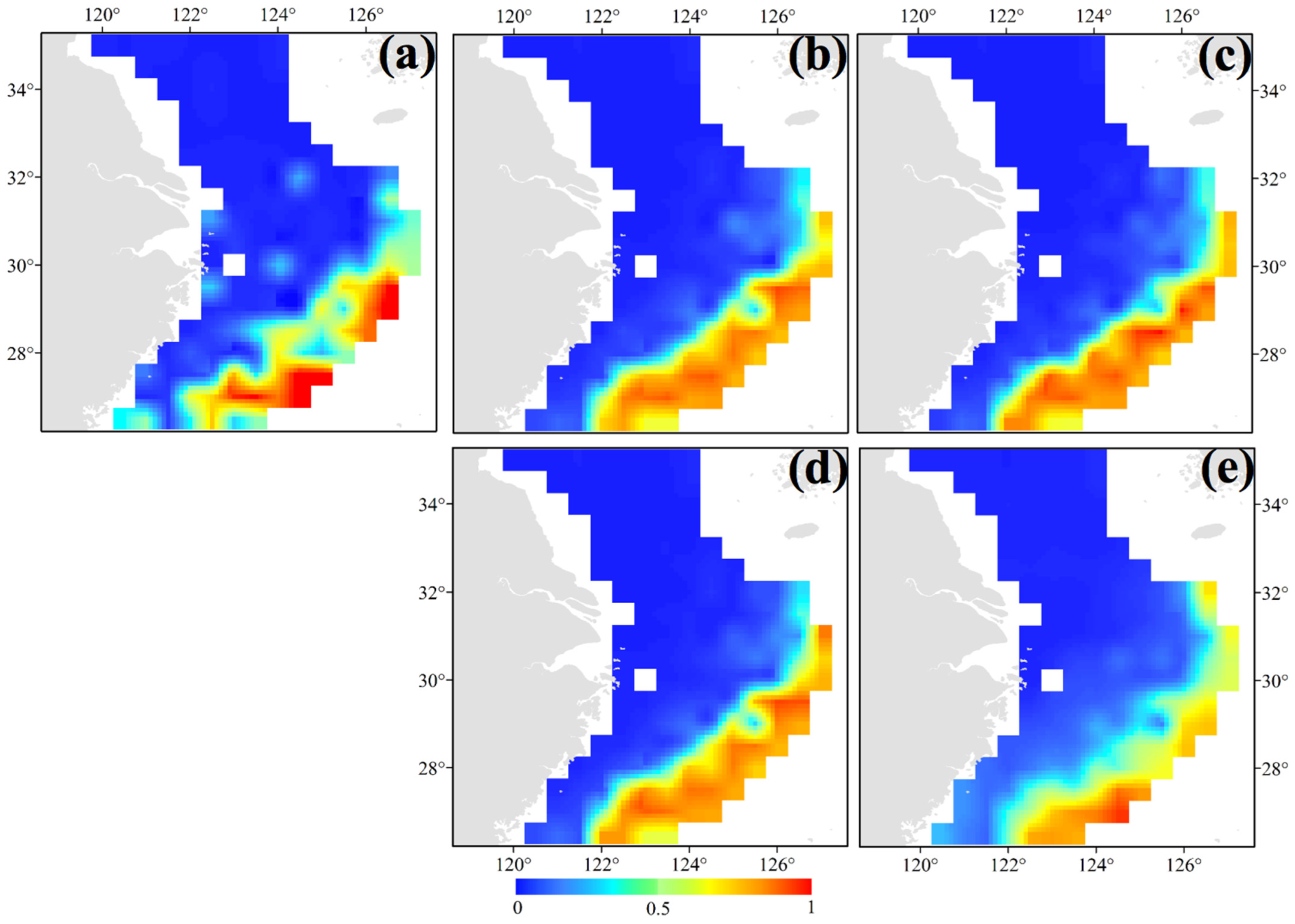
3.2. Seasonal and Spatial Distribution Characteristics of S. kobiensis
3.3. Range of Environmental Variables for S. maindroni and S. kobiensis
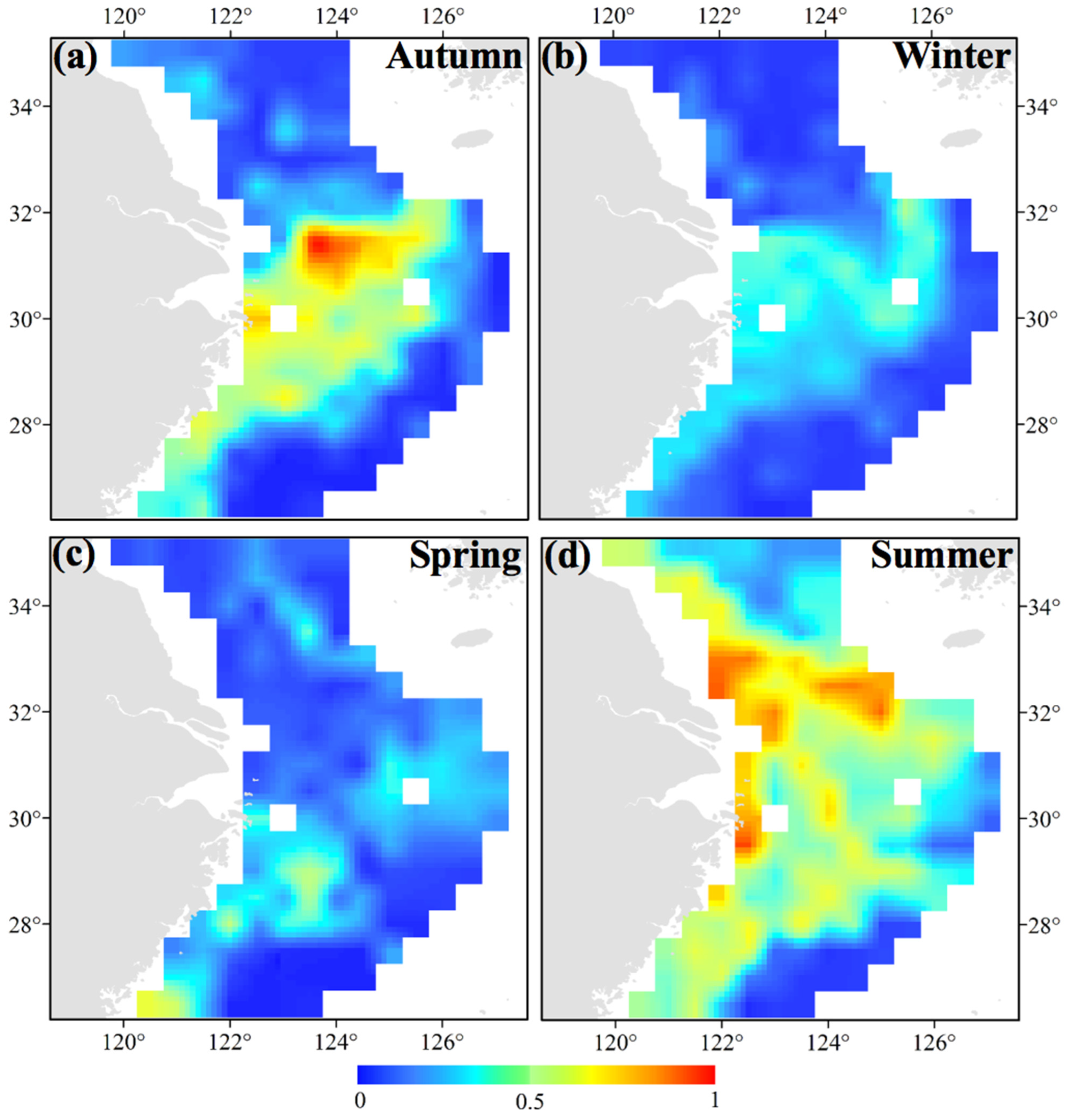
3.4. Most Suitable Habitat Areas for S. maindroni and S. kobiensis in Present and Future Scenarios
4. Conclusions
- (1)
- We found the majority of groups of S. maindroni in the central and southern areas of the East China Sea near the closed fishing lines in spring. In summer, they moved to the southern Yellow Sea near the closed fishing lines and Zhejiang Islands, and in autumn they moved to the central and northern areas of the East China Sea near the closed fishing lines. Finally, in winter they migrated to the northern East China Sea off the Yangtze Estuary areas and the southwest corner of the survey area near the closed fishing lines. Generally, they moved from inshore to offshore areas from spring to winter, which was indicated by the SSS index;
- (2)
- Climate change scenarios indicated that the habitat areas of S. maindroni will shift to the south first and then to the north of the study area with the intensification of CO2 emissions. The habitat area will first expand and then substantially reduce. Generally, the habitat area range in cases of slight CO2 emissions may always be larger than in cases of heavy emissions;
- (3)
- In spring and summer, we found the major groups of S. kobiensis in the southern East China Sea, especially in the southeast offshore corner of the survey area. In autumn, they were distributed in shallow areas, and finally they migrated to the warmer marginal areas in the central and southern East China Sea in winter. Generally, the majority of groups with large numbers of individuals stayed in the overwintering grounds from winter to spring, but the numbers largely decreased in summer when the adults died after releasing eggs. The number of individuals increased in autumn due to the presence of numerous juveniles. Climate change scenarios showed that the rising SST may result in the enlargement of the habitat of this species.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jereb, P.; Roper, C.F.E. “Cephalopods of the world” in An annotated and illustrated catalogue of cephalopod species known to date. In Myopsid and Oegopsid Squids; FAO: Rome, Italy, 2010; Volume 2. [Google Scholar]
- Navarro, J.; Coll, M.; Somes, C.J.; Olson, R.J. Trophic niche of squids: Insights from isotopic data in marine systems worldwide. Deep Sea Res. Part II 2013, 95, 93–102. [Google Scholar] [CrossRef]
- Rodhouse, P.G.; Symon, C.; Hatfield, E.M.C. Early life cycle of cephalopods in relation to the major oceanographic features of the southwest Atlantic Ocean. Mar. Ecol. Prog. Ser. Oldendorf 1992, 89, 183–195. [Google Scholar] [CrossRef]
- Chen, F.; Wei, G.; Li, N.; Fang, Z.; Zhang, H.; Zhou, Y.; Jiang, R. Modelling the spatial distribution of habitats of main cephalopod taxa in inshore waters of Zhejiang Province at spring and autumn. Res. Sq. 2021, 1–24. [Google Scholar] [CrossRef]
- Yan, L.P.; Li, S.F.; Ling, J.Z.; Zheng, Y.J. Study on the resource alteration of commercial cuttlefish in the East China Sea. Mar. Sci. 2007, 4, 27–31, (In Chinese with English abstract). [Google Scholar]
- Li, J.J.; Guo, B.Y.; Wu, C.W. A review of the resource evolvement and the way of restoration of Sepiella maindroni in coastal waters of Zhejiang Province. J. Zhejiang Ocean. Univ. 2011, 30, 381–385+396, (In Chinese with English abstract). [Google Scholar]
- Zhu, W.B.; Xue, L.J.; Lu, Z.H.; Xu, H.X.; Xu, K.D. Cephalopod community structure and its relationship with environmental factors in the southern East China Sea. Oceanol. Limnol. Sin. 2014, 45, 436–442, (In Chinese with English abstract). [Google Scholar]
- Guo, X.; Fan, G.Z.; Jia, G.S. A preliminary study of the feeding habit of Sepiella maindroni De Rechebrune. J. Zhejiang Coll. Fish. 1986, 5, 171–175, (In Chinese with English abstract). [Google Scholar]
- Li, X.X.; Dai, J.S.; Tong, D.Y. An ecological environmental investigation on the egg-attaching area of Sepiella maindroni De Rechebrune. J. Zhejiang Coll. Fish. 1986, 5, 121–124, (In Chinese with English abstract). [Google Scholar]
- Yang, J.M. A study on food and trophic levels of Bohai Sea Invertebrates. Mod. Fish. Inf. 2001, 16, 8–16, (In Chinese with English abstract). [Google Scholar]
- Ni, Z.Y.; Xu, H.X. The preliminary study of the population of the cuttlefish Sepiella maindroni de Rochebrune along the East China Sea. Mar. Sci. 1985, 9, 41–45, (In Chinese with English abstract). [Google Scholar]
- Lei, S.H.; Wu, C.W.; Gao, T.X.; Hao, Z.L.; Zhang, X.M. A comparative study of Sepia esculenta and Sepiella maindroni on embryonic development and ability of salinity tolerance. J. Fish. Sci. China 2011, 18, 350–359, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Wu, Y.Q.; Tang, Z.C. Population composition and migratory distribution of cuttlefish (Sepiella maindroni de Rochebrune) in the Huanghe Estuary and the Laizhou Gulf. J. Fish. China 1990, 14, 149–152, (In Chinese with English abstract). [Google Scholar]
- Zhang, B.L.; Sun, D.Y.; Bi, H.S.; Wu, Y.Q.; Huang, B. Growth and seasonal distribution of Sepiella maindroni in the Jiaozhou Bay and adjacent waters. Mar. Sci. 1997, 5, 61–64, (In Chinese with English abstract). [Google Scholar]
- Tang, Y.M.; Wu, C.W. Biological characteristics of Sepiella maindroni de Rochebrune and changes of the clam-fishing grounds. J. Zhejiang Coll. Fish. 1986, 5, 165–170, (In Chinese with English abstract). [Google Scholar]
- Yi, F.; Wang, C.L.; Song, W.W. Tolerance of Sepiella maindroni larvae to environmental conditions. J. Guangdong Ocean. Univ. 2005, 4, 39–43, (In Chinese with English abstract). [Google Scholar]
- Wu, C.W.; Dong, Z.Y.; Chi, C.F.; Ding, F. Reproductive and spawning habits of Sepiella maindroni off Zhejiang, China. Oceanol. Limnol. Sin. 2010, 41, 39–46, (In Chinese with English abstract). [Google Scholar]
- Chen, F.; Qu, J.Y.; Fang, Z.; Zhang, H.L.; Zhou, Y.D.; Liang, J. Variation of community structure of cephalopods in spring and autumn along the coast of Zhejiang Province. J. Fish. China 2020, 44, 1317–1328, (In Chinese with English abstract). [Google Scholar]
- Ciannelli, L.; Fisher, J.A.D.; Skern-Mauritzen, M.; Hunsicker, M.E.; Hidalgo, M.; Frank, K.T.; Bailey, K.M. Theory, consequences and evidence of eroding population spatial structure in harvested marine fishes: A review. Mar. Ecol. Prog. Ser. 2013, 480, 227–243. [Google Scholar] [CrossRef]
- Puerta, P.; Hidalgo, M.; González, M.; Esteban, A.; Quetglas, A. Role of hydro-climatic and demographic processes on the spatio-temporal distribution of cephalopods in the western Mediterranean. Mar. Ecol. Prog. Ser. 2014, 514, 105–118. [Google Scholar] [CrossRef]
- Jin, Y.; Jin, X.; Gorfine, H.; Wu, Q.; Shan, X. Modeling the oceanographic impacts on the spatial distribution of common cephalopods during autumn in the Yellow Sea. Front. Mar. Sci. 2020, 7, 432. [Google Scholar] [CrossRef]
- Jiang, X.M.; Lu, Z.R.; He, H.J.; Ye, B.L.; Ying, Z.; Wang, C.L. Effects of several ecological factors on the hatching of Sepiella maindroni wild and cultured eggs. Chin. J. Appl. Ecol. 2010, 21, 1321–1326, (In Chinese with English abstract). [Google Scholar]
- Climate Change Center of China Meteorological Administration (Ed.) China Climate Change Blue Book; Science Press: Beijing, China, 2023; Volume 110. (In Chinese) [Google Scholar]
- Xu, M.; Yang, L.; Liu, Z.; Zhang, Y.; Zhang, H. Seasonal and spatial distribution characteristics of Sepia esculenta in the East China Sea Region: Transfer of the central distribution from 29° N to 28° N. Animals 2024, 14, 1412. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Feng, W.; Liu, Z.; Li, Z.; Song, X.; Zhang, H.; Zhang, C.; Yang, L. Seasonal-spatial distribution variations and predictions of Loliolus beka and Loliolus uyii in the East China Sea Region: Implications from Climate Change Scenarios. Animals 2024, 14, 2070. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.; Liu, Z.; Liu, Y.; Zhang, Y.; Yang, L.; Wang, F.; Wu, H.; Cheng, J. Seasonal distribution of the early life stages of the small yellow croaker (Larimichthys polyactis) and its dynamic controls adjacent to the Changjiang River Estuary. Fish. Oceanogr. 2023, 32, 390–404. [Google Scholar] [CrossRef]
- Xu, M.; Liu, Z.; Wang, Y.; Jin, Y.; Yuan, X.; Zhang, H.; Song, X.; Otaki, T.; Yang, L.; Cheng, J. Larval spatiotemporal distribution of six fish species: Implications for sustainable fisheries management in the East China Sea. Sustainability 2022, 14, 14826. [Google Scholar] [CrossRef]
- Xu, M.; Liu, Z.; Song, X.; Wang, F.; Wang, Y.; Yang, L.; Otaki, T.; Shen, J.; Komatsu, T.; Cheng, J. Tidal variations of fish larvae measured using a 15-day continuous ichthyoplankton survey in Subei shoal: Management implications for the red-Crowned Crane (Grus japonensis) population in Yancheng Nature Reserve. Animals 2023, 13, 3088. [Google Scholar] [CrossRef]
- Liu, S.; Tian, Y.; Liu, Y.; Alabia, I.D.; Cheng, J.; Ito, S. Development of a prey-predator species distribution model for a large piscivorous fish: A case study for Japanese Spanish mackerel Scomberomorus niphonius and Japanese anchovy Engraulis japonicus. Deep Sea Res. Part II Top. Stud. Oceanogr. 2023, 207, 105227. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Teschke, K.; Hindell, M.A.; Downey, R.; Woods, B.; Kang, B.; Ma, S.; Zhang, C.; Li, J.; et al. Incorporating mesopelagic fish into the evaluation of conservation areas for marine living resources under climate change scenarios. Mar. Life Sci. Technol. 2024, 6, 68–83. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Vignali, S.; Barras, A.G.; Arlettaz, R.; Braunisch, V. SDMtune: An R package to tune and evaluate species distribution models. Ecol. Evol. 2020, 10, 11488–11506. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Thuiller, W. Editorial commentary on “BIOMOD—Optimizing predictions of species distributions and projecting potential future shifts under global change”. Glob. Chang. Biol. 2014, 20, 3591–3592. [Google Scholar] [CrossRef]
- Pearce, J.; Ferrier, S. Evaluating the predictive performance of habitat models developed using logistic regression. Ecol. Model. 2000, 133, 225–245. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudik, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Alabia, I.D.; Saitoh, S.-I.; Igarashi, H.; Ishikawa, Y.; Usui, N.; Kamachi, M.; Awaji, T.; Seito, M. Ensemble squid habitat model using three-dimensional ocean data. ICES J. Mar. Sci. 2016, 73, 1863–1874. [Google Scholar] [CrossRef]
- Riahi, K.; Van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’Neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O.; et al. The shared socioeconomic pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Chang. 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Boucher, O.; Denvil, S.; Levavasseur, G.; Cozic, A.; Caubel, A.; Foujols, M.-A.; Meurdesoif, Y.; Bony, S.; Flavoni, S.; Idelkadi, A.; et al. IPSL IPSL-CM6A-LR Model Output Prepared for CMIP6 CFMIP amip-p4K-lwoff. World Data Center for Climate (WDCC) at DKRZ. 2023. Available online: https://hdl.handle.net/21.14106/8c1b7f221326e56bd3c57d2da9bf7978a4cf43fd (accessed on 17 September 2024).
- Wieners, K.-H.; Giorgetta, M.; Jungclaus, J.; Reick, C.; Esch, M.; Bittner, M.; Legutke, S.; Schupfner, M.; Wachsmann, F.; Gayler, V.; et al. MPI-M MPI-ESM1.2-LR Model Output Prepared for CMIP6-CMIP Historical; Earth System Grid Federation: Rome, Italy, 2019. [Google Scholar] [CrossRef]
- Wu, C.W.; Zhou, C.; Guo, B.Y.; Zhang, J.S. Study on changes in reproductive biology characteristics of Sepiella maindroni (Rochebrune) offshore Zhejiang. Oceanol. Limnol. Sin. 2012, 43, 689–694, (In Chinese with English abstract). [Google Scholar]
- Cheng, J.S. Studies on fishery biology of squid and its resources in the Yellow Sea. J. Fish. Sci. China 1997, 4, 22–28, (In Chinese with English abstract). [Google Scholar]
- Rosa, A.L.; Yamamoto, J.; Sakurai, Y. Effects of environmental variability on the spawning areas, catch, and recruitment of the Japanese common squid, Todarodes pacificus (Cephalopoda: Ommastrephidae), from the 1970s to the 2000s. ICES J. Mar. Sci. 2011, 68, 1114. [Google Scholar] [CrossRef]
- Zhang, J.S.; Chi, C.F.; Wu, C.W. Biological zero temperature and effective accumulated temperature for embryonic development of Sepiella maindroni. South China Fish. Sci. 2011, 7, 45–49, (In Chinese with English abstract). [Google Scholar]
- Li, Y.M.; Miao, Z.Q. Hydrologic nature of squid spawning grounds in northern Zhejiang Sea. J. Zhejiang Coll. Fish. 1986, 5, 139–145. (In Chinese) [Google Scholar]
- Zheng, X.; Ikeda, M.; Kong, L.; Lin, X.; Li, Q.; Taniguchi, N. Genetic diversity and population structure of the golden cuttlefish, Sepia esculenta (Cephalopoda: Sepiidae) indicated by microsatellite DNA variations. Mar. Ecol. 2009, 30, 448–454. [Google Scholar] [CrossRef]
- Rosa, R.; Dierssen, H.M.; Gonzalez, L.; Seibel, B.A. Ecological biogeography of cephalopod mollusks in the Atlantic Ocean: Historical and contemporary causes of coastal diversity patterns. Glob. Ecol. Biogeogr. 2008, 17, 600–610. [Google Scholar] [CrossRef]
- Hoving, H.J.T.; Robison, B.H. Vampire squid: Detritivores in the oxygen minimum zone. Proc. R. Soc. B 2012, 279, 4559–4567. [Google Scholar] [CrossRef]
- Gutowska, M.A.; Portner, H.O.; Melzner, F. Growth and calcification in the cephalopod Sepia officinalis under elevated seawater pCO2. Mar. Ecol. Prog. Ser. 2008, 373, 303–309. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rodhouse, P.G. Rapid selection effects in a shortlived semelparous squid species exposed to exploitation: Inferences from the optimisation of life-history functions. Evol. Ecol. 1999, 13, 517–537. [Google Scholar] [CrossRef]
- Hoving, H.J.T.; Gilly, W.F.; Markaida, U.; Benoit-Bird, K.J.; West-Brown, Z.; Daniel, P.; Field, J.; Paressenti, L.; Liu, B.; Campos, B. Extreme plasticity in life history strategy allows a migratory predator (jumbo squid) to cope with a changing climate. Glob. Chang. Biol. 2013, 19, 2089–2103. [Google Scholar] [CrossRef]
| Sepiella maindroni | Sepia kobiensis | |||||||
|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | |
| Mean CPUEw at all stations | 21.17 | 17.78 | 14.95 | 13.35 | 66.26 | 37.19 | 18.50 | 25.05 |
| Mean CPUEw at collection stations | 199.02 | 311.08 | 210.91 | 166.09 | 627.30 | 289.26 | 180.69 | 154.48 |
| Value range of CPUEw | 22.30–806.90 | 39.87–1111.20 | 61.04–835.70 | 13.60–311.20 | 13.29–3400.00 | 19.40–1528.36 | 27.69–715.29 | 3.90–672.00 |
| Mean CPUEn at all stations | 0.28 | 0.69 | 0.18 | 0.20 | 4.58 | 1.34 | 3.90 | 3.26 |
| Mean CPUEn at collection stations | 2.64 | 12.13 | 2.59 | 2.54 | 43.38 | 10.44 | 38.08 | 20.13 |
| Value range of CPUEn | 1.00–12.41 | 2.77–30.00 | 1.00–6.00 | 0.97–9.00 | 1.00–200.00 | 1.00–49.09 | 1.00–150.59 | 1.00–170.85 |
| Mean AIW | 90.41 | 32.56 | 86.19 | 109.35 | 27.92 | 31.57 | 14.45 | 21.89 |
| Value range of AIW | 22.30–213.92 | 4.50–65.98 | 23.00–168.57 | 3.40–276.36 | 1.68–129.00 | 4.44–110.40 | 2.38–66.40 | 1.27–73.58 |
| Factor | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| Sepiella maindroni | ||||
| Depth (m) | 19.00–101.00 | 20.00–108.00 | 15.00–93.00 | 46.00–92.00 |
| SST (°C) | 14.88–23.60 | 25.17–27.50 | 19.13–23.08 | 14.56–17.80 |
| SBT (°C) | 11.56–20.16 | 18.43–27.79 | 16.79–21.98 | 14.64–17.27 |
| SSS (‰) | 28.80–34.45 | 27.86–33.83 | 31.88–33.99 | 33.50–34.20 |
| SBS (‰) | 28.95–34.77 | 30.54–34.61 | 32.00–34.57 | 33.54–34.37 |
| SSDO (mg/L) | 7.84–8.35 | 5.32–5.92 | 7.73–8.28 | |
| SBDO (mg/L) | 8.04–8.84 | 3.10–6.12 | 7.81–8.26 | |
| Sepia kobiensis | ||||
| Depth (m) | 56.00–116.00 | 10.00–120.00 | 41.00–115.00 | 60.00–145.00 |
| SST (°C) | 16.72–24.56 | 26.11–29.19 | 20.17–24.82 | 14.94–22.34 |
| SBT (°C) | 15.16–21.93 | 17.23–28.19 | 18.26–21.99 | 15.10–21.55 |
| SSS (‰) | 31.73–34.62 | 32.55–34.30 | 32.71–34.40 | 33.77–34.53 |
| SBS (‰) | 33.51–35.08 | 33.68–34.68 | 32.94–34.71 | 33.89–34.72 |
| SSDO (mg/L) | 5.11–6.54 | 7.10–8.20 | ||
| SBDO (mg/L) | 4.53–6.60 | 7.20–8.17 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Liu, S.; Zhang, H.; Li, Z.; Song, X.; Yang, L.; Tang, B. Seasonal Analysis of Spatial Distribution Patterns and Characteristics of Sepiella maindroni and Sepia kobiensis in the East China Sea Region. Animals 2024, 14, 2716. https://doi.org/10.3390/ani14182716
Xu M, Liu S, Zhang H, Li Z, Song X, Yang L, Tang B. Seasonal Analysis of Spatial Distribution Patterns and Characteristics of Sepiella maindroni and Sepia kobiensis in the East China Sea Region. Animals. 2024; 14(18):2716. https://doi.org/10.3390/ani14182716
Chicago/Turabian StyleXu, Min, Shuhao Liu, Hui Zhang, Zhiguo Li, Xiaojing Song, Linlin Yang, and Baojun Tang. 2024. "Seasonal Analysis of Spatial Distribution Patterns and Characteristics of Sepiella maindroni and Sepia kobiensis in the East China Sea Region" Animals 14, no. 18: 2716. https://doi.org/10.3390/ani14182716
APA StyleXu, M., Liu, S., Zhang, H., Li, Z., Song, X., Yang, L., & Tang, B. (2024). Seasonal Analysis of Spatial Distribution Patterns and Characteristics of Sepiella maindroni and Sepia kobiensis in the East China Sea Region. Animals, 14(18), 2716. https://doi.org/10.3390/ani14182716






