Identification of Reference Gene for Quantitative Gene Expression in Early-Term and Late-Term Cultured Canine Fibroblasts Derived from Ear Skin
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fibroblast Isolation and Culture
2.2. Analysis of Proliferation Potential in Long-Term Expanded Fibroblasts
2.3. Senescence-Associated β-Galactosidase Assay in Long-Term Expanded Fibroblasts
2.4. Candidate Reference Genes and Primers
2.5. RNA Isolation, cDNA Synthesis, and qRT-PCR
2.6. Analysis of Stable Reference Gene Expression
2.7. The Use of Different Reference Genes in the Normalization of GOI
2.8. Statistical Analysis
3. Results
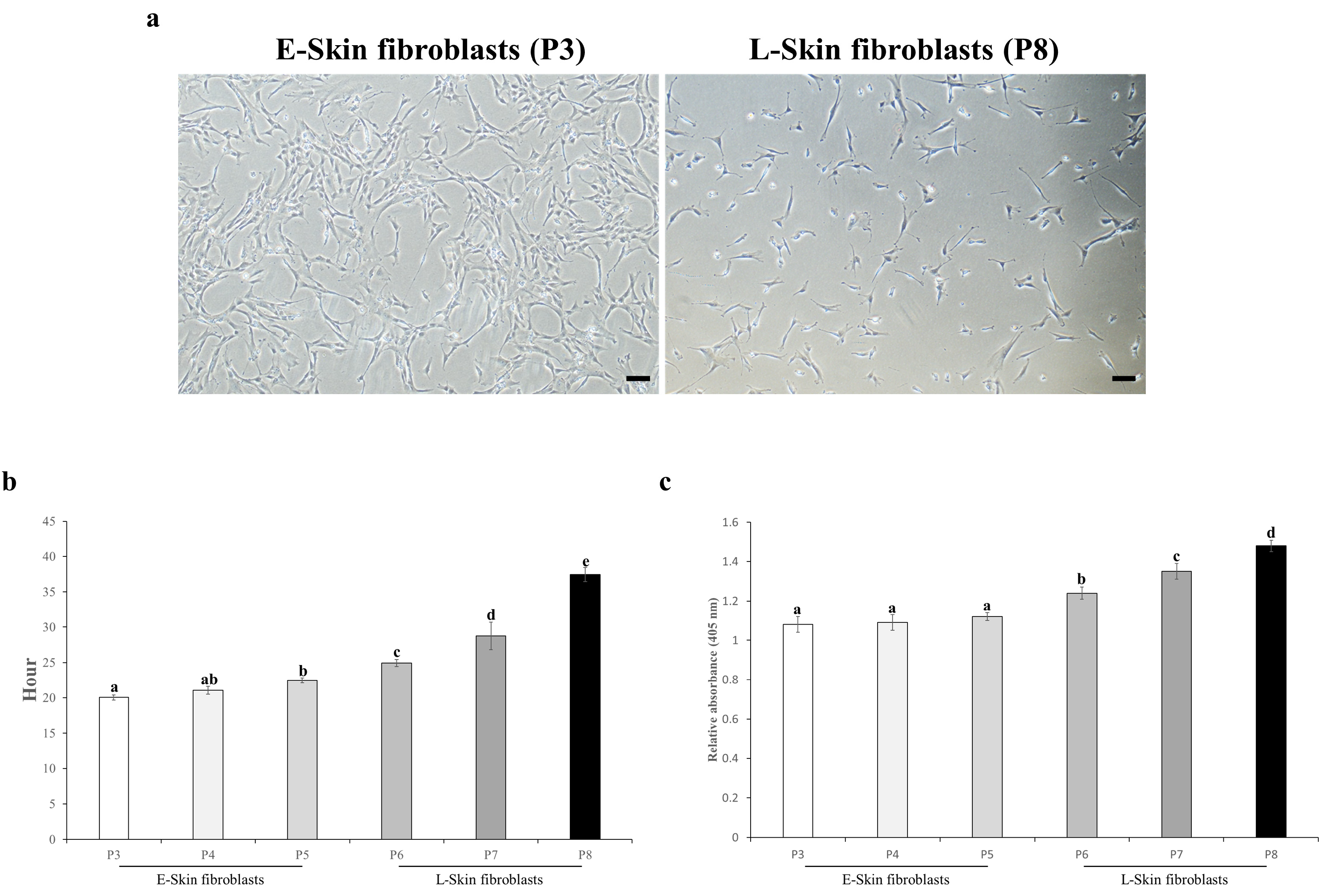
3.1. Cell Properties of Long-Term Expanded Fibroblasts
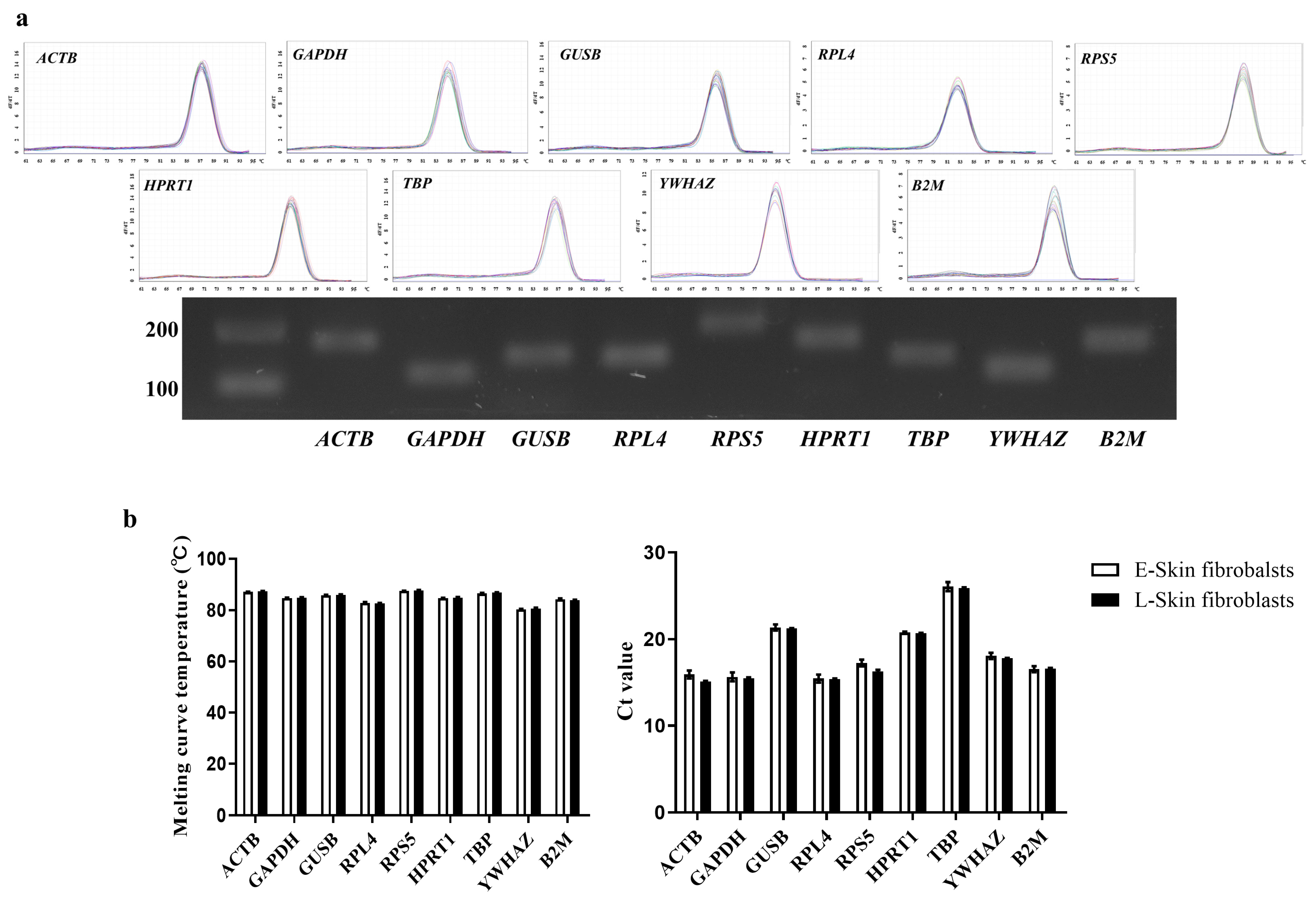
3.2. Evaluation of Primer Efficiency, Amplicon Size, and Ct Values
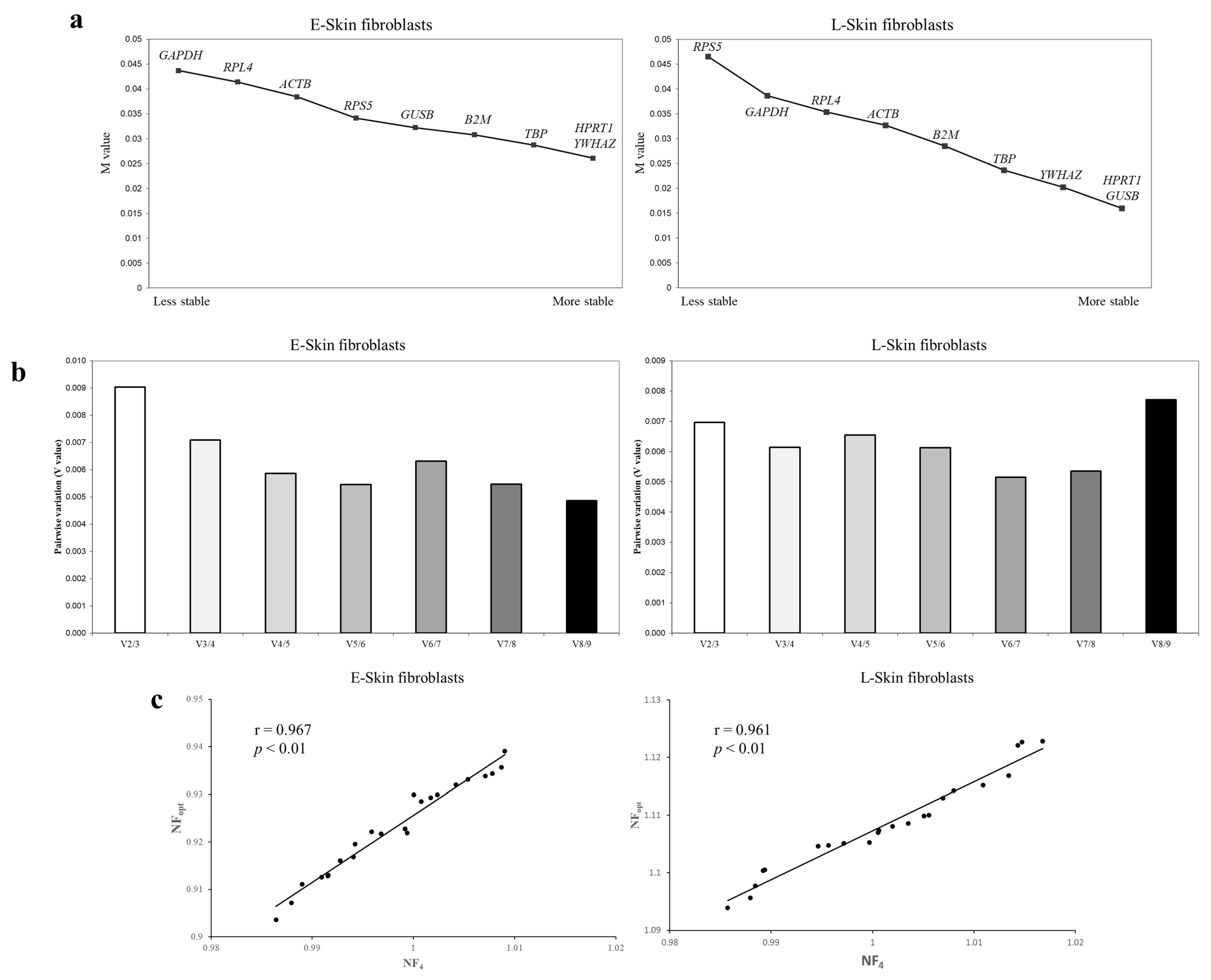
3.3. An Analysis of the Most Stable Reference Gene Using geNorm
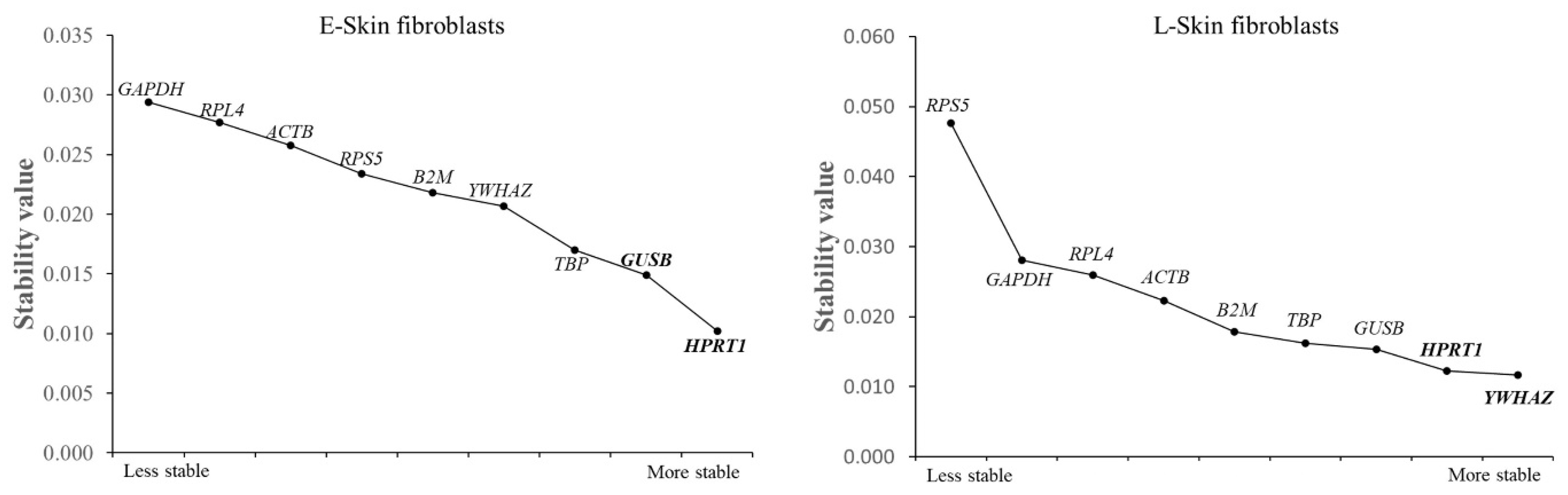
3.4. An Analysis of the Most Stable Reference Gene Using Normfinder
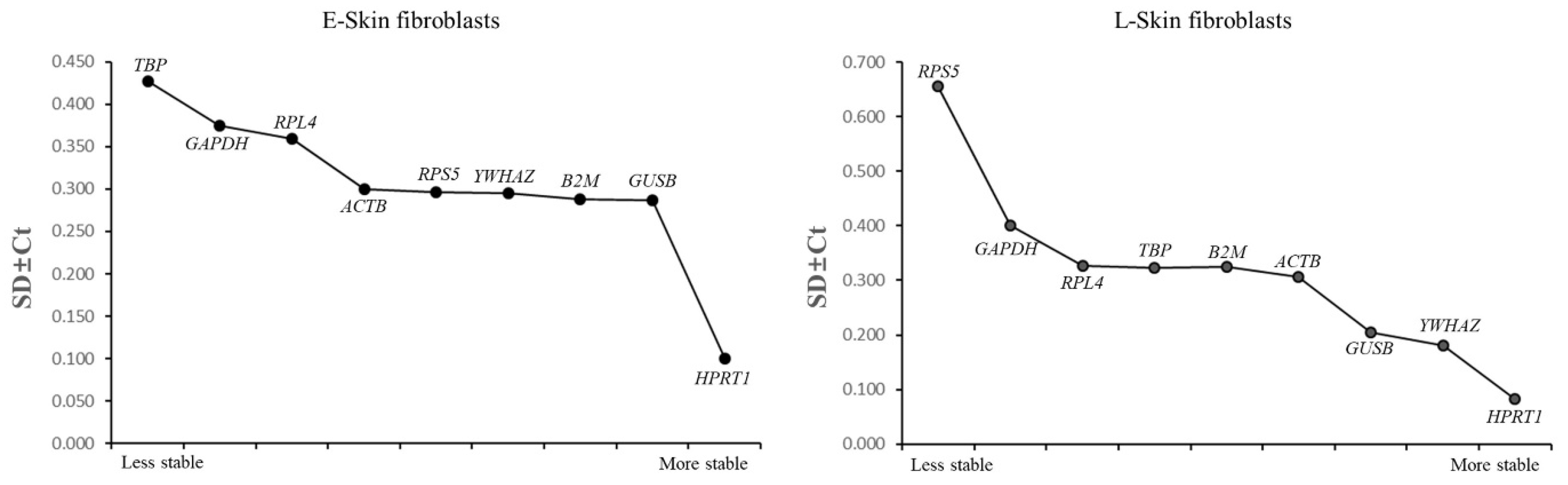
3.5. An Analysis of the Most Stable Reference Gene Using Bestkeeper
3.6. GOI Normalization Using the Most Stable Reference Gene Identified
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darby, I.A.; Hewitson, T.D. Fibroblast differentiation in wound healing and fibrosis. Int. Rev. Cytol. 2007, 257, 143–179. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Yan, X.; Guo, X.; Song, Y.; Wu, B.; Bao, S.; Cao, G.; Guo, J.; Sun, Q. Establishment and biological characteristics of fibroblast cell lines obtained from wild corsac fox. Vitr. Cell Dev. Biol. Anim. 2020, 56, 837–841. [Google Scholar] [CrossRef]
- Francia, M.; Bot, M.; Boltz, T.; De la Hoz, J.F.; Boks, M.; Kahn, R.; Ophoff, R. Fibroblasts as an in vitro model of circadian genetic and genomic studies: A temporal analysis. bioRxiv, 2024. [Google Scholar] [CrossRef]
- Ma, Y.; Lin, Y.; Huang, W.; Wang, X. Direct Reprograming of Mouse Fibroblasts into Dermal Papilla Cells via Small Molecules. Int. J. Mol. Sci. 2022, 23, 4213. [Google Scholar] [CrossRef]
- Dua, S.; Bansal, S.; Gautam, D.; Jose, B.; Singh, P.; Singh, M.K.; De, S.; Kumar, D.; Yadav, P.S.; Kues, W.; et al. Production of MSTN gene-edited embryos of buffalo using the CRISPR/Cas9 system and SCNT. Cell Reprogram. 2023, 25, 121–127. [Google Scholar] [CrossRef]
- Kim, D.E.; Lee, J.H.; Ji, K.B.; Park, K.S.; Kil, T.Y.; Koo, O.; Kim, M.K. Generation of genome-edited dogs by somatic cell nuclear transfer. BMC Biotechnol. 2022, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.; Singh, S.P.; Kharche, S.D.; Goel, A.; Soni, Y.K.; Kaushik, R.; Kose, M.; Kumar, A. Cell culture media dependent in vitro dynamics and culture characteristics of adult caprine dermal fibroblast cells. Sci. Rep. 2023, 13, 13716. [Google Scholar] [CrossRef]
- Nailis, H.; Coenye, T.; Van Nieuwerburgh, F.; Deforce, D.; Nelis, H.J. Development and evaluation of different normalization strategies for gene expression studies in Candida albicans biofilms by real-time PCR. BMC Mol. Biol. 2006, 7, 25. [Google Scholar] [CrossRef]
- Bustin, S.A. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): Trends and problems. J. Mol. Endocrinol. 2002, 29, 23–39. [Google Scholar] [CrossRef]
- Radonić, A.; Thulke, S.; Mackay, I.M.; Landt, O.; Siegert, W.; Nitsche, A. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2004, 313, 856–862. [Google Scholar] [CrossRef]
- Davoren, P.A.; McNeill, R.E.; Lowery, A.J.; Kerin, M.J.; Miller, N. Identification of suitable endogenous control genes for microRNA gene expression analysis in human breast cancer. BMC Mol. Biol. 2008, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Viganò, M.; Rebulla, P.; Giordano, R.; Lazzari, L. What is beyond a qRT-PCR study on mesenchymal stem cell differentiation properties: How to choose the most reliable housekeeping genes. J. Cell. Mol. Med. 2013, 17, 168–180. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Zakrajsek, B.A. Effect of experimental treatment on housekeeping gene expression: Validation by real-time, quantitative RT-PCR. J. Biochem. Biophys. Methods 2000, 46, 69–81. [Google Scholar] [CrossRef]
- Ropenga, A.; Chapel, A.; Vandamme, M.; Griffiths, N.M. Use of reference gene expression in rat distal colon after radiation exposure: A caveat. Radiat. Res. 2004, 161, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Jeon, R.H.; Lee, W.J.; Son, Y.B.; Bharti, D.; Shivakumar, S.B.; Lee, S.L.; Rho, G.J. PPIA, HPRT1, and YWHAZ Genes Are Suitable for Normalization of mRNA Expression in Long-Term Expanded Human Mesenchymal Stem Cells. Biomed. Res. Int. 2019, 2019, 3093545. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Warrington, J.A.; Nair, A.; Mahadevappa, M.; Tsyganskaya, M. Comparison of human adult and fetal expression and identification of 535 housekeeping/maintenance genes. Physiol. Genom. 2000, 2, 143–147. [Google Scholar] [CrossRef]
- Thellin, O.; Zorzi, W.; Lakaye, B.; De Borman, B.; Coumans, B.; Hennen, G.; Grisar, T.; Igout, A.; Heinen, E. Housekeeping genes as internal standards: Use and limits. J. Biotechnol. 1999, 75, 291–295. [Google Scholar] [CrossRef]
- Selvey, S.; Thompson, E.W.; Matthaei, K.; Lea, R.A.; Irving, M.G.; Griffiths, L.R. Beta-actin--an unsuitable internal control for RT-PCR. Mol. Cell Probes. 2001, 15, 307–311. [Google Scholar] [CrossRef]
- Glare, E.M.; Divjak, M.; Bailey, M.J.; Walters, E.H. beta-Actin and GAPDH housekeeping gene expression in asthmatic airways is variable and not suitable for normalising mRNA levels. Thorax 2002, 57, 765–770. [Google Scholar] [CrossRef]
- Hildyard, J.C.W.; Taylor-Brown, F.; Massey, C.; Wells, D.J.; Piercy, R.J. Determination of qPCR reference genes suitable for normalizing gene expression in a canine model of duchenne muscular dystrophy. J. Neuromuscul. Dis. 2018, 5, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.B.; Kang, Y.H.; Lee, H.J.; Jang, S.J.; Bharti, D.; Lee, S.L.; Jeon, B.G.; Park, B.W.; Rho, G.J. Evaluation of odonto/osteogenic differentiation potential from different regions derived dental tissue stem cells and effect of 17β-estradiol on efficiency. BMC Oral Health 2021, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.H.; Ley, S.; Aitken, A. Isoforms of 14-3-3 protein can form homo- and heterodimers in vivo and in vitro: Implications for function as adapter proteins. FEBS Lett. 1995, 368, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.L.; Tayerjiang, J.L.T.; Zhao, X.B.; Wang, H.; Wang, Q.; Yuan, H. Study of optimal scheme of spinal image-guided radiotherapy based on expression of caspase-3 in spinal cord neurons by orthogonal design. Genet. Mol. Res. 2015, 14, 3223–3233. [Google Scholar] [CrossRef] [PubMed]
- Sculley, D.G.; Dawson, P.A.; Emmerson, B.T.; Gordon, R.B. A review of the molecular basis of hypoxanthine-guanine phosphoribosyltransferase (HPRT) deficiency. Hum. Genet. 1992, 90, 195–207. [Google Scholar] [CrossRef]
- Buken, C.; Sahana, J.; Corydon, T.J.; Melnik, D.; Bauer, J.; Wehland, M.; Krüger, M.; Balk, S.; Abuagela, N.; Infanger, M.; et al. Morphological and molecular changes in juvenile normal human fibroblasts exposed to simulated microgravity. Sci. Rep. 2019, 9, 11882. [Google Scholar] [CrossRef]
- Ham, S.; Harrison, C.; Southwick, G.; Temple-Smith, P. Selection of internal control genes for analysis of gene expression in normal and diseased human dermal fibroblasts using quantitative real-time PCR. Exp. Dermatol. 2016, 25, 911–914. [Google Scholar] [CrossRef]
- Toorani, T.; Mackie, P.M.; Mastromonaco, G.F. Validation of reference genes for use in untreated bovine fibroblasts. Sci. Rep. 2021, 11, 10253. [Google Scholar] [CrossRef]
- Fasullo, M.; Endres, L. Nucleotide salvage deficiencies, DNA damage and neurodegeneration. Int. J. Mol. Sci. 2015, 16, 9431–9449. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Kural, K.C.; Tandon, N.; Skoblov, M.; Kel-Margoulis, O.V.; Baranova, A.V. Pathways of aging: Comparative analysis of gene signatures in replicative senescence and stress induced premature senescence. BMC Genom. 2016, 17, 1030. [Google Scholar] [CrossRef] [PubMed]
- Ohori, M.; Nakayama, Y.; Ogasawara-Shimizu, M.; Toyoshiba, H.; Nakanishi, A.; Aparicio, S.; Araki, S. Gene regulatory network analysis defines transcriptome landscape with alternative splicing of human umbilical vein endothelial cells during replicative senescence. BMC Genom. 2021, 22, 869. [Google Scholar] [CrossRef] [PubMed]
- Marthandan, S.; Priebe, S.; Hemmerich, P.; Klement, K.; Diekmann, S. Long-term quiescent fibroblast cells transit into senescence. PLoS ONE 2014, 9, e115597. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Su, H.; Chen, F.; Ma, M.; Yu, W.; Ye, G.; Cen, S.; Mi, R.; Wu, X.; et al. Effects of long-term culture on the biological characteristics and RNA profiles of human bone-marrow-derived mesenchymal stem cells. Mol. Ther. Nucleic Acids 2021, 26, 557–574. [Google Scholar] [CrossRef]
- Brinkhof, B.; Spee, B.; Rothuizen, J.; Penning, L.C. Development and evaluation of canine reference genes for accurate quantification of gene expression. Anal. Biochem. 2006, 356, 36–43. [Google Scholar] [CrossRef]
- Naz, H.; Islam, A.; Waheed, A.; Sly, W.S.; Ahmad, F.; Hassan, I. Human β-glucuronidase: Structure, function, and application in enzyme replacement therapy. Rejuvenation Res. 2013, 16, 352–363. [Google Scholar] [CrossRef]
- Kang, J.; Brajanovski, N.; Chan, K.T.; Xuan, J.; Pearson, R.B.; Sanij, E. Ribosomal proteins and human diseases: Molecular mechanisms and targeted therapy. Signal Transduct. Target. Ther. 2021, 6, 323. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, S.I.; Van Acker, Z.P.; Haagdorens, M.; Pintelon, I.; Koppen, C.; Zakaria, N. Selecting appropriate reference genes for quantitative real-time polymerase chain reaction studies in isolated and cultured ocular surface epithelia. Sci. Rep. 2019, 9, 19631. [Google Scholar] [CrossRef]
- Dheda, K.; Huggett, J.F.; Chang, J.S.; Kim, L.U.; Bustin, S.A.; Johnson, M.A.; Rook, G.; Zumla, A. The implications of using an inappropriate reference gene for real-time reverse transcription PCR data normalization. Anal. Biochem. 2005, 344, 141–143. [Google Scholar] [CrossRef]
- Provenzano, M.; Mocellin, S. Complementary techniques: Validation of gene expression data by quantitative real time PCR. Adv. Exp. Med. Biol. 2007, 593, 66–73. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choe, Y.H.; Han, J.H.; Hwang, G.; Choi, M.Y.; Thakur, G.; Jo, C.H.; Oh, S.J.; Lee, W.J.; Rho, G.J.; et al. HPRT1 most suitable reference gene for accurate normalization of mRNA expression in canine dermal tissues with radiation therapy. Genes 2022, 13, 1928. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.H.; Hildyard, J.C.W.; Wells, D.J.; Piercy, R.J. Identification of quantitative polymerase chain reaction reference genes suitable for normalising gene expression in the brain of normal and dystrophic mice and dogs. Wellcome Open Res. 2021, 6, 84. [Google Scholar] [PubMed]
- Zornhagen, K.W.; Kristensen, A.T.; Hansen, A.E.; Oxboel, J.; Kjaer, A. Selection of suitable reference genes for normalization of genes of interest in canine soft tissue sarcomas using quantitative real-time polymerase chain reaction. Vet. Comp. Oncol. 2015, 13, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Huh, J.W.; Kim, Y.H.; Lee, S.R.; Kim, S.H.; Kim, S.U.; Kim, H.S.; Kim, M.K.; Chang, K.T. Selection of internal reference genes for normalization of quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis in the canine brain and other organs. Mol. Biotechnol. 2013, 54, 47–57. [Google Scholar] [CrossRef]
- Pisamai, S.; Rungsipipat, A.; Kalpravidh, C.; Suriyaphol, G. Selection of reference genes for real-time polymerase chain reaction in canine oral tumor and cancer. Thai J. Vet. Med. 2016, 46, 295–304. [Google Scholar] [CrossRef]
- Wood, S.H.; Clements, D.N.; McEwan, N.A.; Nuttall, T.; Carter, S.D. Reference genes for canine skin when using quantitative real-time PCR. Vet. Immunol. Immunopathol. 2008, 126, 392–395. [Google Scholar] [CrossRef]
- Ostrowska-Podhorodecka, Z.; Ding, I.; Norouzi, M.; McCulloch, C.A. Impact of vimentin on regulation of cell signaling and matrix remodeling. Front. Cell Dev. Biol. 2022, 10, 869069. [Google Scholar] [CrossRef]
- Wu, H.; Yao, Z.; Li, H.; Zhang, L.; Zhao, Y.; Li, Y.; Wu, Y.; Zhang, Z.; Xie, J.; Ding, F.; et al. Improving dermal fibroblast-to-epidermis communications and aging wound repair through extracellular vesicle-mediated delivery of Gstm2 mRNA. J. Nanobiotechnol. 2024, 22, 307. [Google Scholar] [CrossRef]
- Ivaska, J.; Pallari, H.-M.; Nevo, J.; Eriksson, J.E. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell Res. 2007, 313, 2050–2062. [Google Scholar] [CrossRef]
- Mendez, M.G.; Kojima, S.; Goldman, R.D. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010, 24, 1838–1851. [Google Scholar] [CrossRef]
- Vuoriluoto, K.; Haugen, H.; Kiviluoto, S.; Mpindi, J.-P.; Nevo, J.; Gjerdrum, C.; Tiron, C.; Lorens, J.B.; Ivaska, J. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene 2011, 30, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Dave, J.M.; Bayless, K.J. Vimentin as an integral regulator of cell adhesion and endothelial sprouting. Microcirculation 2014, 21, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Sliogeryte, K.; Gavara, N. Vimentin plays a crucial role in fibroblast ageing by regulating biophysical properties and cell migration. Cells 2019, 8, 1164. [Google Scholar] [CrossRef]

| Information of Primers | Standard Curve Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Gene Name (Symbol) | Sequence | Base Pair | Accession | R2 | M | B | E |
| Beta-actin (ACTB) | F: GCACTCTTCCAACCTTCTTTCC R: GCTGTGATTTCCTTCTGCATCC | 179 | AF021873.2 | 0.993 | −3.489 | 34.252 | 1.01 |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | F: GGAGAAAGCTGCCAAATATGACG R: ACTGTTGAAGTCACAGGAGACC | 118 | NM_001003142.2 | 0.991 | −3.436 | 35.514 | 0.98 |
| Beta-glucuronidase (GUSB) | F: ATCTGTAGTCATGTGGTCTGTAGC R: GGTCTGCTTCATAGTTGGAATTGG | 149 | AF019759.1 | 0.996 | −3.332 | 33.257 | 0.99 |
| Ribosomal protein 4 (RPL4) | F: AATGAGAAACCGTCGTCGTATCC R: GGAGCAAGTTTCAGAATGTTCAGC | 141 | NM_001252409.1 | 0.992 | −3.355 | 39.041 | 1.01 |
| Ribosomal protein S5 (RPS5) | F: TGAAGGAGAAGTATGCCAAGTACC R: GAGCAGATGGATGATCTCGAAGG | 188 | XM_533568.5 | 0.995 | −3.435 | 39.145 | 0.97 |
| Hypoxanthine phosphoribosyl transferase 1 (HPRT1) | F: GACTGAAGAGCTACTGTAATGACC R: TCTTTGGATTATGCTCCTTGACC | 168 | NM_001003357.2 | 0.996 | −3.412 | 36.915 | 0.98 |
| TATA box-binding protein (TBP) | F: ATCTGGTATCCCTTACGCTTCG R: GCAAGAGAGTCTGGTTTGTTTCC | 137 | XM_849432.4 | 0.995 | −3.498 | 36.972 | 1.02 |
| Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide (YWHAZ) | F: GTGAAGAGTCATACAAAGACAGCA R: CCCTCCTTCTCCTGCTTCAG | 110 | XM_014118550.1 | 0.992 | −3.511 | 37.145 | 1.01 |
| Beta-2 microglobulin (B2M) | F: AGATGAAAGCAGAACAGACAGACC R: GTTGTCTCGGTCCCACTTAACG | 161 | JQ733515.1 | 0.999 | −3.475 | 35.221 | 0.99 |
| Vimentin | F: AAGTTTGCCGACCTCTCTGA R: TTCGACGGCAAAGTTCTCTT | 183 | NM_001287023.1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-Y.; Jeong, Y.-W.; Choe, Y.-H.; Oh, S.-J.; Miah, R.; Lee, W.-J.; Lee, S.-L.; Bok, E.-Y.; Yoo, D.-S.; Son, Y.-B. Identification of Reference Gene for Quantitative Gene Expression in Early-Term and Late-Term Cultured Canine Fibroblasts Derived from Ear Skin. Animals 2024, 14, 2722. https://doi.org/10.3390/ani14182722
Lee S-Y, Jeong Y-W, Choe Y-H, Oh S-J, Miah R, Lee W-J, Lee S-L, Bok E-Y, Yoo D-S, Son Y-B. Identification of Reference Gene for Quantitative Gene Expression in Early-Term and Late-Term Cultured Canine Fibroblasts Derived from Ear Skin. Animals. 2024; 14(18):2722. https://doi.org/10.3390/ani14182722
Chicago/Turabian StyleLee, Sang-Yun, Yeon-Woo Jeong, Yong-Ho Choe, Seong-Ju Oh, Rubel Miah, Won-Jae Lee, Sung-Lim Lee, Eun-Yeong Bok, Dae-Sung Yoo, and Young-Bum Son. 2024. "Identification of Reference Gene for Quantitative Gene Expression in Early-Term and Late-Term Cultured Canine Fibroblasts Derived from Ear Skin" Animals 14, no. 18: 2722. https://doi.org/10.3390/ani14182722





