Comparative Analysis of the Potential Adaptability of Tibetan Dzo and Yellow Cattle Based on Blood Indices, Metabolites, and Fecal Microbiota
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Blood Sample and Fecal Sample Preparation
2.3. Analyses of Blood Samples
2.4. Metabolomics Analysis
2.5. Fecal Microbiome Analysis
2.6. Statistical Analysis
3. Results
3.1. Blood-Based Physiological and Biochemical Indices in the MG and HG Groups
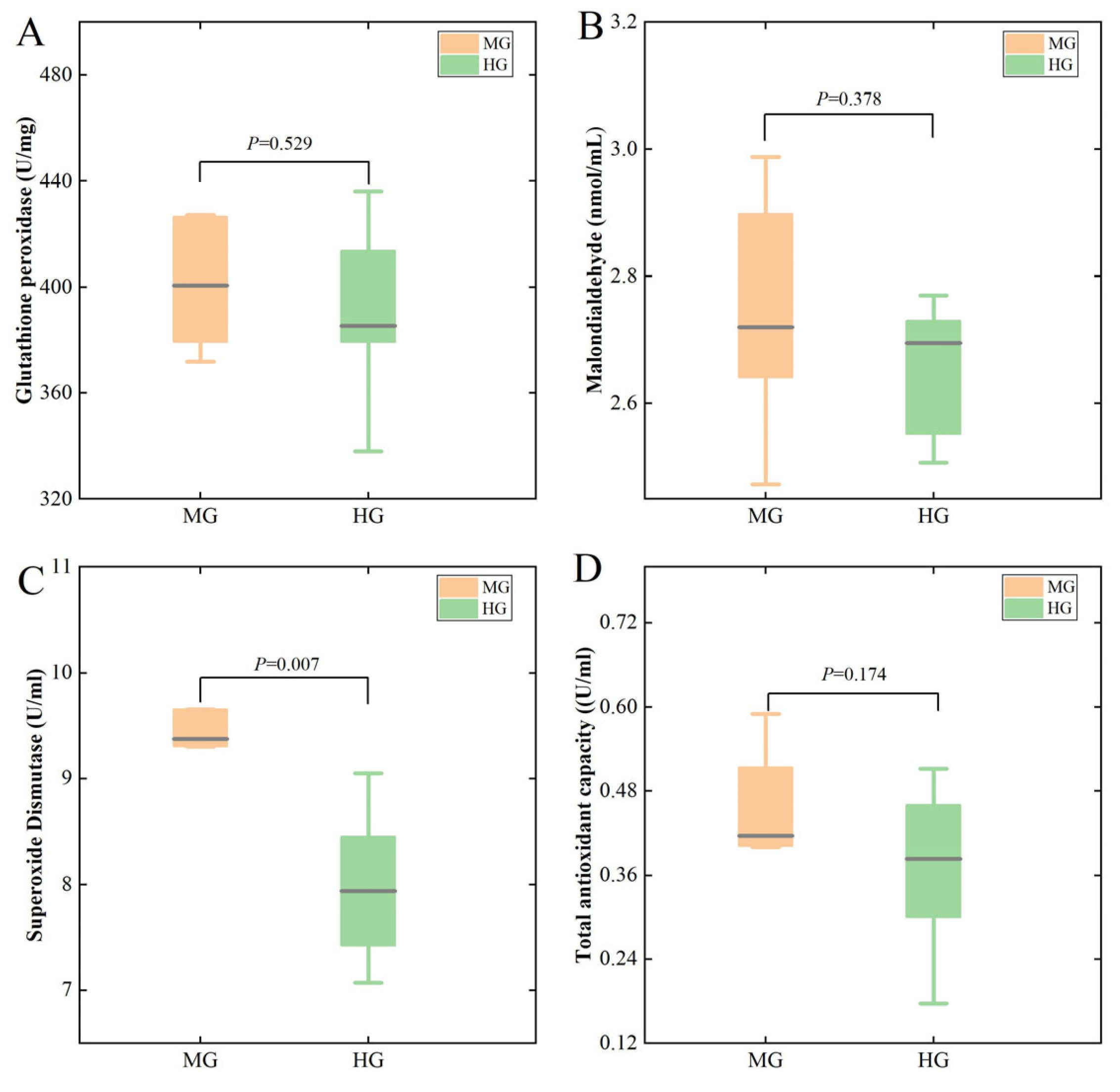
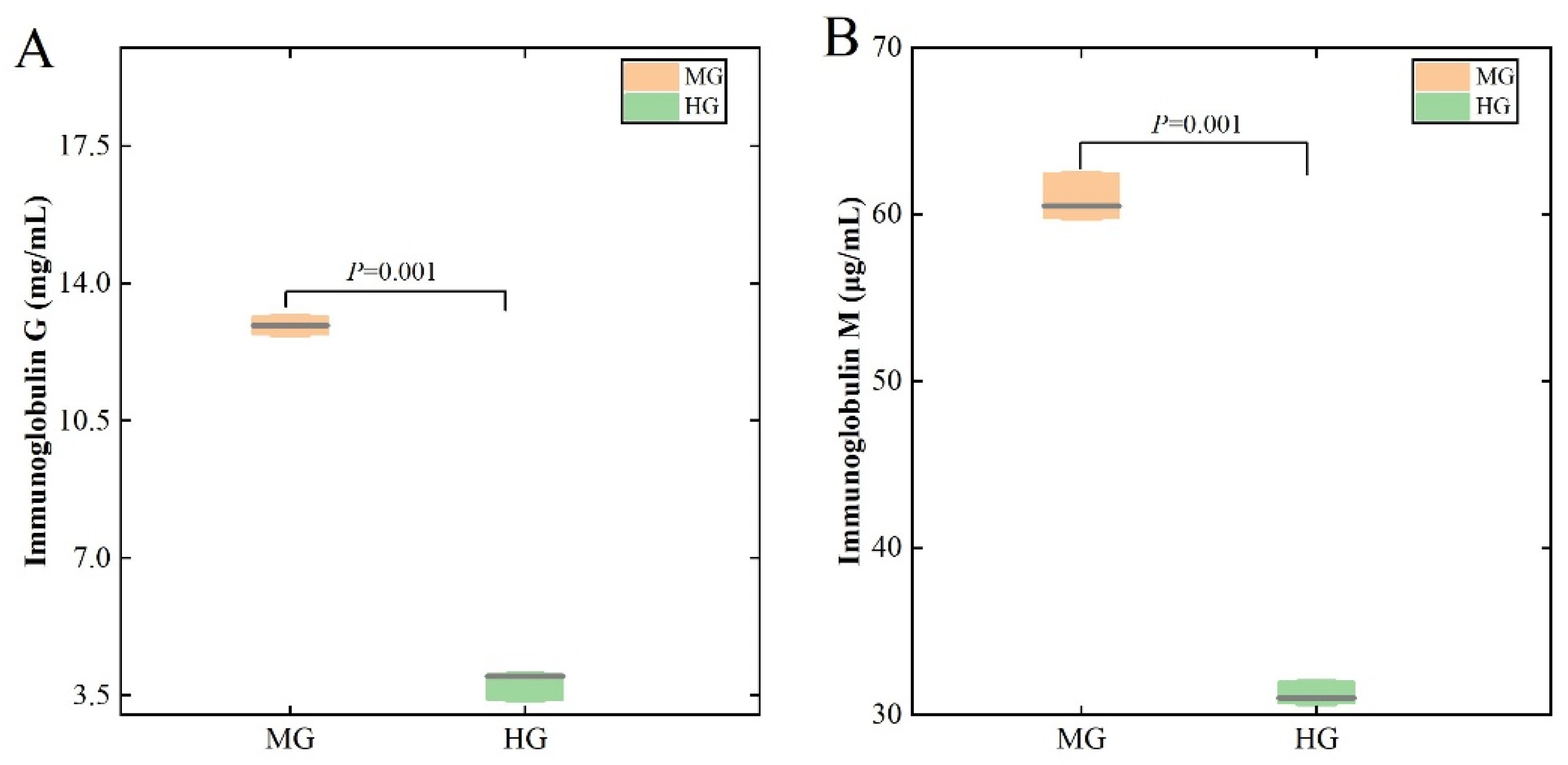
3.2. Antioxidant and Immune Indicators in the MG and HG Groups
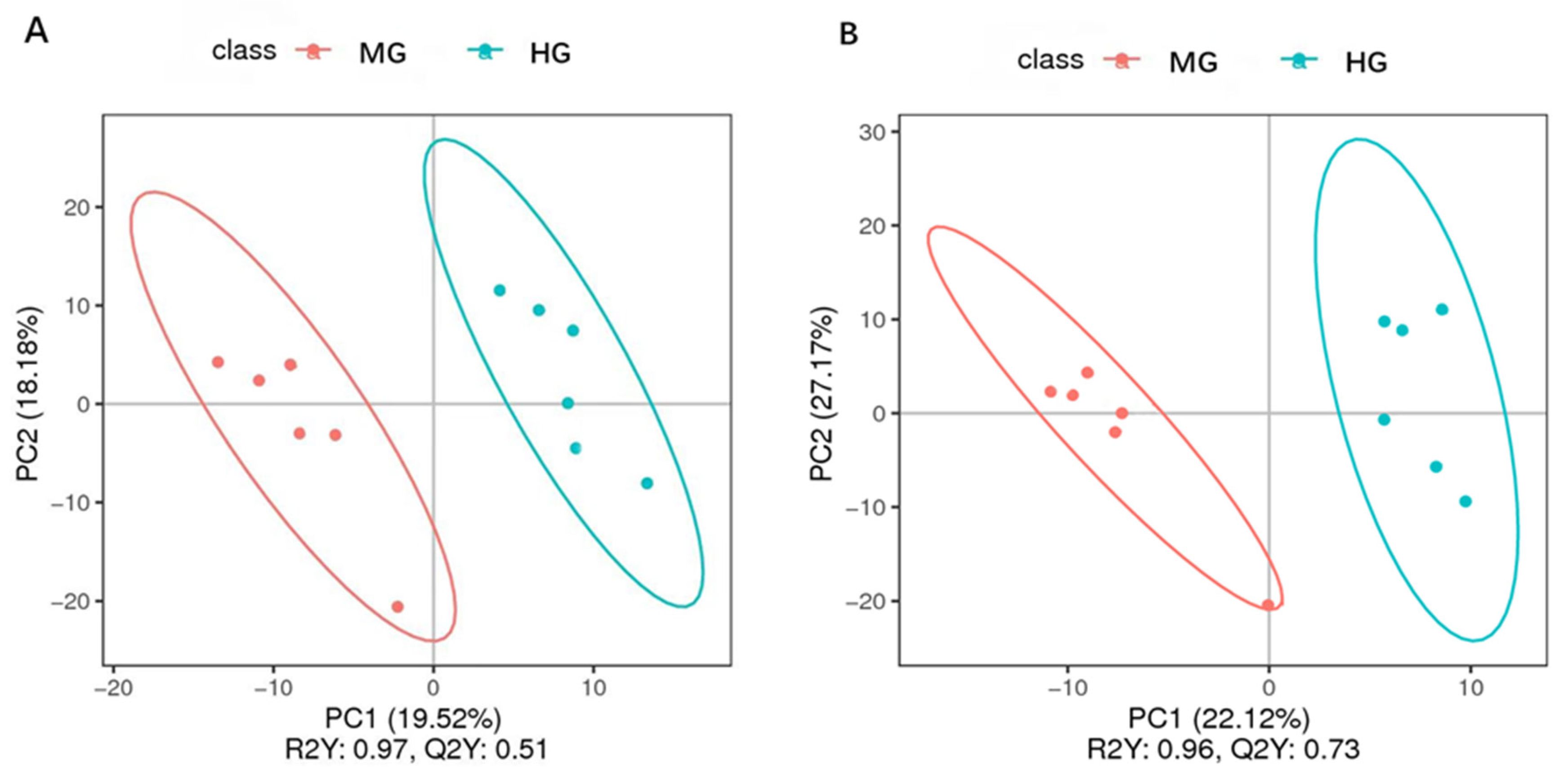
3.3. Blood Metabolite Profiling of the MG and HG Groups
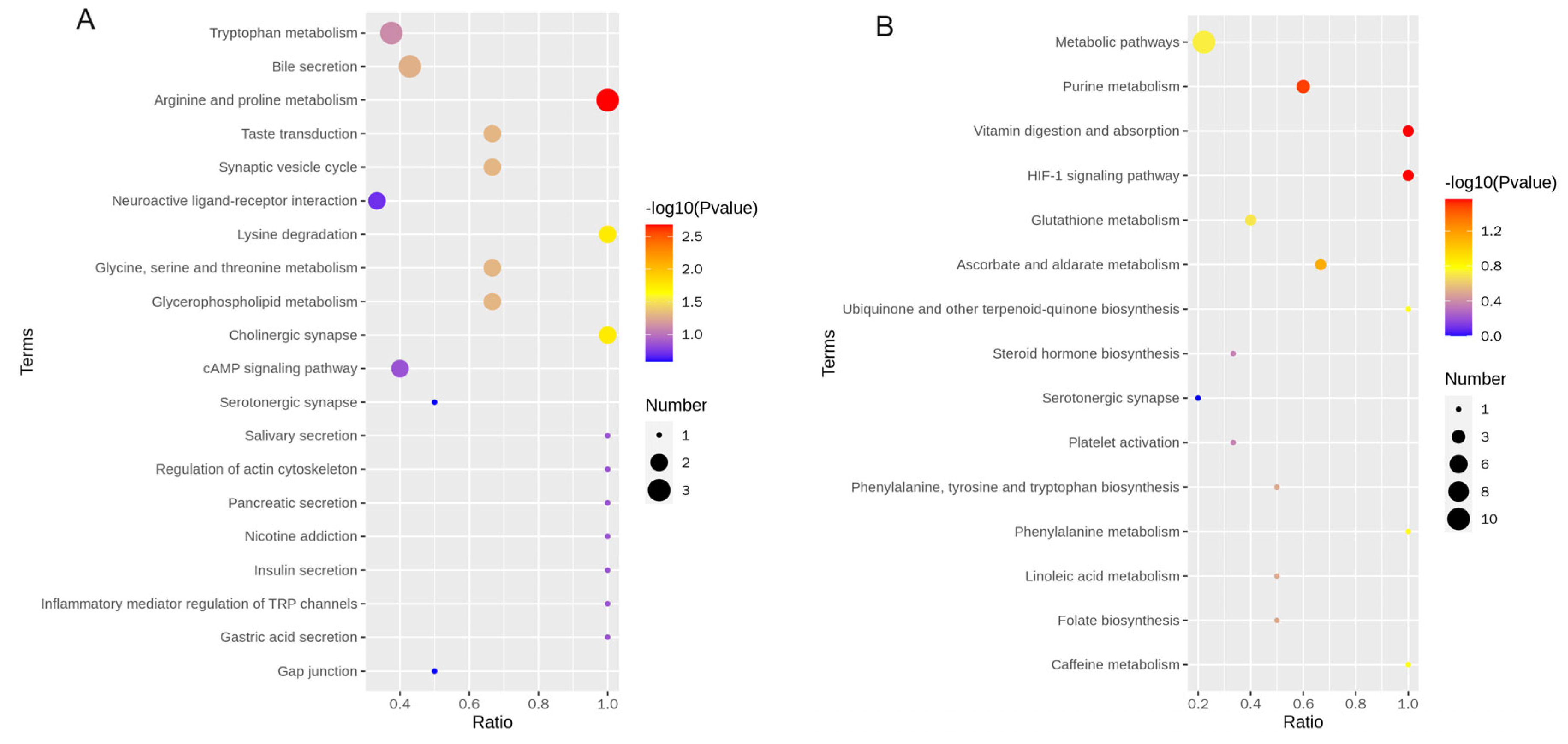
3.4. Identification of Differential Metabolites and Metabolic Pathways
3.5. Fecal Microbiome Profiling of the MG and HG Groups
3.5.1. Number of OTUs
3.5.2. Alpha Diversity Analysis
3.5.3. PCoA Analysis
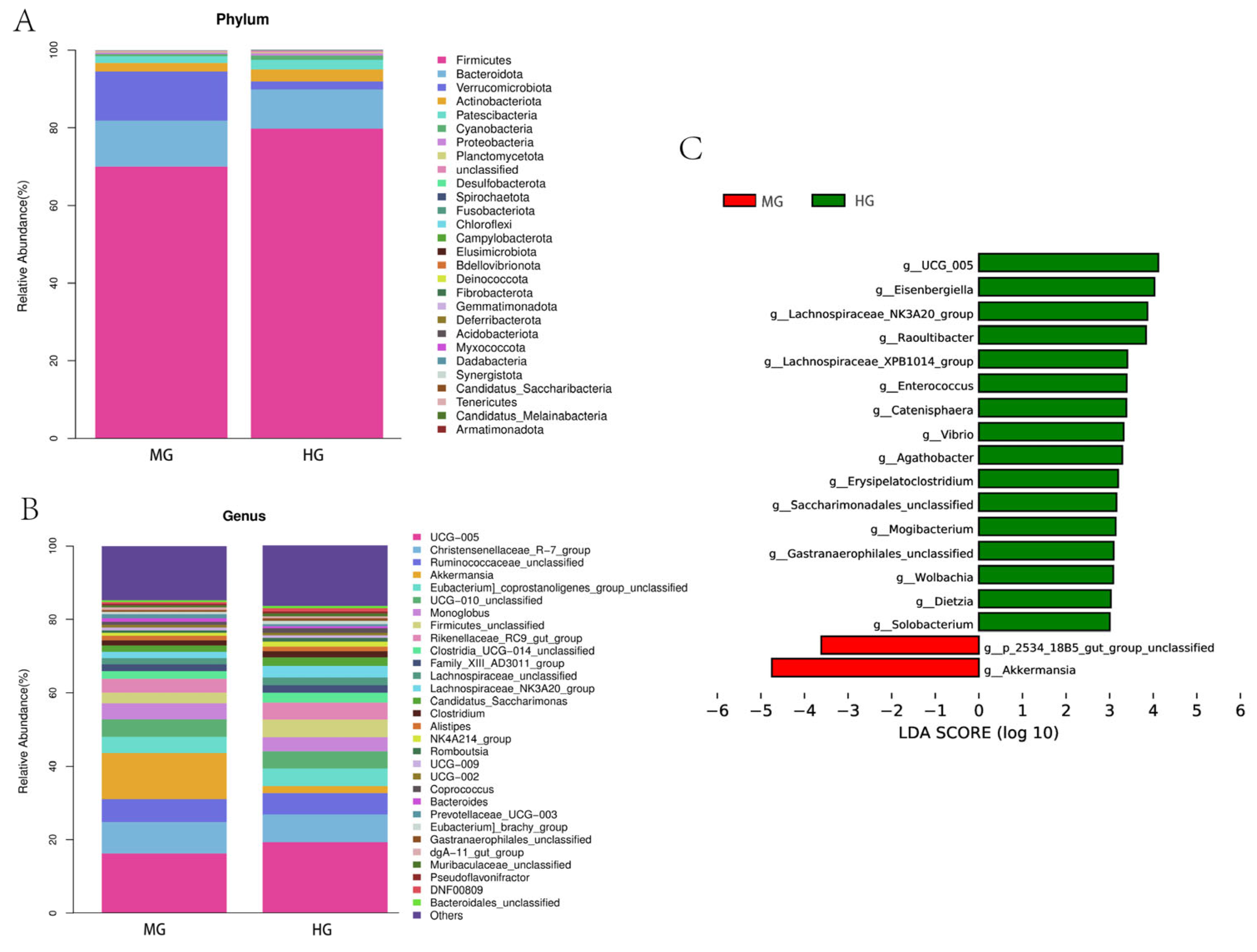
3.5.4. Analysis of the Phylum and Genus Level
3.5.5. Differences in Fecal Bacterial Composition Were Analyzed between the MG and HG Groups
4. Discussion
4.1. The Differences in the Blood-Based Physiological and Biochemical Characteristics of the MG and HG Groups
4.2. The Differences in the Blood Metabolite Profiles of the MG and HG Groups
4.2.1. The Differences in the Lipid Metabolism Levels of the MG and HG Groups
4.2.2. The Differences in Amino Acid Metabolism and Nucleotide Metabolites in the MG and HG Groups
4.2.3. KEGG Enrichment Analysis of Differential Metabolites
4.3. The Differences in the Fecal Microbiome Profiles of the MG and HG Groups
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Xu, D.; Wang, L.; Hao, J.; Wang, J.; Zhou, X.; Wang, W.; Qiu, Q.; Huang, X.; Zhou, J.; et al. Convergent evolution of rumen microbiomes in high-altitude mammals. Curr. Biol. 2016, 26, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.X.; Xin, J.W.; Zhang, C.F.; Dawayangla; Luosang; Zhang, Q.; Pingcuozhandui; Li, C.; Zhu, Y.; Cao, H.W.; et al. Whole-genome resequencing provides insights into the evolution and divergence of the native domestic yaks of the Qinghai-Tibet Plateau. BMC Evol. Biol. 2020, 27, 137. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wang, X.; Guo, S.; Kang, Y.; Pei, J.; Guo, X. F1 Male Sterility in cattle-yak examined through changes in testis tissue and transcriptome profiles. Animals 2022, 12, 2711. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; He, J.J.; Li, Z.Q.; Wang, Z.X.; Zhang, J.H. Comparative analysis of physiological and biochemical indexes of blood in Dazu black goats and other goats. Heilongjiang Anim. Sci. Vet. Med. 2011, 3, 8–10. [Google Scholar]
- Wang, G.H.; Xu, W.; Wang, J.J.; Yang, Y.J.; Jiang, H.Z. Comparative study on blood physiological and biochemical indexes of main meat sheep breeds in Jilin Province. Chin. Agric. Sci. Bull. 2023, 39, 82–87. [Google Scholar]
- Cui, C.; Liang, Y.J.; Yang, S.Y.; Miu, L.S.; Xiao, C.; Liu, Y.; Jin, H.G.; Wei, B.D.; Cao, Y. Study on blood biochemical indexes and dietary nutrient digestibility of 6-month-old Australian Han, Du Han hybrid sheep and small-tailed Han sheep. Chin. J. Anim. Sci. 2023, 59, 262–266. [Google Scholar]
- Li, F.; Li, C.; Chen, Y.; Liu, J.; Zhang, C.; Irving, B.; Fitzsimmons, C.; Plastow, G.; Guan, L.L. Host genetics influence the rumen microbiota and heritable rumen microbial features associate with feed efficiency in cattle. Microbiome 2019, 7, 92–108. [Google Scholar] [CrossRef]
- Sha, Y.; Hu, J.; Shi, B.; Dingkao, R.; Wang, J.; Li, S.; Zhang, W.; Luo, Y.; Liu, X. Characteristics and functions of the rumen microbial community of cattle-yak at different ages. BioMed Res. Int. 2020, 2020, 3482692. [Google Scholar] [CrossRef]
- Donaldson, G.; Lee, S.; Mazmanian, S. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef]
- Liu, J.H.; Bian, G.R.; Zhu, W.Y.; Mao, S.Y. High-grain feeding causes strong shifts in ruminal epithelial bacterial community and expression of Toll-like receptor genes in goats. Front. Microbiol. 2015, 6, 167–176. [Google Scholar] [CrossRef]
- Klein-Jöbstl, D.; Schornsteiner, E.; Mann, E.; Wagner, M.; Drillich, M.; Schmitz-Esser, S. Pyrosequencing reveals diverse fecal microbiota in Simmental calves during early development. Front. Microbiol. 2014, 5, 622. [Google Scholar]
- Oikonomou, G.; Teixeira, A.G.V.; Foditsch, C.; Bicalho, M.L.; Machado, V.S.; Bicalho, R.C. Fecal microbial diversity in pre-weaned dairy calves as described by pyrosequencing of metagenomic 16S rDNA. Associations of Faecalibacterium species with health and growth. PLoS ONE 2013, 8, e63157. [Google Scholar] [CrossRef]
- Li, H.; Qi, Y.; Jasper, H. Preventing age-related decline of gut compartmentalization limits microbiota dysbiosis and extends lifespan. Cell Host Microbe 2016, 19, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Li, D. Study on the Histological Structure and Distributive Changes of Mucosal Immunity-Associated Cells in Large Intestine of the Yak. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2013. [Google Scholar]
- Mi, J.D. Dynamics in Rumen and Distribution along Gastrointestinal Tracts of Bacteria and Methanogen in Yak. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2020. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC Int.: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.M.; Pu, Y.; Liu, L.; Zhao, X.C.; Xu, S.S.; Chai, J.B.; Wang, C.W.; Chai, M.L.; Sun, F. Effects of guanidine acetic acid on growth performance and blood indices of Chinese Holstein bulls. Heilongjiang Anim. Sci. Vet. Med. 2022, 13, 47–51. [Google Scholar]
- Cui, H.; Liu, L.; Liu, X.; Wang, Y.; Luo, N.; Tan, X.; Zhu, Y.; Liu, R.; Zhao, G.; Wen, J. A selected population study reveals the biochemical mechanism of intramuscular fat deposition in chicken meat. J. Anim. Sci. Biotechnol. 2022, 13, 54. [Google Scholar] [CrossRef]
- Yuan, P.; Zhang, X.F.; Guan, J.Q.; An, T.W.; Li, H.D.; Tan, W.; Xie, R.Q.; Luo, X.L.; Jiang, M.F. Effects of low altitude house-feeding on growth performance, slaughter performance and muscle quality of yak and cattle-yak. Chin. J. Anim. Nutr. 2021, 33, 4791–4800. [Google Scholar]
- Liu, X.X.; Wang, L.; Liu, L.Z.; Zhao, Y.H.; Zhang, X.; Wu, J.; Zhao, Y.M. Study on the meat quality traits of the F1 and F2 generations of Japanese Black-haired cattle and Yanbian cattle hybridization. Anim. Husb. Vet. Med. 2023, 55, 1–6. [Google Scholar]
- Huang, J.; Bao, K.; Zhang, T.T.; Li, G.Y.; Zhou, N.; Wang, K.Y.; Yang, F.H. Effects of low protein diet supplemented lysine and methionine on growth performance and serum biochemical indices of weaner Sika deer. Chin. J. Anim. Nutr. 2014, 26, 2714–2721. [Google Scholar]
- Saker, K.E.; Fike, J.H.; Veit, H.; Ward, D.L. Brown seaweed-(Tasco) treated conserved forage enhances antioxidant status and immune function in heat-stressed wether lambs. J. Anim. Physiol. Anim. Nutr. 2004, 88, 122–130. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Shariatmadari, F.; Karimi Torshizi, M.A.; Ahmadi, H.; Scholey, D. Plectranthus amboinicus and rosemary (Rosmarinus officinalis L.) essential oils effects on performance, antioxidant activity, intestinal health, immune response, and plasma biochemistry in broiler chickens. Food Sci. Nutr. 2023, 11, 3939–3948. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Ji, X.; Huang, J.; Liao, A.; Pan, L.; Hou, Y.; Hui, M.; Guo, W. Immunity improvement and gut microbiota remodeling of mice by wheat germ globulin. World J. Microb. Biot. 2021, 37, 64. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Sun, W.; Yi, K.; Yang, L.; Chi, F.; Luo, Z.; Wang, J.; Zhang, J.; Wang, W.; Yang, T.; et al. Comparison of muscle lipidomes between cattle-yak, yak, and cattle using UPLC–MS/MS. J. Food Compos. Anal. 2021, 103, 104113–104120. [Google Scholar] [CrossRef]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Y.; Jiang, R.; Nie, C.; Zeng, Z.; Zhao, N.; Huang, C.; Shao, Q.; Ding, C.; Qing, C.; et al. miR-132 inhibits lipopolysaccharide-induced inflammation in alveolar macrophages by the cholinergic anti-inflammatory pathway. Exp. Lung Res. 2015, 41, 261–269. [Google Scholar] [CrossRef]
- Rahbar, B.; Taghizadeh, A.; Paya, H.; Daghigh, K.H. Conjugated linoleic acid (CLA) supplementation effects on performance, metabolic parameters and reproductive traits in lactating Holstein dairy cows. Vet. Res. Forum. 2021, 12, 297–304. [Google Scholar]
- Yousef, M.S.; Marey, M.A.; Hambruch, N.; Hayakawa, H.; Shimizu, T.; Hussien, H.A.; Abdel-Razek, A.K.; Pfarrer, C.; Miyamoto, A. sperm binding to oviduct epithelial cells enhances TGFB1 and IL10 expressions in epithelial cells as well as neutrophils in vitro: Prostaglandin E2 as a main regulator of anti-Inflammatory response in the bovine oviduct. PLoS ONE 2016, 11, e0162309. [Google Scholar] [CrossRef]
- Zhu, Q.; Dong, Y.; Li, X.; Ni, C.; Huang, T.; Sun, J.; Ge, R.S. Dehydroepiandrosterone and its CYP7B1 metabolite 7α-Hydroxydehydroepiandrosterone regulates 11β-Hydroxysteroid dehydrogenase 1 directions in rat Leydig cells. Front. Endocrinol. 2019, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yu, L.; Zhao, J.; Ma, H. Effect of dehydroepiandrosterone on the immune function of mice in vivo and in vitro. Mol. Immunol. 2019, 112, 283–290. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, S.; Zhou, Y.; Yang, D.; Li, J.; Cui, Z. The effect of supplemental concentrate feeding on the morphological and functional development of the pancreas in early weaned Yak calves. Animals 2022, 12, 2563. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, J.; Cao, W.; Wu, X.; Jia, G.; Zhao, H.; Chen, X.; Wu, C.; Wang, J. Effects of spermine on liver barrier function, amino acid transporters, immune status, and apoptosis in piglets. RSC Adv. 2019, 9, 11054–11062. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Schenkel, L.C.; Yokich, M.; Bakovic, M. Adaptations to excess choline in insulin resistant and Pcyt2 deficient skeletal muscle. Biochem. Cell Biol. 2017, 95, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, Y. Liver-specific reduction of Mfn2 protein by RNAi results in impaired glycometabolism and lipid homeostasis in BALB/c mice. J. Huazhong Univ. Sci. Technol. Med. Sci. 2009, 29, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Katafuchi, A.; Shimamoto, S.; Kawaguchi, M.; Tomonaga, S.; Nakashima, K.; Ishihara, S.; Ohtsuka, A.; Ijiri, D. Effects of delaying post-hatch feeding on the plasma metabolites of broiler chickens revealed by targeted and untargeted metabolomics. J. Poult. Sci. 2023, 60, 2023032. [Google Scholar] [CrossRef]
- Maes, M.; Mihaylova, I.; Ruyter, M.D.; Kubera, M.; Bosmans, E. The immune effects of TRYCATs (tryptophan catabolites along the IDO pathway): Relevance for depression and other conditions characterized by tryptophan depletion induced by inflammation. Neuro Endocrinol. Lett. 2007, 28, 826–831. [Google Scholar]
- Wang, G.; Huang, S.; Wang, Y.; Cai, S.; Yu, H.; Liu, H.; Zeng, X.; Zhang, G.; Qiao, S. Bridging intestinal immunity and gut microbiota by metabolites. Cell Mol. Life Sci. 2019, 76, 3917–3937. [Google Scholar] [CrossRef]
- Roussel, G.; Bessede, A.; Klein, C.; Maitre, M.; Mensah-Nyagan, A.G. Xanthurenic acid is localized in neurons in the central nervous system. Neuroscience 2016, 329, 226–238. [Google Scholar] [CrossRef]
- Elolimy, A.; Alharthi, A.; Zeineldin, M.; Parys, C.; Loor, J.J. Residual feed intake divergence during the preweaning period is associated with unique hindgut microbiome and metabolome profiles in neonatal Holstein heifer calves. J. Anim. Sci. Biotechnol. 2020, 11, 13. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The role of vitamin E in immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef]
- Liu, X.; Sha, Y.; Lv, W.; Guo, X.; Pu, X.; Wang, J.; Li, S.; Hu, J.; Luo, Y. Multi-omics reveals that the rumen transcriptome, microbiome, and its metabolome co-regulate cold season adaptability of Tibetan Sheep. Front. Microbiol. 2022, 13, 859601. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Huang, Y.; Zhang, X.; Wang, S.; Zou, Z.; Wang, G.; Wang, Y.; Zhang, Z. Cloning, characterization, hypoxia and heat shock response of hypoxia inducible factor-1 (HIF-1) from the small abalone Haliotis diversicolor. Gene 2014, 534, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Albanese, C.T.; Rowe, M.I. Necrotizing enterocolitis. Semin. Pediatr. Surg. 1995, 4, 200–206. [Google Scholar]
- Clauss, M.; Hume, I.D.; Hummel, J. Evolutionary adaptations of ruminants and their potential relevance for modern production systems. Animal 2010, 4, 979–992. [Google Scholar] [CrossRef]
- Ley, R.E.; Lozupone, C.A.; Hamady, M.; Knight, R.; Gordon, J.I. Worlds within worlds: Evolution of the vertebrate gut microbiota. Nat. Rev. Microbiol. 2008, 6, 776–788. [Google Scholar] [CrossRef]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila gen. nov., sp nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Wu, Q.; Shi, F.; Niu, J.; Zhang, T.; Degen, A.A.; Fang, Q.; Ding, L.; Shang, Z.; Zhang, Z.; et al. Seasonal dynamics of diet-gut microbiota interaction in adaptation of yaks to life at high altitude. NPJ Biofilms Microbiomes 2021, 7, 38. [Google Scholar] [CrossRef]
- Chevalier, C.; Stojanovic, O.; Colin, D.J.; Suarez-Zamorano, N.; Tarallo, V.; Veyrat-Durebex, C.; Rigo, D.; Fabbiano, S.; Stevanović, A.; Hagemann, S. Gut microbiota orchestrates energy homeostasis during cold. Cell 2015, 163, 1360–1374. [Google Scholar] [CrossRef]
- Yang, B.; Le, J.; Wu, P.; Liu, J.; Guan, L.L.; Wang, J. Alfalfa intervention alters rumen microbial community development in hu lambs during early life. Front. Microbiol. 2018, 9, 574–586. [Google Scholar] [CrossRef]
- Garrity, G.; Boone, D.R.; Castenholz, R.W. Bergey’s Manual of Systematic Bacteriology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]



| Item | Diet |
|---|---|
| Ingredients | |
| Corn | 14.60 |
| Wheat bran | 3.70 |
| Soybean meal | 3.60 |
| Rapeseed meal | 1.95 |
| Wheat | 3.70 |
| CaHPO4 | 0.15 |
| NaCl | 0.30 |
| Premix 1 | 2.00 |
| Oat hay | 25.00 |
| Corn silage | 45.00 |
| Total | 100.00 |
| Chemical composition | |
| CP | 10.91 |
| EE | 1.68 |
| ADF | 35.2 |
| NDF | 54.26 |
| P | 0.16 |
| Ca | 0.83 |
| Ash | 9.51 |
| Metabolic energy (MJ/Kg) 2 | 8.96 |
| Item | Dzo | Yellow Cattle | p-Value |
|---|---|---|---|
| WBC (109/L) | 10.04 ± 3.91 | 9.06 ± 1.82 | 0.589 |
| Neu (109/L) | 3.66 ± 1.95 | 3.24 ± 0.94 | 0.645 |
| Lym (109/L) | 5.73 ± 1.64 | 5.13 ± 0.81 | 0.436 |
| Mon (109/L) | 0.15 ± 0.14 | 0.11 ± 0.04 | 0.509 |
| Eos (109/L) | 0.50 ± 0.50 | 0.59 ± 0.38 | 0.748 |
| Neu (%) | 35.02 ± 11.81 | 35.40 ± 6.45 | 0.946 |
| Lym (%) | 59.50 ± 12.30 | 57.27 ± 6.95 | 0.707 |
| Mon (%) | 1.30 ± 0.70 | 1.18 ± 0.29 | 0.714 |
| Eos (%) | 4.18 ± 2.66 | 6.15 ± 3.35 | 0.287 |
| RBC (1012/L) | 8.18 ± 1.46 | 8.03 ± 1.39 | 0.858 |
| HGB (g/L) | 142.33 ± 20.61 | 141.33 ± 12.72 | 0.921 |
| HCT (%) | 42.73 ± 7.01 | 41.63 ± 2.99 | 0.731 |
| MCV (fL) | 52.48 ± 3.91 | 52.65 ± 5.84 | 0.955 |
| MCH (pg) | 17.52 ± 1.29 | 17.85 ± 1.73 | 0.713 |
| MCHC (g/L) | 334.17 ± 7.08 | 339.50 ± 8.60 | 0.268 |
| RDW-CV (%) | 21.50 ± 1.86 | 21.13 ± 1.30 | 0.700 |
| RDW-SD (fL) | 41.30 ± 5.68 | 40.30 ± 3.52 | 0.722 |
| PLT (109/L) | 311.00 ± 126.85 | 317.67 ± 67.24 | 0.912 |
| MPV (fL) | 5.97 ± 0.43 | 6.25 ± 0.64 | 0.389 |
| PDW (%) | 15.78 ± 0.40 | 15.63 ± 0.27 | 0.467 |
| PCT (%) | 0.18 ± 0.07 | 0.20 ± 0.04 | 0.642 |
| Item | Dzo | Yellow Cattle | p-Value |
|---|---|---|---|
| AST (U/L) | 55.52 ± 11.96 | 64.12 ± 18.98 | 0.370 |
| ALT (U/L) | 24.28 ± 8.53 | 27.67 ± 12.40 | 0.594 |
| Glu-G (mmol/L) | 3.07 ± 1.16 | 3.66 ± 0.54 | 0.291 |
| TBA (μmol/L) | 16.40 ± 13.89 | 11.95 ± 3.14 | 0.462 |
| γ-GT (U/L) | 18.05 ± 4.37 | 23.63 ± 14.57 | 0.390 |
| UREA (mmol/L) | 4.55 ± 0.29 | 4.88 ± 0.77 | 0.348 |
| T-Bil-V (mol/L) | 2.16 ± 1.09 | 1.80 ± 0.38 | 0.470 |
| TC (mmol/L) | 2.23 ± 0.35 | 2.96 ± 0.88 | 0.091 |
| LDL-C (mmol/L) | 0.34 ± 0.08 | 0.41 ± 0.31 | 0.069 |
| HDL-C (mmol/L) | 1.34 ± 0.19 | 1.40 ± 0.24 | 0.624 |
| UA (μmol/L) | 30.93 ± 6.48 | 38.60 ± 9.36 | 0.130 |
| Ca (mmol/L) | 1.97 ± 0.29 | 1.80 ± 0.13 | 0.232 |
| P (mmol/L) | 1.97 ± 0.55 | 2.36 ± 0.78 | 0.340 |
| Mg (mmol/L) | 0.99 ± 0.10 | 1.01 ± 0.06 | 0.707 |
| Chemical Denomination | Formula | p-Value | VIP | m. z | Retention Time (min) | Trend | Metabolic Pathway |
|---|---|---|---|---|---|---|---|
| Lipid metabolism-related metabolites | |||||||
| PC (16:1e/6:0) | C30H60NO7P | 2.00 × 10−4 | 1.57 | 578.42 | 11.38 | up | |
| PC (14:1e/2:0) | C24H48NO7P | 1.60 × 10−2 | 1.84 | 494.32 | 8.67 | up | |
| PC (18:5e/2:0) | C28H48NO7P | 3.20 × 10−2 | 1.57 | 542.32 | 8.46 | up | |
| PC (16:2e/2:0) | C26H50NO7P | 4.10 × 10−2 | 1.45 | 520.34 | 9.19 | up | |
| PC (20:5e/2:0) | C30H52NO7P | 4.80 × 10−2 | 1.51 | 570.35 | 9.17 | up | |
| PC (22:3e/12:0) | C42H80NO7P | 4.10 × 10−2 | 1.08 | 742.57 | 10.54 | down | |
| PC (18:3e/2:0) | C28H52NO7P | 3.70 × 10−2 | 1.71 | 546.35 | 9.20 | down | |
| PC (18:1/18:3) | C44H80NO8P | 3.40 × 10−2 | 1.54 | 826.56 | 11.78 | down | |
| SM (d14:1/16:2) | C35H67N2O6P | 7.00 × 10−3 | 1.18 | 643.48 | 11.73 | up | |
| LPC 15:1 | C23H46NO7P | 3.40 × 10−2 | 1.74 | 480.31 | 8.66 | up | |
| LPC 14:1 | C22H44NO7P | 2.80 × 10−2 | 1.47 | 510.28 | 8.01 | up | |
| LPC 16:1 | C24H48NO7P | 4.20 × 10−2 | 1.53 | 538.32 | 8.87 | up | |
| LPC 18:2 | C26H50NO7P | 7.00 × 10−3 | 1.48 | 564.33 | 9.17 | up | |
| LPC 16:0 | C24H50NO7P | 9.00 × 10−3 | 1.79 | 540.33 | 9.51 | up | |
| LPC 20:5 | C28H48NO7P | 1.20 × 10−2 | 1.55 | 586.32 | 8.63 | up | |
| LPC 20:1 | C28H56NO7P | 2.30 × 10−2 | 1.72 | 594.38 | 10.32 | up | |
| LPC 20:3 | C28H52NO7P | 4.20 × 10−2 | 1.40 | 590.35 | 9.48 | down | |
| LPE 16:0 | C21H44NO7P | 3.00 × 10−3 | 2.17 | 452.28 | 9.45 | up | |
| LPE 17:0 | C22H46NO7P | 1.00 × 10−2 | 1.60 | 466.29 | 9.74 | up | |
| LPE 16:1 | C21H42NO7P | 3.90 × 10−2 | 1.25 | 450.26 | 8.83 | up | |
| LPE 18:1 | C23H46NO7P | 3.20 × 10−2 | 1.62 | 478.29 | 8.87 | up | |
| LPE 20:3 | C25H46NO7P | 1.00 × 10−3 | 1.87 | 502.29 | 9.45 | down | |
| LPA 16:0 | C19H39O7P | 4.00 × 10−3 | 1.47 | 409.24 | 9.51 | up | |
| LPG 18:1 | C24H47O9P | 4.10 × 10−2 | 1.30 | 511.30 | 6.58 | down | |
| Choline | C5H13NO | 1.40 × 10−2 | 1.44 | 104.11 | 1.28 | down | Cholinergic synapse Glycerophospholipid metabolism |
| Acetylcholine | C7H15NO2 | 6.00 × 10−3 | 1.74 | 146.12 | 1.37 | up | Cholinergic synapse Glycerophospholipid metabolism Insulin secretion cAMP signaling pathway Neuroactive ligand–receptor interaction |
| Linoleic acid | C18H32O2 | 3.00 × 10−3 | 1.44 | 279.23 | 9.17 | up | Linoleic acid metabolism |
| Tetrahydrocorticosterone | C21H34O4 | 6.00 × 10−3 | 1.40 | 349.24 | 7.97 | up | Steroid hormone biosynthesis |
| 11-transleukotriene C4 | C30H47N3O9S | 2.20 × 10−2 | 1.44 | 624.30 | 5.02 | down | |
| Dehydroepiandrosterone (DHEA) | C19H28O2 | 6.00 × 10−4 | 1.89 | 271.21 | 8.42 | up | |
| Prostaglandin G2 | C20H32O6 | 2.50 × 10−2 | 1.69 | 367.21 | 7.85 | down | Platelet activation |
| Amino acids metabolism-related metabolites | |||||||
| Xanthurenic acid | C10H7NO4 | 5.00 × 10−4 | 2.29 | 206.04 | 5.30 | up | Tryptophan metabolism |
| Indole-3-lactic acid | C11H11NO3 | 3.00 × 10−3 | 1.06 | 206.08 | 5.65 | up | |
| Serotonin | C10H12N2O | 1.20 × 10−2 | 1.14 | 177.10 | 1.60 | down | Tryptophan metabolism Inflammatory mediator regulation of TRP channels cAMP signaling pathway |
| Creatinine | C4H7N3O | 1.30 × 10−2 | 1.20 | 114.07 | 1.32 | up | Arginine and proline metabolism |
| Creatine | C4H9N3O2 | 2.40 × 10−2 | 1.90 | 132.08 | 1.37 | down | Arginine and proline metabolism Glycine, serine and threonine metabolism |
| N6,N6,N6-Trimethyl-l-lysine | C9H20N2O2 | 4.10 × 10−2 | 1.36 | 189.16 | 1.25 | down | Lysine degradation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Zhang, G.; Sun, M.; Xia, M.; Shi, R.; Jin, Y.; Zhang, X. Comparative Analysis of the Potential Adaptability of Tibetan Dzo and Yellow Cattle Based on Blood Indices, Metabolites, and Fecal Microbiota. Animals 2024, 14, 2728. https://doi.org/10.3390/ani14182728
Li K, Zhang G, Sun M, Xia M, Shi R, Jin Y, Zhang X. Comparative Analysis of the Potential Adaptability of Tibetan Dzo and Yellow Cattle Based on Blood Indices, Metabolites, and Fecal Microbiota. Animals. 2024; 14(18):2728. https://doi.org/10.3390/ani14182728
Chicago/Turabian StyleLi, Kenan, Guorui Zhang, Mengjiao Sun, Maolin Xia, Ruizhi Shi, Yanmei Jin, and Xiaoqing Zhang. 2024. "Comparative Analysis of the Potential Adaptability of Tibetan Dzo and Yellow Cattle Based on Blood Indices, Metabolites, and Fecal Microbiota" Animals 14, no. 18: 2728. https://doi.org/10.3390/ani14182728





