Characterizing Marine Medaka (Oryzias melastigma) Haploid Embryonic Stem Cells: A Valuable Tool for Marine Fish Genetic Research
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Viruses
2.2. Induced Gynogenesis
2.3. Cell Culture
2.4. Cell Colony Formation
2.5. Cytogenetic Analyses
2.6. Alkaline Phosphatase (AP) Staining
2.7. Growth Curves
2.8. RNA Extraction and qPCR Analysis
2.9. Embryoid Body (EB) Formation
2.10. Cell Transplantation
2.11. Cell Transfection and Transduction
2.12. Statistics
3. Results
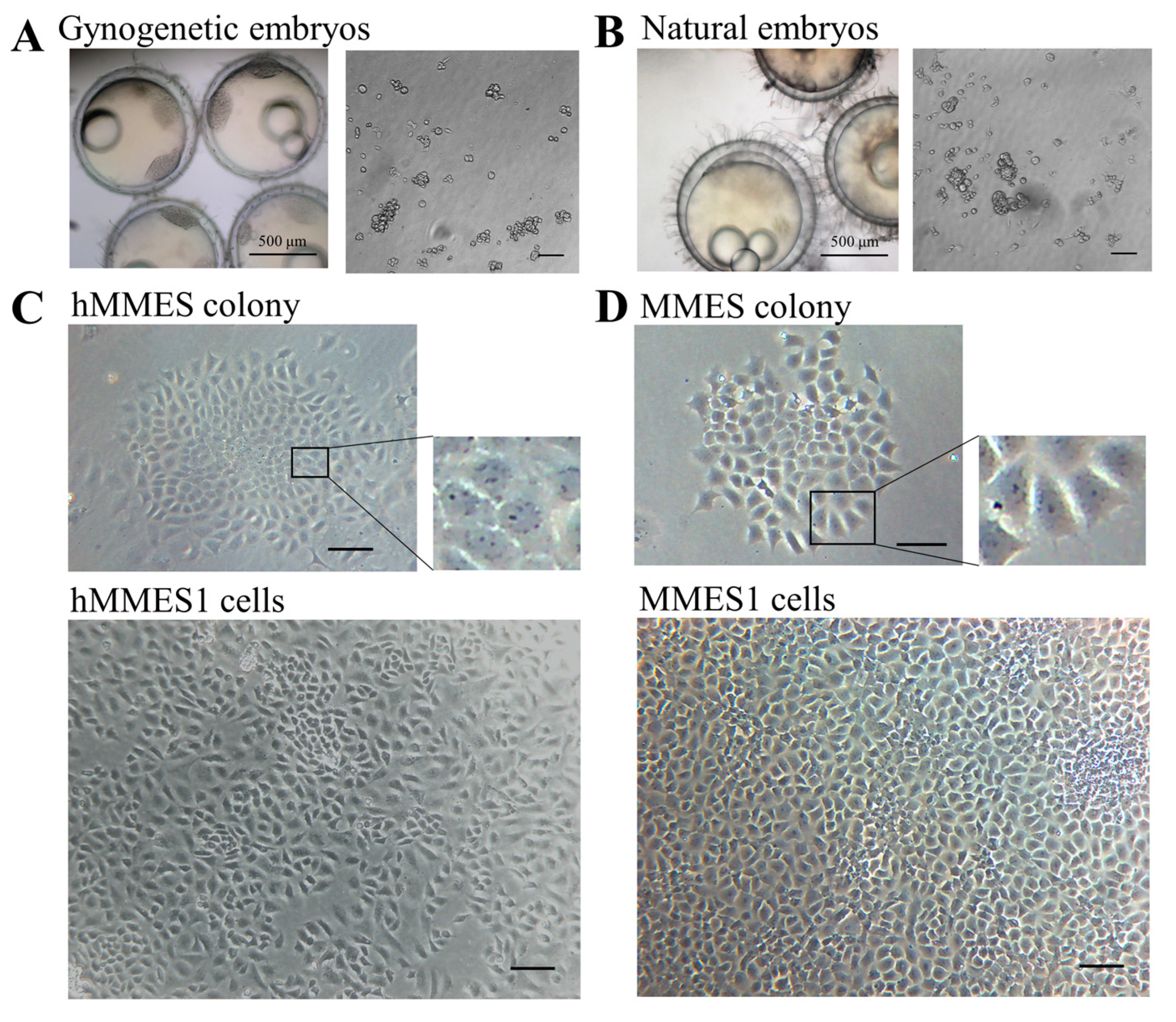
3.1. Derivation of Marine Medaka Haploid ESCs
3.2. Karyotype, Pluripotency, and Growth Kinetics of hMMES1 Cells
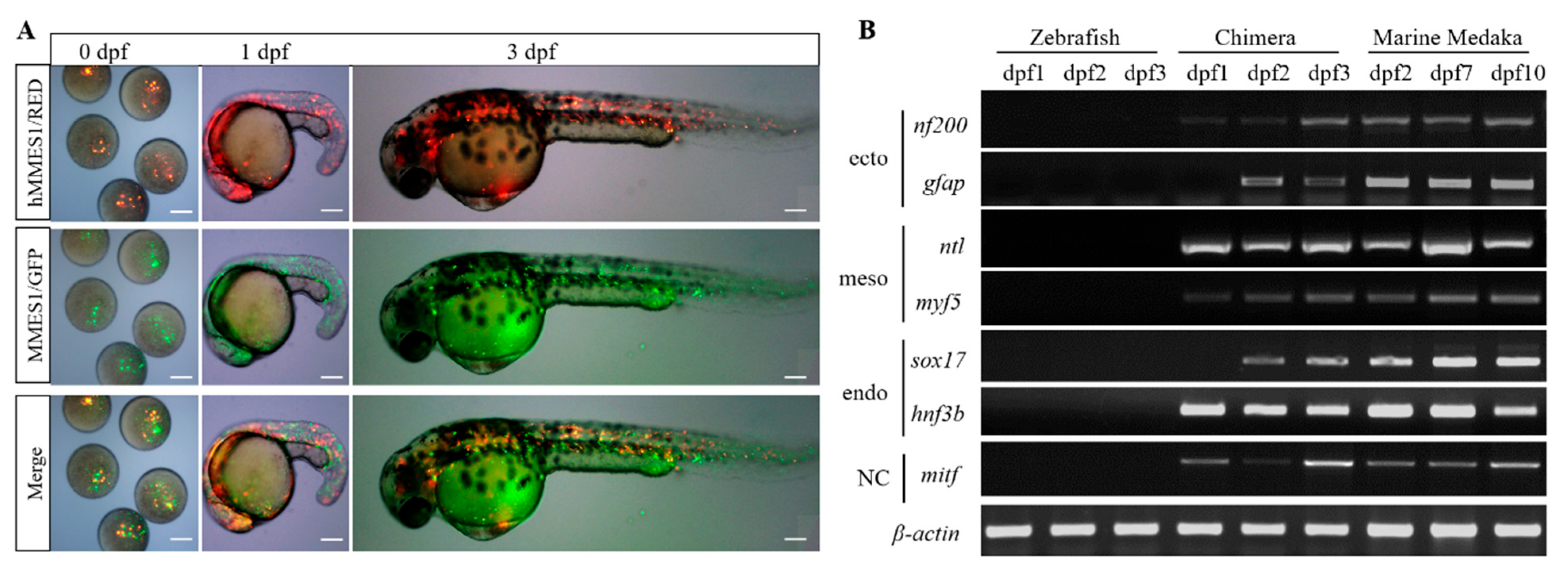
3.3. Induced Differentiation of hMMES1 Cells In Vitro and In Vivo
3.4. Interordinal Chimera Formation of hMMES1 Cells in Zebrafish
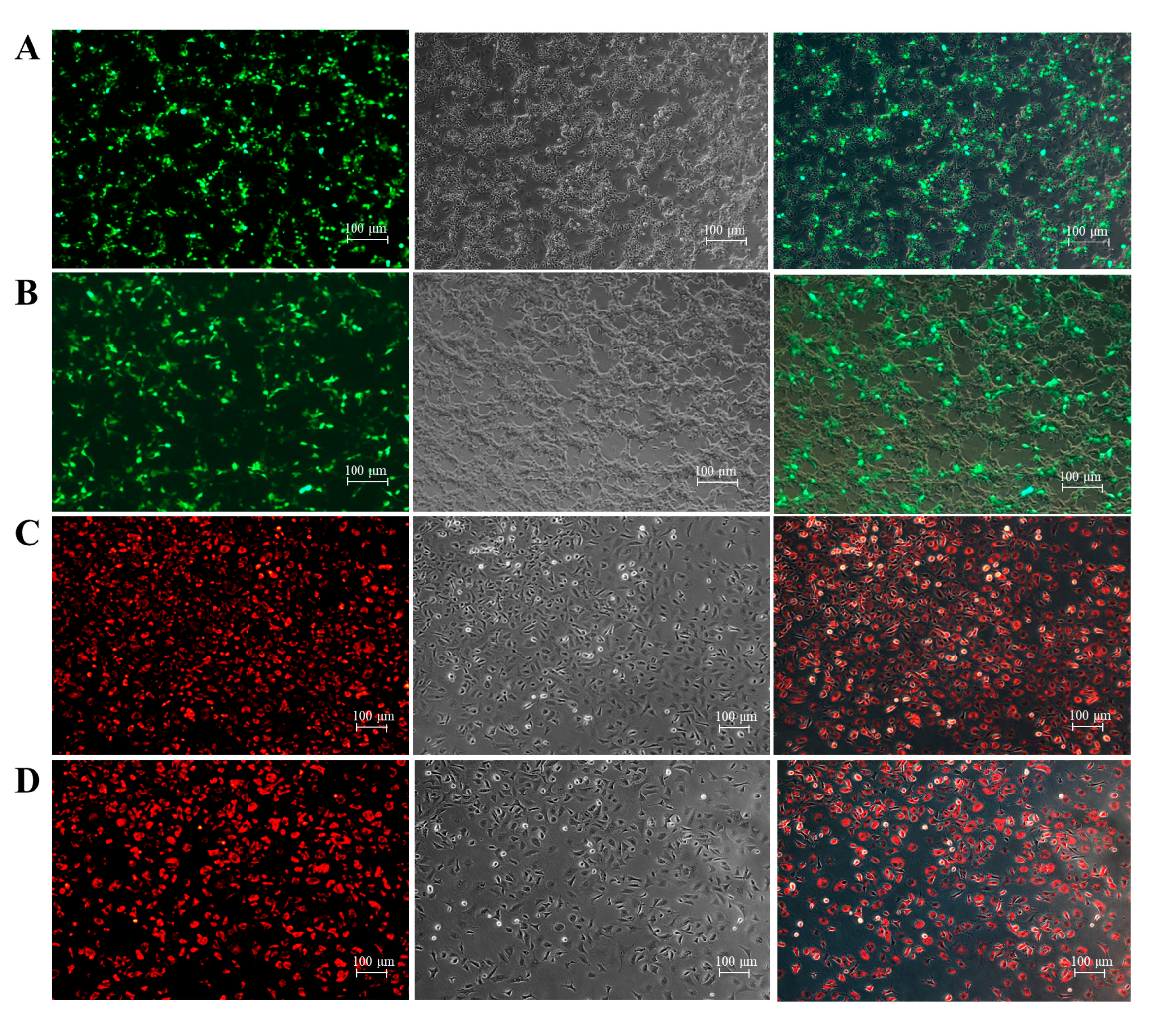
3.5. Cell Transfection and Transduction Efficiency
3.6. Viral Susceptibility of hMMES1 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, L.F.; Li, J.S. Generation of genetically modified mice using CRISPR/Cas9 and haploid embryonic stem cell systems. Zool. Res. 2016, 37, 205–213. [Google Scholar]
- Carette, J.E.; Guimaraes, C.P.; Varadarajan, M.; Park, A.S.; Wuethrich, I.; Godarova, A.; Kotecki, M.; Cochran, B.H.; Spooner, E.; Ploegh, H.L.; et al. Haploid genetic screens in human cells identify host factors used by pathogens. Science 2009, 326, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Elling, U.; Taubenschmid, J.; Wirnsberger, G.; O’Malley, R.; Demers, S.-P.; Vanhaelen, Q.; Shukalyuk, A.I.; Schmauss, G.; Schramek, D.; Schnuetgen, F.; et al. Forward and reverse genetics through derivation of haploid mouse embryonic stem cells. Cell Stem Cell 2011, 9, 563–574. [Google Scholar] [CrossRef]
- Leeb, M.; Walker, R.; Mansfield, B.; Nichols, J.; Smith, A.; Wutz, A. Germline potential of parthenogenetic haploid mouse embryonic stem cells. Development 2012, 139, 3301–3305. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Hong, N.; Hong, Y. Generation of medaka fish haploid embryonic stem cells. Science 2009, 326, 430–433. [Google Scholar] [CrossRef]
- Leeb, M.; Wutz, A. Derivation of haploid embryonic stem cells from mouse embryos. Nature 2011, 479, 131–134. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Li, T.; Jiang, M.G.; Wan, H.; Luo, G.Z.; Feng, C.; Cui, X.; Teng, F.; Yuan, Y.; et al. Genetic modification and screening in rat using haploid embryonic stem cells. Cell Stem Cell 2014, 14, 404–414. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Z.; Ma, Y.; Zhong, C.; Yin, Q.; Zhou, C.; Shi, L.; Cai, Y.; Zhao, H.; Wang, H.; et al. Generation of haploid embryonic stem cells from Macaca fascicularis monkey parthenotes. Cell Res. 2013, 23, 1187–1200. [Google Scholar] [CrossRef]
- Sagi, I.; Chia, G.; Golan-Lev, T.; Peretz, M.; Weissbein, U.; Sui, L.; Sauer, M.V.; Yanuka, O.; Egli, D.; Benvenisty, N. Derivation and differentiation of haploid human embryonic stem cells. Nature 2016, 532, 107–111. [Google Scholar] [CrossRef]
- Lee, D.; Ryu, J.H.; Lee, S.T.; Nam, Y.K.; Kim, D.S.; Gong, S.P. Identification of embryonic stem cell activities in an embryonic cell line derived from marine medaka (Oryzias dancena). Fish Physiol. Biochem. 2015, 41, 1569–1576. [Google Scholar] [CrossRef]
- Zilova, L.; Weinhardt, V.; Tavhelidse, T.; Schlagheck, C.; Thumberger, T.; Wittbrodt, J. Fish primary embryonic pluripotent cells assemble into retinal tissue mirroring in vivo early eye development. Elife 2021, 10, e66998. [Google Scholar] [CrossRef] [PubMed]
- Son, M.J.; Gong, S.P. Feeder cell-dependent primary culture of single blastula-derived embryonic cell lines from marine medaka (Oryzias dancena). In Vitro Cell. Dev. Biol. Anim. 2022, 58, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Kim, J.; Choi, I.Y.; Raisuddin, S.; Au, D.W.; Leung, K.M.; Wu, R.S.; Rhee, J.S.; Lee, J.S. Omics of the marine medaka (Oryzias melastigma) and its relevance to marine environmental research. Mar. Environ. Res. 2016, 113, 141–152. [Google Scholar] [CrossRef]
- Ye, R.R.; Lei, E.N.; Lam, M.H.; Chan, A.K.; Bo, J.; van de Merwe, J.P.; Fong, A.C.; Yang, M.M.; Lee, J.S.; Segner, H.E.; et al. Gender-specific modulation of immune system complement gene expression in marine medaka Oryzias melastigma following dietary exposure of BDE-47. Environ. Sci. Pollut. Res. Int. 2011, 19, 2477–2487. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Jia, K.T.; Yi, M.S. Complete genome sequence of a fish nervous necrosis virus isolated from Sea perch (Lateolabrax japonicus) in China. Genome. Announc. 2015, 3, 10–1128. [Google Scholar] [CrossRef]
- Liu, Y.; Tran, B.N.; Wang, F.; Ounjai, P.; Wu, J.; Hew, C.L. Visualization of assembly intermediates and budding vacuoles of singapore grouper iridovirus in grouper embryonic cells. Sci. Rep. 2016, 6, 18696. [Google Scholar] [CrossRef]
- Yi, M.; Hong, N.; Hong, Y. Derivation and characterization of haploid embryonic stem cell cultures in medaka fish. Nat. Protoc. 2010, 5, 1418–1430. [Google Scholar] [CrossRef]
- Le, Y.; Li, Y.; Jin, Y.; Jia, P.; Jia, K.; Yi, M. Establishment and characterization of a brain cell line from sea perch, Lateolabrax japonicus. In Vitro Cell. Dev. Biol. Anim. 2017, 53, 834–840. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, P.; Liu, W.; Jia, K.; Yi, M. Screening for antiviral medaka haploid embryonic stem cells by genome wide mutagenesis. Mar. Biotechnol. 2019, 21, 186–195. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Lin, F.; Zhang, L.; Li, Y.; Ge, R.; Hong, Y. Gene transfer and genome-wide insertional mutagenesis by retroviral transduction in fish stem cells. PLoS ONE 2015, 10, e0127961. [Google Scholar] [CrossRef]
- Hong, N.; Chen, S.; Ge, R.; Song, J.; Yi, M.; Hong, Y. Interordinal chimera formation between medaka and zebrafish for analyzing stem cell differentiation. Stem Cells Dev. 2012, 21, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Bandín, I.; Souto, S. Betanodavirus and VER Disease: A 30-year Research Review. Pathogens 2020, 9, 106–116. [Google Scholar] [CrossRef]
- Yuan, Y.; Hong, Y. Subcellular redistribution and sequential recruitment of macromolecular components during SGIV assembly. Protein Cell 2016, 7, 651–661. [Google Scholar] [CrossRef]
- Yilmaz, A.; Peretz, M.; Sagi, I.; Benvenisty, N. Haploid human embryonic stem cells: Half the genome, double the value. Cell Stem Cell 2016, 19, 569–572. [Google Scholar] [CrossRef][Green Version]
- Pillay, S.; Carette, J.E. Hunting viral receptors using haploid cells. Annu Rev Virol 2015, 2, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Carette, J.E.; Raaben, M.; Wong, A.C.; Herbert, A.S.; Obernosterer, G.; Mulherkar, N.; Kuehne, A.I.; Kranzusch, P.J.; Griffin, A.M.; Ruthel, G.; et al. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 2011, 477, 340–343. [Google Scholar] [CrossRef]
- Guimaraes, C.P.; Carette, J.E.; Varadarajan, M.; Antos, J.; Popp, M.W.; Spooner, E.; Brummelkamp, T.R.; Ploegh, H.L. Identification of host cell factors required for intoxication through use of modified cholera toxin. J. Cell Biol. 2011, 195, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jia, K.; Jia, P.; Xiang, Y.; Lu, X.; Liu, W.; Yi, M. Marine medaka heat shock protein 90ab1 is a receptor for red-spotted grouper nervous necrosis virus and promotes virus internalization through clathrin-mediated endocytosis. PLoS Pathog. 2020, 16, e1008668. [Google Scholar] [CrossRef]
- Chen, J.J.; Lei, K. The known, unknown, and unknown unknowns of cell-cell communication in planarian regeneration. Zool. Res. 2023, 44, 981–992. [Google Scholar] [CrossRef]
- Guo, L.Y.; Wei, J.K.; Yang, S.C.; Wang, Z.B. Glaucoma model for stem cell transplantation research in New Zealand white rabbits. Zool. Res. 2012, 33, 225–230. [Google Scholar] [CrossRef][Green Version]
- Chen, S.-L.; Sha, Z.-X.; Ye, H.-Q. Establishment of a pluripotent embryonic cell line from sea perch (Lateolabrax japonicus) embryos. Aquaculture 2003, 218, 141–151. [Google Scholar] [CrossRef]
- Zhang, W.W.; Jia, P.; Lu, X.B.; Chen, X.Q.; Weng, J.H.; Jia, K.T.; Yi, M.S. Capsid protein from red-spotted grouper nervous necrosis virus induces incomplete autophagy by inactivating the HSP90ab1-AKT-MTOR pathway. Zool. Res. 2022, 43, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, P.; Liu, J.; Ni, S.; Yu, Y.; Yang, M.; Wei, S.; Qin, Q. Establishment and characterization of a mid-kidney cell line derived from golden pompano Trachinotus ovatus, a new cell model for virus pathogenesis and toxicology studies. In Vitro Cell. Dev. Biol. Anim. 2017, 53, 320–327. [Google Scholar] [CrossRef]
- Qin, Q.W.; Wu, T.H.; Jia, T.L.; Hegde, A.; Zhang, R.Q. Development and characterization of a new tropical marine fish cell line from grouper, Epinephelus coioides susceptible to iridovirus and nodavirus. J. Virol. Methods 2006, 131, 58–64. [Google Scholar] [CrossRef]
- Kuo, H.C.; Wang, T.Y.; Hsu, H.H.; Chen, P.P.; Lee, S.H.; Chen, Y.M.; Tsai, T.J.; Wang, C.K.; Ku, H.T.; Lee, G.-B.; et al. Nervous necrosis virus replicates following the embryo development and dual infection with iridovirus at juvenile stage in grouper. PLoS ONE 2012, 7, e36183. [Google Scholar] [CrossRef]
- Gomez, D.K.; Sato, J.; Mushiake, K.; Isshiki, T.; Okinaka, Y.; Nakai, T. PCR-based detection of betanodaviruses from cultured and wild marine fish with no clinical signs. J. Fish Dis. 2004, 27, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhu, Y.; Hew, C.L. Quantitative study of proteomic alterations in a zebrafish (Danio rerio) cell line infected with the singapore grouper iridovirus (SGIV). Virus Res. 2015, 199, 62–67. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, X.; Zhang, L.; Zhu, Y.; Huang, Y.; Qin, Q.; Hong, Y. Medaka haploid embryonic stem cells are susceptible to Singapore grouper iridovirus as well as to other viruses of aquaculture fish species. J. Gen. Virol. 2013, 94, 2352–2359. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Chen, H.; Liu, W.; Jia, K.; Yi, M. Characterizing Marine Medaka (Oryzias melastigma) Haploid Embryonic Stem Cells: A Valuable Tool for Marine Fish Genetic Research. Animals 2024, 14, 2739. https://doi.org/10.3390/ani14182739
Zhang W, Chen H, Liu W, Jia K, Yi M. Characterizing Marine Medaka (Oryzias melastigma) Haploid Embryonic Stem Cells: A Valuable Tool for Marine Fish Genetic Research. Animals. 2024; 14(18):2739. https://doi.org/10.3390/ani14182739
Chicago/Turabian StyleZhang, Wanwan, Huiquan Chen, Wei Liu, Kuntong Jia, and Meisheng Yi. 2024. "Characterizing Marine Medaka (Oryzias melastigma) Haploid Embryonic Stem Cells: A Valuable Tool for Marine Fish Genetic Research" Animals 14, no. 18: 2739. https://doi.org/10.3390/ani14182739
APA StyleZhang, W., Chen, H., Liu, W., Jia, K., & Yi, M. (2024). Characterizing Marine Medaka (Oryzias melastigma) Haploid Embryonic Stem Cells: A Valuable Tool for Marine Fish Genetic Research. Animals, 14(18), 2739. https://doi.org/10.3390/ani14182739






