Establishment of an Indirect ELISA Method for the Detection of the Bovine Rotavirus VP6 Protein
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell and Serum Samples
2.2. Construction and Characterization of the VP6 Recombinant Plasmid
2.3. Transfection, Purification, and Western Blot Analysis of Recombinant Proteins
2.4. Preparation of Mouse Polyclonal Antibodies
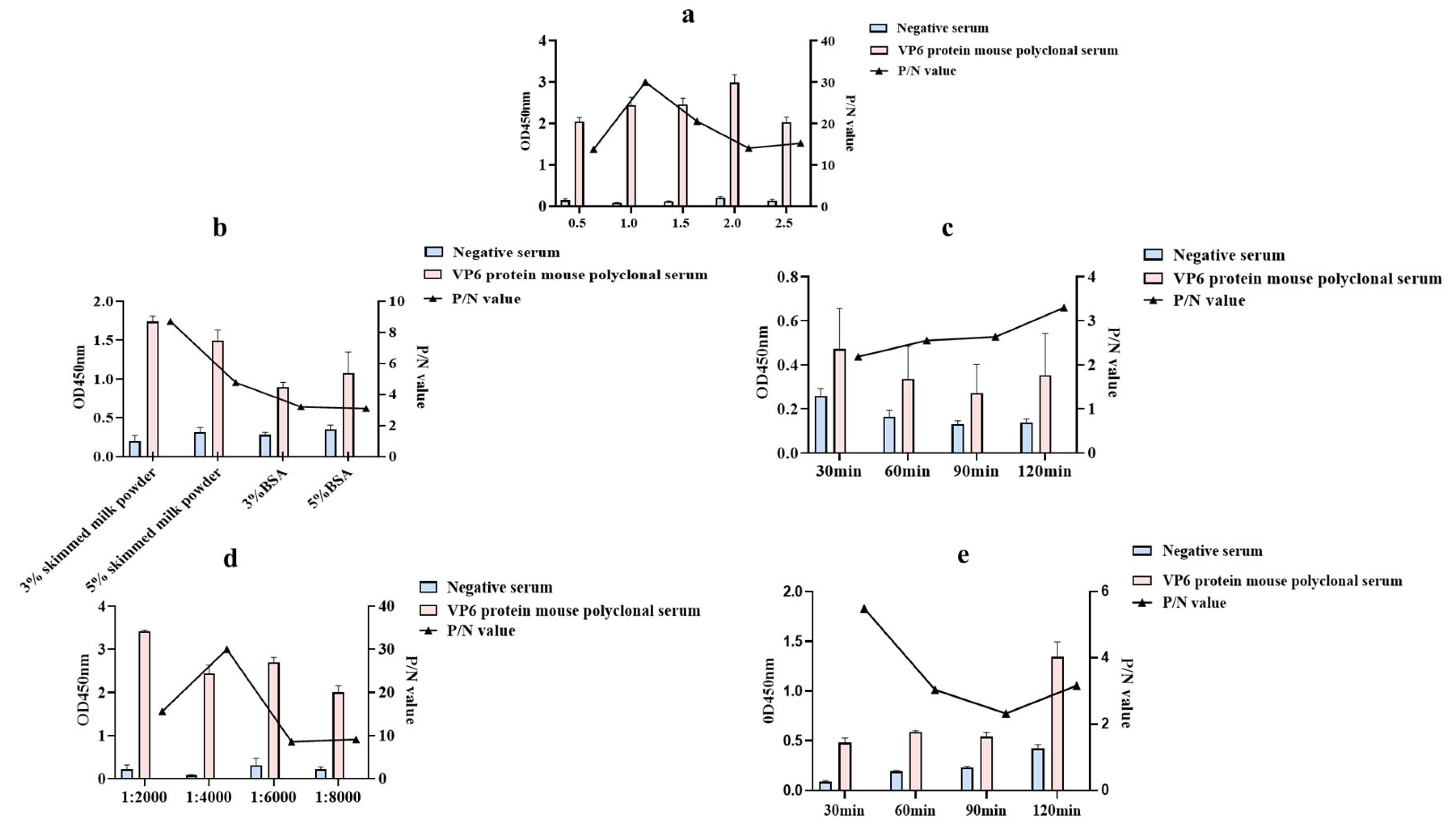
2.5. Establishment of an Indirect ELISA Based on the VP6 Protein
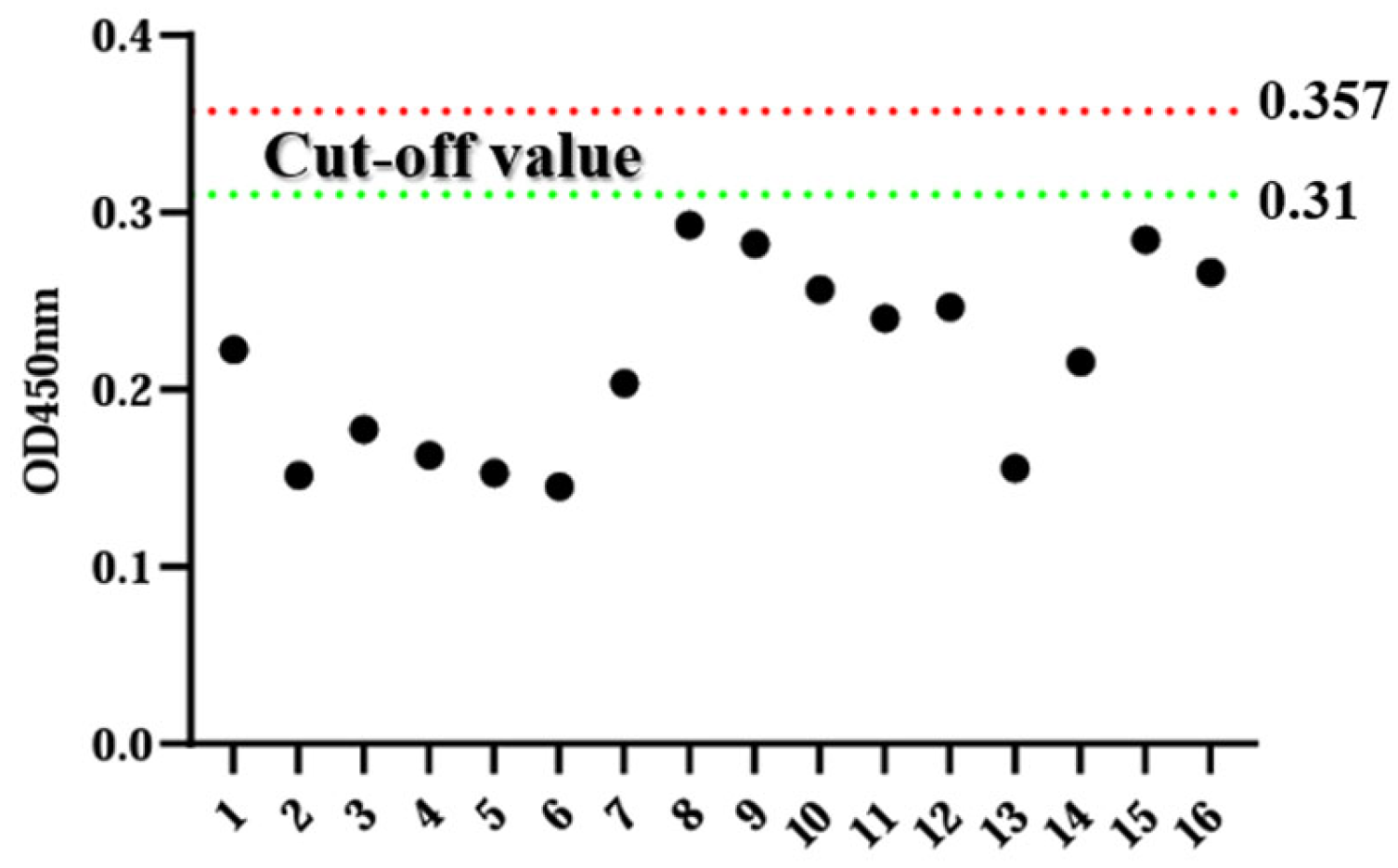
2.6. Determination of Critical Values
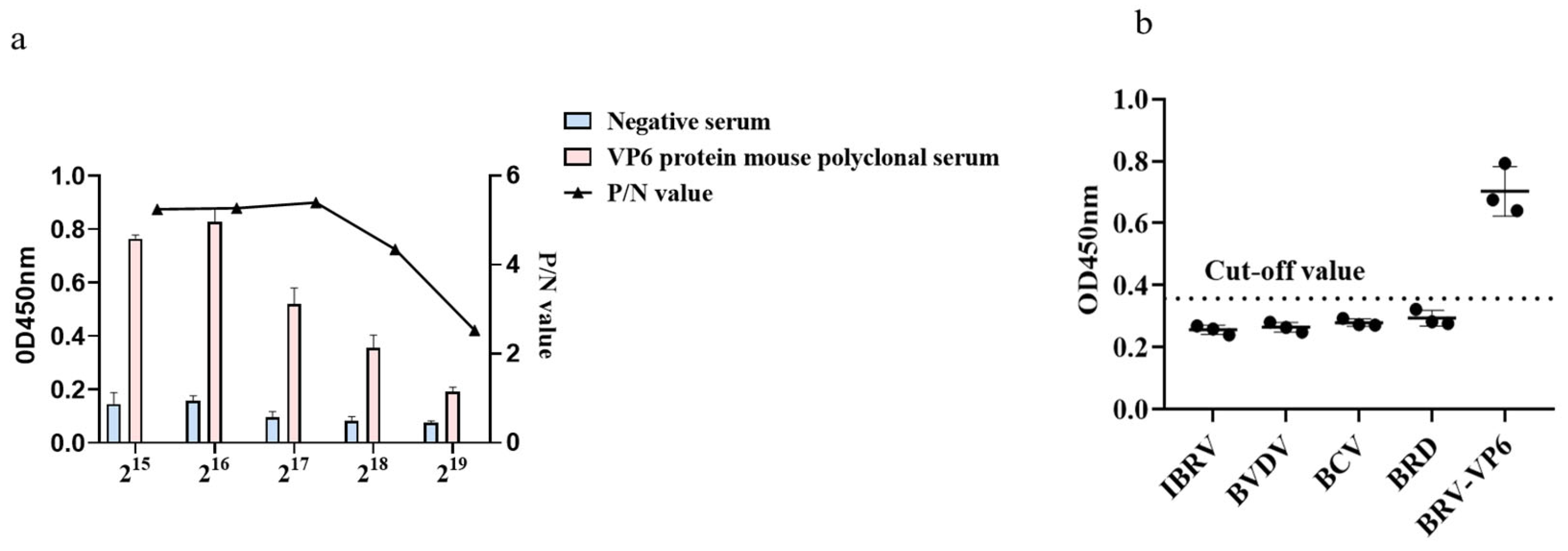
2.7. Sensitivity and Specificity Tests
2.8. Repeat Experiments within and between Batches
2.9. Preliminary Application of ELISA in Clinical Samples
3. Results
3.1. Enzymatic Digestion of Recombinant Plasmids and Protein Expression
3.2. Establishment of an Indirect ELISA Method Based on a Polyclonal Antibody against the VP6 Protein
3.3. Determination of Critical Values
3.4. Validation of the Sensitivity and Specificity of an Indirect ELISA Based on a Murine Polyclonal Antibody against the VP6 Protein
3.5. Stability Verification
3.6. Preliminary Application of ELISA in Clinical Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acres, S.D. Enterotoxigenic Escherichia coli infections in newborn calves: A review. J. Dairy Sci. 1985, 68, 229–256. [Google Scholar] [CrossRef]
- Tzipori, S. The relative importance of enteric pathogens affecting neonates of domestic animals. Adv. Vet. Sci. Comp. Med. 1985, 29, 103–206. [Google Scholar]
- Paredes, A.M.; Brown, D.T.; Rothnagel, R.; Chiu, W.; Schoepp, R.J.; Johnston, R.E.; Prasad, B. Three-dimensional structure of a membrane-containing virus. Proc. Natl. Acad. Sci. USA 1993, 90, 9095–9099. [Google Scholar] [CrossRef]
- Al-Kubati, A.A.; Hussen, J.; Kandeel, M.; Al-Mubarak, A.I.; Hemida, M.G. Recent advances on the bovine viral diarrhea virus molecular pathogenesis, immune response, and vaccines development. Front. Vet. Sci. 2021, 8, 665128. [Google Scholar] [CrossRef]
- Bertoni, E.; Aduriz, M.; Bok, M.; Vega, C.; Saif, L.; Aguirre, D.; Cimino, R.O.; Miño, S.; Parreño, V. First report of group A rotavirus and bovine coronavirus associated with neonatal calf diarrhea in the northwest of Argentina. Trop. Anim. Health Prod. 2020, 52, 2761–2768. [Google Scholar] [CrossRef]
- Gichile, A. Review on the epidemiology of Bovine Rotavirus and its public health significance. Int. J. Vet. Sci. Res. 2022, 8, 5–10. [Google Scholar]
- Kim, D.-U.; Jeong, S.-A.; Lee, S.-C.; Cho, K.-O.; Kang, S.-Y. Production and characterization of VP6-specific monoclonal antibodies against bovine group C rotavirus. J. Biomed. Transl. Res. 2016, 17, 58–64. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, Y.; Wang, X.; Dong, J.; Wang, B.; Han, C.; Yu, J.; Li, D. Oral administration of plant-based rotavirus VP6 induces antigen-specific IgAs, IgGs and passive protection in mice. Vaccine 2010, 28, 6021–6027. [Google Scholar] [CrossRef]
- Lappalainen, S.; Pastor, A.R.; Tamminen, K.; López-Guerrero, V.; Esquivel-Guadarrama, F.; Palomares, L.A.; Vesikari, T.; Blazevic, V. Immune responses elicited against rotavirus middle layer protein VP6 inhibit viral replication in vitro and in vivo. Hum. Vaccin. Immunother. 2014, 10, 2039–2047. [Google Scholar] [CrossRef]
- Li, Z.; Cui, K.; Wang, H.; Liu, F.; Huang, K.; Duan, Z.; Wang, F.; Shi, D.; Liu, Q. A milk-based self-assemble rotavirus VP6–ferritin nanoparticle vaccine elicited protection against the viral infection. J. Nanobiotechnol. 2019, 17, 13. [Google Scholar] [CrossRef]
- Athanassious, R.; Marsolais, G.; Assaf, R.; Dea, S.; DescOteaux, J.-P.; Dulude, S.; Montpetit, C. Detection of bovine coronavirus and type A rotavirus in neonatal calf diarrhea and winter dysentery of cattle in Quebec: Evaluation of three diagnostic methods. Can. Vet. J. 1994, 35, 163. [Google Scholar]
- Czerny, C.-P.; Eichhorn, W. Characterization of monoclonal and polyclonal antibodies to bovine enteric coronavirus: Establishment of an efficient ELISA for antigen detection in feces. Vet. Microbiol. 1989, 20, 111–122. [Google Scholar] [CrossRef]
- Lucchelli, A.; Kang, S.; Jayasekera, M.; Parwani, A.; Zeman, D.; Saif, L. A survey of G6 and G10 serotypes of group A bovine rotaviruses from diarrheic beef and dairy calves using monoclonal antibodies in ELISA. J. Vet. Diagn. Investig. 1994, 6, 175–181. [Google Scholar] [CrossRef]
- De Beer, M.; Peenze, I.; da Costa Mendes, V.; Steele, A. Comparison of electron microscopy, enzyme-linked immunosorbent assay and latex agglutination for the detection of bovine rotavirus in faeces. J. South Afr. Vet. Assoc. 1997, 68, 93–96. [Google Scholar] [CrossRef]
- Haikala, O.J.; Kokkonen, J.O.; Leinonen, M.K.; Nurmi, T.; Mäntyjärvi, R.; Sarkkinen, H.K. Rapid detection of rotavirus in stool by latex agglutination: Comparison with radioimmunoassay and electron microscopy and clinical evaluation of the test. J. Med. Virol. 1983, 11, 91–97. [Google Scholar] [CrossRef]
- Wernike, K.; Aebischer, A.; Michelitsch, A.; Hoffmann, D.; Freuling, C.; Balkema-Buschmann, A.; Graaf, A.; Müller, T.; Osterrieder, N.; Rissmann, M. Multi-species ELISA for the detection of antibodies against SARS-CoV-2 in animals. Transbound. Emerg. Dis. 2021, 68, 1779–1785. [Google Scholar] [CrossRef]
- Al-Yousif, Y.; Anderson, J.; Chard-Bergstrom, C.; Bustamante, A.; Muenzenberger, M.; Austin, K.; Kapil, S. Evaluation of a latex agglutination kit (Virogen Rotatest) for detection of bovine rotavirus in fecal samples. Clin. Diagn. Lab. Immunol. 2001, 8, 496–498. [Google Scholar] [CrossRef]
- Al-Yousif, Y.; Al-Majhdi, F.; Chard-Bergstrom, C.; Anderson, J.; Kapil, S. Development, characterization, and diagnostic applications of monoclonal antibodies against bovine rotavirus. Clin. Diagn. Lab. Immunol. 2000, 7, 288–292. [Google Scholar] [CrossRef]
- Mayameei, A.; Mohammadi, G.; Yavari, S.; Afshari, E.; Omidi, A. Evaluation of relationship between Rotavirus and Coronavirus infections with calf diarrhea by capture ELISA. Comp. Clin. Pathol. 2010, 19, 553–557. [Google Scholar] [CrossRef]
- Garaicoechea, L.; Bok, K.; Jones, L.R.; Combessies, G.; Odeon, A.; Fernandez, F.; Parreno, V. Molecular characterization of bovine rotavirus circulating in beef and dairy herds in Argentina during a 10-year period (1994–2003). Vet. Microbiol. 2006, 118, 1–11. [Google Scholar] [CrossRef]
- Gray, A.C.; Bradbury, A.; Dübel, S.; Knappik, A.; Plückthun, A.; Borrebaeck, C.A.K. Reproducibility: Bypass animals for antibody production. Nature 2020, 581, 262. [Google Scholar] [CrossRef]
- Midgley, S.E.; Bányai, K.; Buesa, J.; Halaihel, N.; Hjulsager, C.K.; Jakab, F.; Kaplon, J.; Larsen, L.E.; Monini, M.; Poljšak-Prijatelj, M. Diversity and zoonotic potential of rotaviruses in swine and cattle across Europe. Vet. Microbiol. 2012, 156, 238–245. [Google Scholar] [CrossRef]
- Falcone, E.; Tarantino, M.; Di Trani, L.; Cordioli, P.; Lavazza, A.; Tollis, M. Determination of bovine rotavirus G and P serotypes in Italy by PCR. J. Clin. Microbiol. 1999, 37, 3879–3882. [Google Scholar] [CrossRef]
- Lanz Uhde, F.; Kaufmann, T.; Sager, H.; Albini, S.; Zanoni, R.; Schelling, E.; Meylan, M. Prevalence of four enteropathogens in the faeces of young diarrhoeic dairy calves in Switzerland. Vet. Rec. 2008, 163, 362–366. [Google Scholar] [CrossRef]
- Haryadi, R.; Ho, S.; Kok, Y.J.; Pu, H.X.; Zheng, L.; Pereira, N.A.; Li, B.; Bi, X.; Goh, L.-T.; Yang, Y. Optimization of heavy chain and light chain signal peptides for high level expression of therapeutic antibodies in CHO cells. PLoS ONE 2015, 10, e0116878. [Google Scholar] [CrossRef]
- Berlec, A.; Štrukelj, B. Current state and recent advances in biopharmaceutical production in Escherichia coli, yeasts and mammalian cells. J. Ind. Microbiol. Biotechnol. 2013, 40, 257–274. [Google Scholar] [CrossRef]
- Jenkins, N.; Murphy, L.; Tyther, R. Post-translational modifications of recombinant proteins: Significance for biopharmaceuticals. Mol. Biotechnol. 2008, 39, 113–118. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Peng, J.-Y.; Cheng, Y.-H.; Chang, Y.-C.; Wu, Y.-T.; Tsai, P.-S.; Chiou, H.-Y.; Jeng, C.-R.; Chang, H.-W. Development and comparison of enzyme-linked immunosorbent assays based on recombinant trimeric full-length and truncated spike proteins for detecting antibodies against porcine epidemic diarrhea virus. BMC Vet. Res. 2019, 15, 421. [Google Scholar] [CrossRef]
- Tripathi, N.K.; Shrivastava, A. Recent developments in bioprocessing of recombinant proteins: Expression hosts and process development. Front. Bioeng. Biotechnol. 2019, 7, 420. [Google Scholar] [CrossRef]
- Lalonde, M.E.; Durocher, Y. Therapeutic glycoprotein production in mammalian cells. J. Biotechnol. 2017, 251, 128–140. [Google Scholar] [CrossRef]

| Batch | VP6 Mouse Polyclonal Antibody | Average OD450 nm | Standard Deviation SD | Coefficient of Variation/% CV |
|---|---|---|---|---|
| Within batch | Immune VP6 mouse polyclonal antibody | 0.600 | 0.033 | 5.5% |
| Negative mouse antibody | 0.233 | 0.006 | 2.6% | |
| Between batches | Immune VP6 mouse polyclonal antibody | 0.525 | 0.045 | 8.6% |
| Negative mouse antibody | 0.219 | 0.012 | 5.5% |
| BRV Positive Samples | BRV Negative Sample | ||
|---|---|---|---|
| iELISA Positive | iELISA Negative | iELISA Positive | iELISA Negative |
| 21 | 3 | 0 | 40 |
| Sensitivity = [21/21 + 3] = 87.5% | |||
| Specificity = [40/40] = 100% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Liu, Q.; Wang, P.; Zhang, G.; Jiang, L.; Zhang, S.; Zeng, J.; Yu, Y.; Wang, Y.; Li, Y. Establishment of an Indirect ELISA Method for the Detection of the Bovine Rotavirus VP6 Protein. Animals 2024, 14, 271. https://doi.org/10.3390/ani14020271
Niu X, Liu Q, Wang P, Zhang G, Jiang L, Zhang S, Zeng J, Yu Y, Wang Y, Li Y. Establishment of an Indirect ELISA Method for the Detection of the Bovine Rotavirus VP6 Protein. Animals. 2024; 14(2):271. https://doi.org/10.3390/ani14020271
Chicago/Turabian StyleNiu, Xiaoxia, Qiang Liu, Pu Wang, Gang Zhang, Lingling Jiang, Sinong Zhang, Jin Zeng, Yongtao Yu, Yujiong Wang, and Yong Li. 2024. "Establishment of an Indirect ELISA Method for the Detection of the Bovine Rotavirus VP6 Protein" Animals 14, no. 2: 271. https://doi.org/10.3390/ani14020271





