Use of New Ultrasonography Methods for Detecting Neoplasms in Dogs and Cats: A Review
Abstract
Simple Summary
Abstract
1. Introduction to Elastography
2. Applicability of Elastography
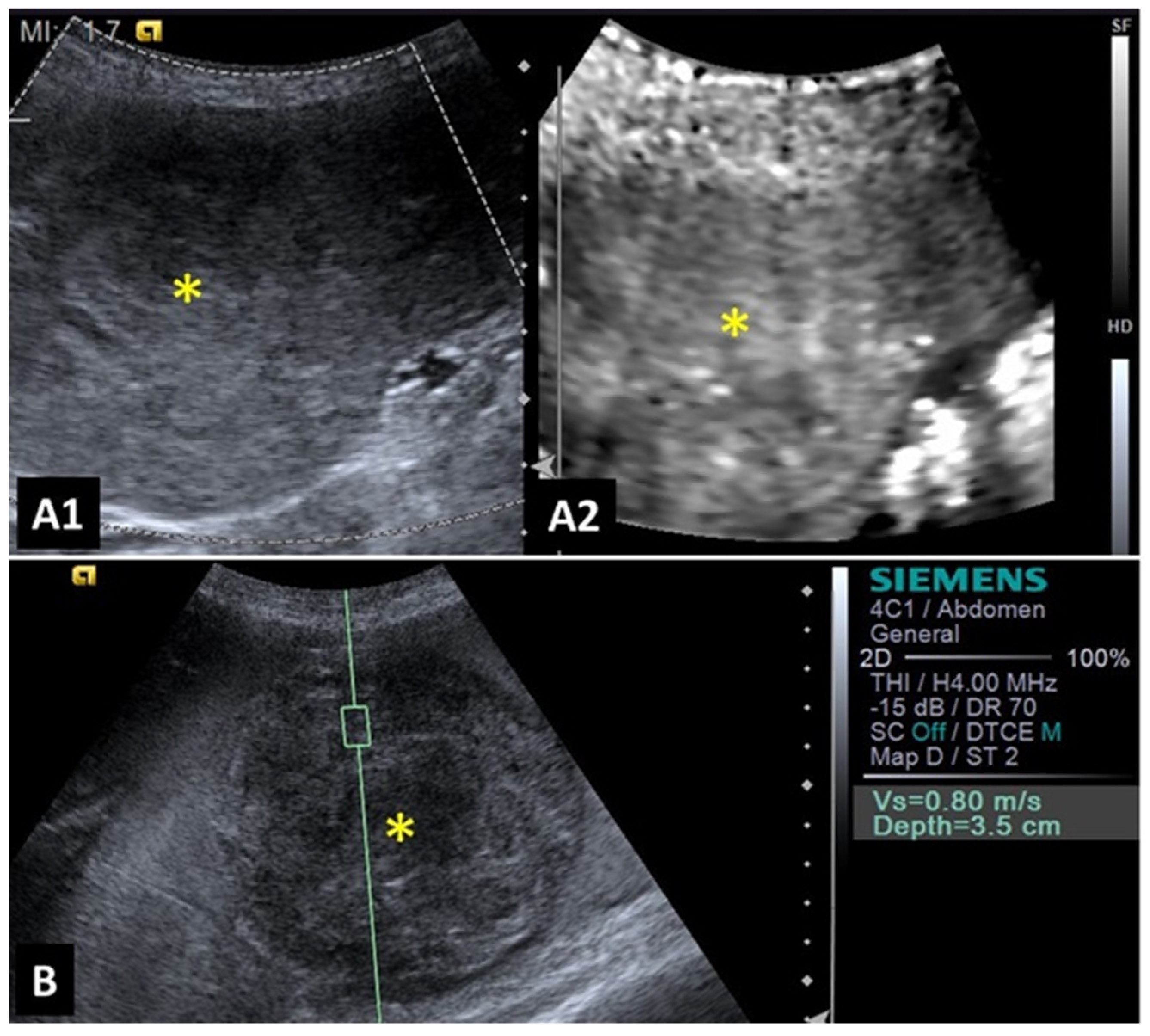
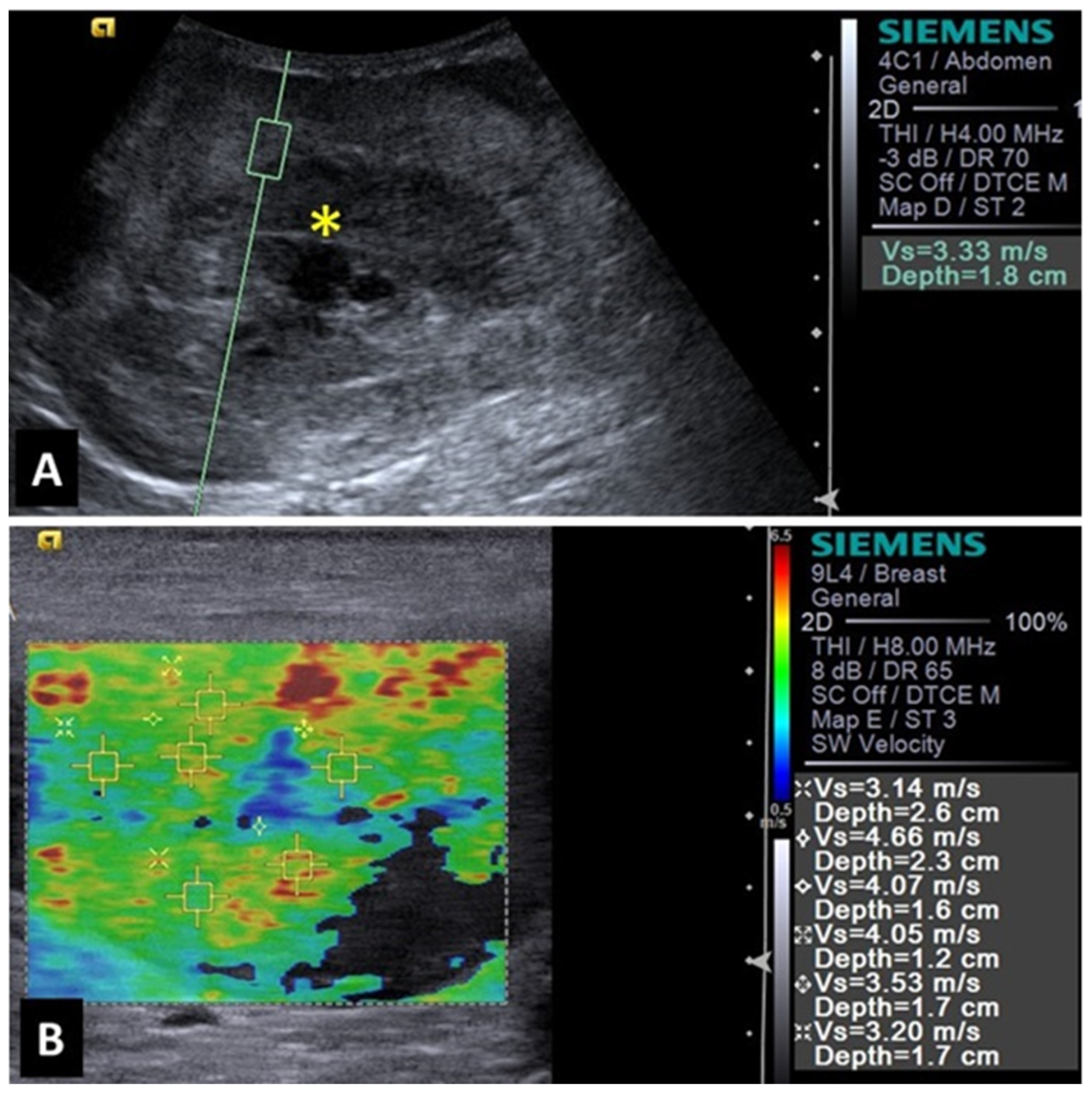
2.1. Mammary Glands
2.2. Lymph Nodes
2.3. Spleen
2.4. Cutaneous Nodules
2.5. Liver
2.6. Prostate and Testes
3. Introduction to Contrast-Enhanced Ultrasonography (CEUS)
4. Applicability of CEUS
4.1. Male Reproductive Tract
4.2. Mammary Glands
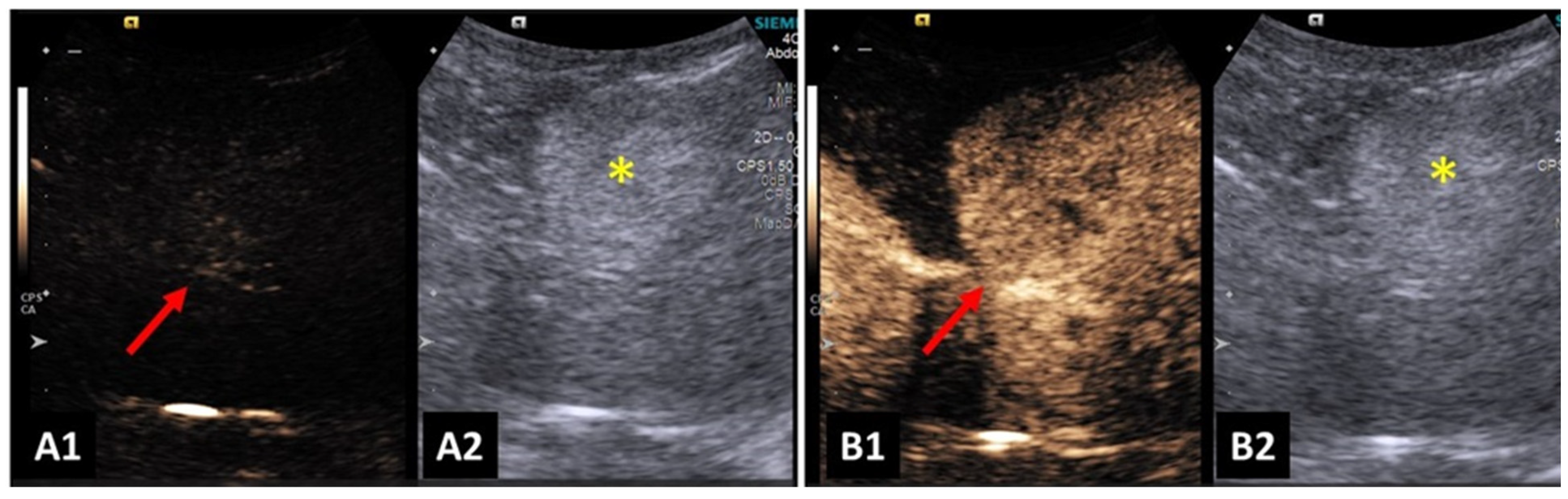
4.3. Kidneys and Urinary Bladder
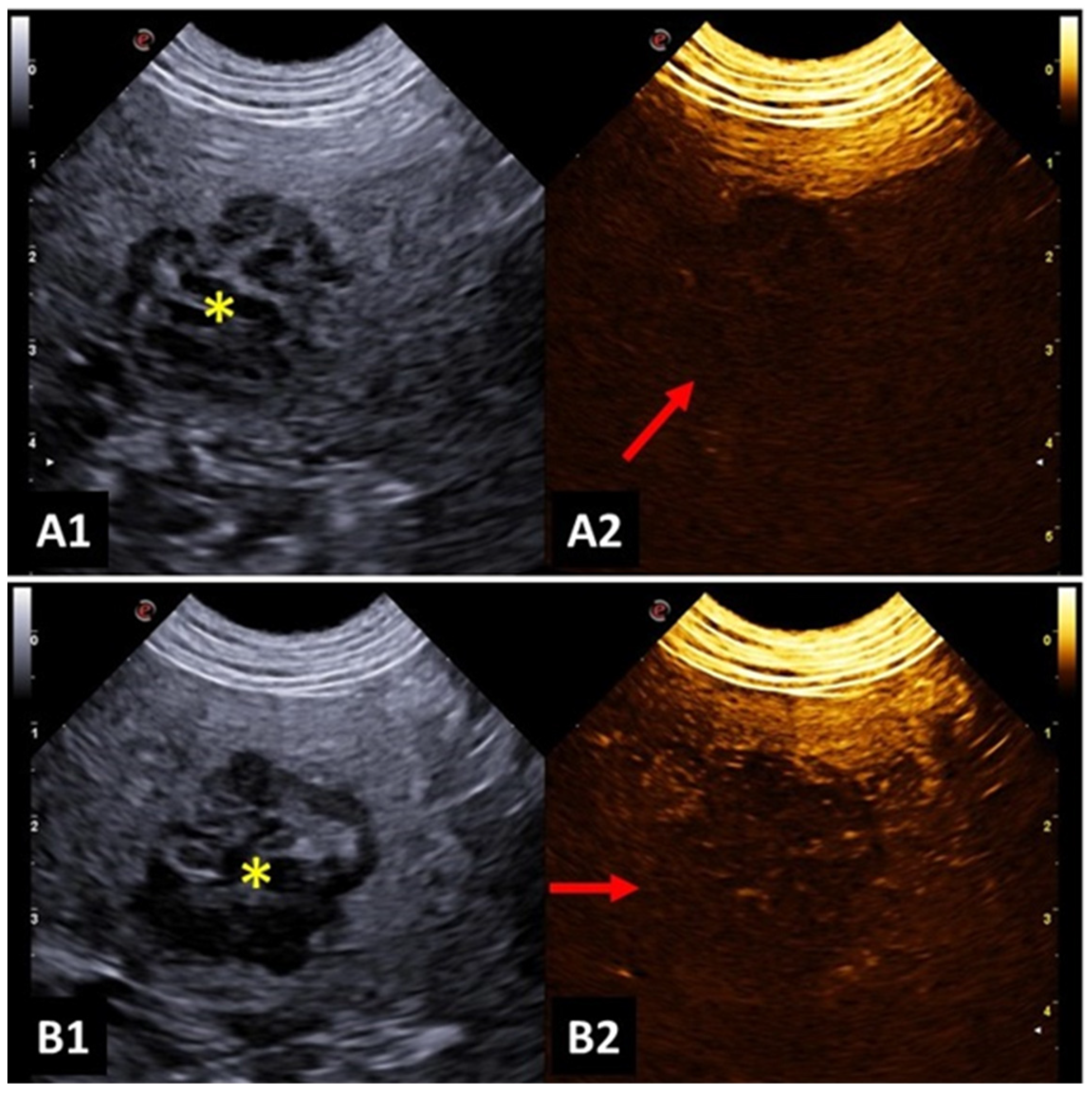
4.4. Lymph Nodes
4.5. Spleen
4.6. Gastrointestinal Tract
4.7. Liver and Biliary System
4.8. Adrenal Glands
4.9. Pancreas
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feliciano, M.A.R.; Maronezi, M.C.; Pavan, L.; Castanheira, T.L.; Simões, A.P.; Carvalho, C.; Canola, J.C.; Vicente, W.R. ARFI elastography as a complementary diagnostic method for mammary neoplasia in female dogs—Preliminary results. J. Small Anim. Pract. 2014, 55, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Appleby, R.B.; Vaden, S.L.; Monteith, G.; Seiler, G.S. Shear wave elastography evaluation of cats with chronic kidney disease. Vet. Radiol. Ultrasound 2023, 64, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Maronezi, M.C.; Feliciano, M.A.R.; Vicente, W.R.R. Elastografia e Ultrassonografia contrastada. In Ultrassonografia em Cães e Gatos, 1st ed.; Feliciano, M.A.R., Assis, A.R., Vicente, W.R.R., Eds.; MedVet: São Paulo, Brazil, 2019; pp. 33–56. [Google Scholar]
- Massimini, M.; Della Salda, L.; Di Francesco, L.; Contri, A. Strain and Shear-Wave Elastography and Their Relationship to Histopathological Features of Canine Mammary Nodular Lesions. Vet. Sci. 2022, 9, 506. [Google Scholar] [CrossRef] [PubMed]
- Sporea, I.; Bota, S.; Jurchis, A.; Sirli, R.; Gradinaru-Tascau, O.; Popescu, A.; Ratiu, I.; Szilaski, M. Acoustic Radiation force impulse and supersonic shear imaging versus transient elastography for liver fibrosis assessment. Ultrasound Med. Biol. 2013, 39, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.F.; Cintra, T.C.F.; Chammas, M.C. Elastography: Principles and considerations for clinical research in veterinary medicine. J. Vet. Med. Anim. Health 2015, 7, 99–110. [Google Scholar] [CrossRef][Green Version]
- Boozari, B.; Potthoff, A.; Mederacke, I.; Hahn, A.; Reising, A.; Rifai, K.; Wedemeyer, H.; Bahr, M.; Kubicka, S.; Manns, M.; et al. Evaluation of sound speed for detection of liver fibrosis. Prospective comparison with transient elastography and histology. J. Ultrasound Med. 2010, 29, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Menzilcioglu, M.S.; Duymus, M.; Citil, S.; Avcu, S.; Gungor, G.; Sahin, T.; Boysan, S.N.; Altunoren, O.; Sarica, A. Strain wave elastography for evaluation of renal parenchyma in chronic kidney disease. Br. J. Radiol. 2015, 88, 20140714. [Google Scholar] [CrossRef]
- Domoslawska, A.; Zdunczyk, S.; Jurczak, A.; Janowski, T. Elastography as a diagnostic tool in the prostate tumour detection in Labrador retriever. Andrologia 2018, 50, e13139. [Google Scholar] [CrossRef]
- Cintra, C.A.; Feliciano, M.A.R.; Santos, V.J.C.; Maronezi, M.C.; Cruz, I.K.; Gasser, B.; Silva, P.; Crivellenti, L.Z.; Uscategui, R.A.R. Applicability of ARFI elastography on the evaluation of canine prostatic alterations detected by B-mode and Doppler ultrasonography. Arq. Bras. Med. Veterinária Zootec. 2020, 72, 2135–2140. [Google Scholar] [CrossRef]
- Feliciano, M.A.R.; Maronezi, M.C.; Simões, A.P.R.; Uscategui, R.R.; Maciel, G.S.; Carvalho, C.F.; Canola, J.C.; Vicente, W.R.R. Acoustic radiation force impulse elastography of prostate and testes of healthy dogs: Preliminary results. J. Small Anim. Pract. 2015, 56, 320–324. [Google Scholar] [CrossRef]
- Assawarachan, S.N.; Chuchalermporn, P.; Maneesaay, P.; Thengchaisri, N. Evaluation of hepatobiliary ultrasound scores in healthy dogs and dogs with liver diseases. Vet. World 2019, 12, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Ohta, H.; Shimbo, G.; Osuga, T.; Sasaki, N.; Morishita, K.; Kagawa, Y.; Takiguchi, M. Usefulness of noninvasive shear wave elastography for the assessment of hepatic fibrosis in dogs with hepatic disease. J. Vet. Intern. Med. 2019, 33, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Favril, S.; Stock, E.; Broeckx, B.J.G.; Devriendt, N.; de Rooster, H.; Vanderperren, K. Shear wave elastography of lymph nodes in dogs with head and neck cancer: A pilot study. Vet. Comp. Oncol. 2022, 20, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Uscategui, R.A.R.; Maronezi, M.C.; Gasser, B.; Pavan, L.; Gatto, I.R.H.; Almeida, V.T.; Vicente, W.R.R.; Feliciano, M.A.R. Ultrasonography for lymph nodes metastasis identification in bitches with mammary neoplasms. Sci. Rep. 2018, 8, 17708. [Google Scholar] [CrossRef] [PubMed]
- Azizi, G.; Keller, J.M.; Mayo, M.L.; Piper, K.; Puett, D.; Earp, K.M.; Malchoff, C.D. Shear Wave Elastography and Cervical Lymph Nodes: Predicting Malignancy. Ultrasound Med. Biol. 2016, 42, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.B.; Li, J.; Xiao, W.W.; Zhao, C.; Lu, T.X.; Han, F. The value of shear wave elastography in predicting undiagnosed small cervical lymph node metastasis in nasopharyngeal carcinoma: A preliminary study. Eur. J. Radiol. 2018, 103, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Seiler, G.S.; Griffith, E. Comparisons between elastographic stiffness scores for benign versus malignant lymph nodes in dogs and cats. Vet. Radiol. Ultrasound 2018, 59, 79–88. [Google Scholar] [CrossRef]
- Choi, M.; Yoon, J.; Choi, M. Semi-quantitative strain elastography may facilitate pre-surgical prediction of mandibular lymph nodes malignancy in dogs. J. Vet. Sci. 2019, 20, e62. [Google Scholar] [CrossRef]
- Choi, M.; Yoon, J.; Choi, M. Contrast-enhanced ultrasound sonography combined with strain elastography to evaluate mandibular lymph nodes in clinically healthy dogs and those with head and neck tumors. Vet. J. 2020, 257, 105447. [Google Scholar] [CrossRef]
- Belotta, A.F.; Gomes, M.C.; Rocha, N.S.; Melchert, A.; Giuffrida, R.; Silva, J.P.; Mamprim, M.J. Sonography and sonoelastography in the detection of malignancy in superficial lymph nodes of dogs. J. Vet. Intern. Med. 2019, 33, 1403–1413. [Google Scholar] [CrossRef]
- Febo, E.; Del Signore, F.; Bernabo, N.; Paolini, A.; Simeoni, F.; De Bonis, A.; Rosto, M.; Canal, S.; Vignoli, M. Ultrasonography and Sonoelastography Characteristics of Benign vs. Malignant Mesenteric Lymph Nodes in Cats: An Update. Animals 2023, 13, 2664. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, M.A.R.; Uscategui, R.A.R.; Maronezi, M.C.; Simões, A.P.R.; Silva, P.; Gasser, B.; Pavan, L.; Carvalho, C.F.; Canola, J.C.; Vicente, W.R.R. Ultrasonography methods for predicting malignancy in canine mammary tumors. PLoS ONE 2017, 12, e0178143. [Google Scholar] [CrossRef]
- Glinska-Suchocka, K.; Jankowski, M.; Kubiak, K.; Spuzak, J.; Dzimira, S.; Nicpon, J. Application of shear wave elastography in the diagnosis of mammary gland neoplasm in dogs. Pol. J. Vet. Sci. 2013, 16, 477–482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feliciano, M.A.R.; Maronezi, M.C.; Brito, M.B.S.; Simões, A.P.R.; Maciel, G.S.; Castanheira, T.L.L.; Garrudo, E.; Uscategui, R.R.; Miceli, N.G.; Vicente, W.R.R. Doppler and Elastography as complementary diagnostic methods for mammary neoplasms in female cats. Arq. Bras. Med. Vet. Zootec. 2015, 67, 935–939. [Google Scholar] [CrossRef]
- Collivignarelli, F.; Tamburro, R.; Aste, G.; Falerrno, I.; Del Signore, F.; Simeoni, F.; Patsikas, M.; Gianfelici, J.; Terragni, R.; Attorri, V.; et al. Lymphatic drainage mapping with indirect lymphography for canine mammary tumors. Animals 2021, 11, 1115. [Google Scholar] [CrossRef] [PubMed]
- Holen, I.; Speirs, V.; Morrissey, B.; Blyth, K. In vivo models in breast cancer research: Progress, challenges and future directions. Dis. Models Mech. 2017, 10, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Liptak, J.M.; Boston, S.E. Nonselective lymph node dissection and sentinel lymph node mapping and biopsy. Vet. Clin. Anim. 2019, 49, 793–807. [Google Scholar] [CrossRef]
- Grimes, J.A.; Secrest, S.A.; Wallace, M.L.; Laver, T.; Schmiedt, C.W. Use of indirect computed tomography lymphangiography to determine metastatic status of sentinel lymph nodes in dogs with a pre-operative diagnosis of melanoma or mast cell tumour. Vet. Comp. Oncol. 2020, 18, 818–824. [Google Scholar] [CrossRef]
- Alvarez-Sanchez, A.; Townsend, K.L.; Newsom, L.; Milovancev, M.; Gorman, E.; Russeell, D.S. Comparison of indirect computed tomographic lymphography and near-infrared fluorescence sentinel lymph node mapping for integumentary canine mast cell tumors. Vet. Surg. 2023, 52, 416–427. [Google Scholar] [CrossRef]
- Lapsley, J.; Hayes, G.M.; Janvier, V.; Newman, A.W.; Peters-Kenneddy, J.; Balkman, C.; Sumner, J.P.; Johnson, P. Influence of locoregional lymph node aspiration cytology vs sentinel lymph node mapping and biopsy on disease stage assignment in dogs with integumentary mast cell tumors. Vet. Surg. 2021, 50, 133–141. [Google Scholar] [CrossRef]
- Haas, S.; Linden, D.; Cole, R.; Smith, A.; Schleis, S.; Matz, B. Indirect lymphography for sentinel lymph node detection in dogs with mast cell tumors. Can. Vet. J. 2023, 64, 142–148. [Google Scholar] [PubMed]
- De Bonis, A.; Collivignarelli, F.; Paolini, A.; Falerno, I.; Rinaldi, V.; Tamburro, R.; Bianchi, A.; Terragni, R.; Gianfelici, J.; Frescura, P.; et al. Sentinel lymph node mapping with indirect lymphangiography for canine mast cell tumour. Vet. Sci. 2022, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Matthews, B.J.; Beavis, A.L. The Role of sentinel lymph node mapping in high-grade endometrial cancer. Curr. Treat. Options Oncol. 2022, 23, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.M.; Haynes, A.M.; Klocke, E.; Higginbotham, M.L.; Thomson, E.M.; Weng, H.Y.; Millard, H.A.T. Occurrence and clinicopathologic features of splenic neoplasia based on body weight: 325 dogs (2003–2013). J. Am. Anim. Hosp. Assoc. 2016, 52, 220–226. [Google Scholar] [CrossRef]
- Maronezi, M.C.; Carneiro, R.K.; da Cruz, I.C.K.; de Oliveira, A.P.L.; De Nardi, A.B.; Pavan, L.; Del’Aguila-Silva, P.; Uscategui, R.A.R.; Feliciano, M.A.R. Accuracy of B-mode ultrasound and ARFI elastography in predicting malignancy of canine splenic lesions. Sci. Rep. 2022, 12, 4252. [Google Scholar] [CrossRef]
- Barella, G.; Lodi, M.; Faverzani, S. Role of strain elastography in differentiating malignant hypoechoic splenic lesions in dogs: Preliminary results. Bulg. J. Vet. Med. 2017, 20, 255–263. [Google Scholar] [CrossRef]
- Cruz, I.C.K.; Carneiro, R.K.; de Nardi, A.B.; Uscategui, R.A.R.; Bortoluzzi, E.M.; Feliciano, M.A.R. Malignancy prediction of cutaneous and subcutaneous neoplasms in canines using B-mode ultrasonography, Doppler, and ARFI elastography. BMC Vet. Res. 2022, 18, 10. [Google Scholar] [CrossRef]
- Brizzi, G.; Crepaldi, P.; Roccabianca, P.; Morabito, S.; Zini, E.; Auriemma, E.; Zanna, G. Strain elastography for the assessment of skin nodules in dogs. Vet. Dermatol. 2021, 32, 272-e75. [Google Scholar] [CrossRef]
- Longo, M.; Bavcar, S.; Handel, I.; Smith, S.; Liuti, T. Real-time elastosonography of lipomatous vs. malignant subcutaneous neoplasms in dogs: Preliminary results. Vet. Radiol. Ultrasound 2018, 59, 198–202. [Google Scholar] [CrossRef]
- Huaijantug, S.; Yatmark, P.; Phophug, P.; Worapakdee, M.; Phutrakul, A.; Julapanthong, P.; Chuaychoo, K. Quantitative ultrasound elastography and serum ferritin level in dogs with liver tumors. J. Adv. Vet. Anim. Res. 2020, 7, 575–584. [Google Scholar] [CrossRef]
- Brito, M.B.S.; Feliciano, M.A.R.; Coutinho, L.N.; Simões, A.P.R.; Maronezi, M.C.; Garcia, P.H.S.; Uscategui, R.R.; de Almeida, V.T.; Crivelaro, R.M.; Vicente, W.R.R. ARFI Elastography of healthy adult felines testes. Acta Sci. Vet. 2015, 43, 1303–1307. [Google Scholar]
- Feliciano, M.A.R.; Maronezi, M.C.; Simões, A.P.R.; Maciel, G.S.; Pavan, L.; Gasser, B.; Silva, P.; Uscategui, R.R.; Carvalho, C.F.; Canola, J.C.; et al. Acoustic radiation force impulse (ARFI) elastography of testicular disorders in dogs: Preliminary results. Arq. Bras. Med. Veterinária Zootec. 2016, 68, 283–291. [Google Scholar] [CrossRef]
- Patton, H.M.; Johnson, B.F.; Smorodinsky, E.; Sirlin, C.B. Imaging and Noninvasive Diagnosis of Liver Disease: Computerized Tomography, Ultrasound, Magnetic Resonance Imaging, and Emerging Techniques. In Zakim and Boyer’s Hepatology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 216–254. [Google Scholar] [CrossRef]
- Volta, A.; Manfredi, S.; Vignoli, M.; Russo, M.; England, G.C.; Rossi, F.; Bigliardi, E.; Di Ianni, F.; Parmigiani, E.; Bresciani, C.; et al. Use of contrast-enhanced ultrasonography in chronic pathologic canine testes. Reprod. Domest. Anim. 2014, 49, 202–209. [Google Scholar] [CrossRef]
- Quartuccio, M.; Mangano, C.; Macri, F.; Rizzo, M.; Di Pietro, S.; Pugliese, M.; Mazzullo, G.; Cristarella, S.; De Majo, M. Contrast-enhanced ultrasound evaluation of testicular interstitial cell tumors in conscious non-sedated dogs. Vet. Med. 2018, 63, 125–130. [Google Scholar] [CrossRef]
- Hillaert, A.; Stock, E.; Duchateau, L.; de Rooster, H.; Devriendt, N.; Vanderperren, K. B-Mode and Contrast-Enhanced Ultrasonography Aspects of Benign and Malignant Superficial Neoplasms in Dogs: A Preliminary Study. Animals 2022, 12, 2765. [Google Scholar] [CrossRef]
- Orlandi, R.; Vallesi, E.; Boiti, C.; Polisca, A.; Bargellini, P.; Troisi, A. Characterization of Testicular Tumor Lesions in Dogs by Different Ultrasound Techniques. Animals 2022, 12, 210. [Google Scholar] [CrossRef]
- Sinagra, L.; Orlandi, R.; Caspanello, T.; Troisi, A.; Iannelli, N.M.; Vallesi, E.; Pettina, G.; Bargellini, P.; de Majo, M.; Boiti, C.; et al. Contrast-Enhanced Ultrasonography (CEUS) in Imaging of the Reproductive System in Dogs: A Literature Review. Animals 2023, 13, 1615. [Google Scholar] [CrossRef]
- Vanderperren, K.; Saunders, J.H.; Van der Vekens, E.; Wydooghe, E.; de Rooster, H.; Duchateau, L.; Stock, E. B-mode and contrast-enhanced ultrasonography of the mammary gland during the estrous cycle of dogs. Anim. Reprod. Sci. 2018, 199, 15–23. [Google Scholar] [CrossRef]
- Choi, S.Y.; Jeong, W.C.; Lee, Y.W.; Choi, H.J. Contrast enhanced ultrasonography of kidney in conscious and anesthetized beagle dogs. J. Vet. Med. Sci. 2016, 78, 239–244. [Google Scholar] [CrossRef]
- Russo, M.; Vignoli, M.; England, G.C.W. B-mode and contrast enhanced ultrasonographic findings in canine prostatic disorders. Reprod. Domest. Anim. 2012, 47, 238–242. [Google Scholar] [CrossRef]
- Vignoli, M.; Russo, M.; Catone, G.; Rossi, F.; Attanasi, G.; Terragni, R.; Saunders, J.; England, G. Assessment of vascular perfusion kinetics using contrast-enhanced ultrasound for the diagnosis of prostate disease in dogs. Reprod. Domest. Anim. 2011, 46, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, M.A.R.; Ramirez, R.A.U.; Maronezi, M.C.; Maciel, G.S.; Avante, M.L.; Senhorello, I.L.S.; Mucédola, T.; GAsser, B.; Carvalho, C.F.; Vicente, W.R.R. Accuracy of four ultrasonography techniques in predicting histopathological classification of canine mammary carcinomas. Vet. Radiol. Ultrasound 2018, 59, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Tan, G.J.S.; Wansaicheong, G.K.L. Contrast enhanced ultrasound of kidneys. Pictorial essay. Med. Ultrason. 2011, 13, 150–156. [Google Scholar] [PubMed]
- Haers, H.; Vignoli, M.; Paes, G.; Rossi, F.; Taeymans, O.; Daminet, S.; Saunders, J.H. Contrast harmonic ultrasonographic appearance of focal space-occupying renal lesions. Vet. Radiol. Ultrasound 2010, 51, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Spediacci, C.; Manfredi, M.; Sala, G.; Liuti, T.; Israeliantz, N.; Zani, D.D.; Di Giancamillo, M.; Longo, M. Fall time may be a reliable discriminator between neoplastic and non-neoplastic urinary bladder lesions in dogs undergoing contrast-enhanced ultrasound: A pilot study. Vet. Radiol. Ultrasound 2022, 63, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Macrì, F.; Di Pietro, S.; Mangano, C.; Pugliese, M.; Mazzullo, G.; Iannelli, N.M.; Angileri, V.; Morabito, S.; De Majo, M. Quantitative evaluation of canine urinary bladder transitional cell carcinoma using contrast-enhanced ultrasonography. BMC Vet. Res. 2018, 14, 84. [Google Scholar] [CrossRef]
- Salwei, R.M.; O’Brien, R.T.; Matheson, J.S. Characterization of lymphomatous lymph nodes in dogs using contrast harmonic and Power Doppler ultrasound. Vet. Radiol. Ultrasound 2005, 46, 411–416. [Google Scholar] [CrossRef]
- Rossi, F.; Leone, V.F.; Vignoli, M.; Laddaga, E.; Terragni, R. Use of contrast-enhanced ultrasound for characterization of focal splenic lesions. Vet. Radiol. Ultrasound 2008, 49, 154–164. [Google Scholar] [CrossRef]
- Taeymans, O.; Penninck, D. Contrast enhanced sonographic assessment of feeding vessels as a discriminator between malignant vs. benign focal splenic lesions. Vet. Radiol. Ultrasound 2011, 52, 457–461. [Google Scholar] [CrossRef]
- Simeoni, F.; Del Signore, F.; Aste, G.; Bargellini, P.; Rubini, G.; Terragni, R.; Tamburro, R.; Falerno, I.; de Pasquale, F.; Russo, M.; et al. B-Mode and Contrast Enhanced Ultrasonography Features of Gastric Inflammatory and Neoplastic Diseases in Dogs. Animals 2021, 11, 670. [Google Scholar] [CrossRef]
- Seiler, G.; Maï, W. The Stomach. In Bsava Manual of Canine and Feline Abdominal Imaging; O’Brien, R., Barr, F., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2009; pp. 87–109. [Google Scholar]
- Simeoni, F.; Del Signore, F.; Terragni, R.; Tamburro, R.; Aste, G.; Vignoli, M. Diagnostic imaging of Gastrointestinal Tumors in Dogs and Cats: A Review. Am. J. Anim. Vet. Sci. 2020, 15, 89–101. [Google Scholar] [CrossRef]
- Nisa, K.; Lim, S.Y.; Shinohara, M.; Osuga, T.; Yokoyama, N.; Tamura, M.; Nagata, N.; Sasaoka, K.; Dermlim, A.; Leela-Arporn, R.; et al. Evaluation of duodenal perfusion by contrast-enhanced ultrasonography in dogs with chronic inflammatory enteropathy and intestinal lymphoma. J. Vet. Intern. Med. 2019, 33, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Simeoni, F.; Terragni, R.; Rubini, G.; Tamburro, R.; Del Signore, F.; Falerno, I.; Aste, G.; Russo, M.; Mastromatteo, G.; Vignoli, M. B-Mode and Contrast Enhanced Ultrasonography Features of Gastric Inflammatory and Neoplastic Diseases in Cats. Animals 2020, 10, 1444. [Google Scholar] [CrossRef] [PubMed]
- Banzato, T.; Rubini, G.; Orlandi, R.; Bargellini, P.; Bonsembiante, F.; Zotti, A. Contrast-enhanced ultrasound features of hepatocellular carcinoma in dogs. Vet. Rec. 2020, 186, 187. [Google Scholar] [CrossRef] [PubMed]
- Chammas, M.C.; Bordini, A.L. Contrast-enhanced ultrasonography for the evaluation of malignant focal liver lesions. Ultrasonography 2022, 41, 4–24. [Google Scholar] [CrossRef]
- Burti, S.; Zotti, A.; Rubini, G.; Orlandi, R.; Bargellini, P.; Bonsembiante, F.; Banzato, T. Contrast-enhanced ultrasound features of malignant focal hepatic masses in dogs. Sci. Rep. 2020, 10, 6076. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, V.; Grazhdani, H.; Fioravanti, C.; Rosignuolo, M.; Calliada, F.; Messineo, D.; Bernieri, M.G.; Redler, A.; Catalano, C.; D’Ambrosio, F. Liver metastases: Contrast-enhanced ultrasound compared with computed tomography and magnetic resonance. World J. Gastroenterol. 2014, 20, 9998–10007. [Google Scholar] [CrossRef]
- Banzato, T.; Burti, S.; Rubini, G.; Orlandi, R.; Bargellini, P.; Bonsembiante, F.; Zotti, A. Contrast-enhanced ultrasonography features of hepatobiliary neoplasms in cats. Vet. Rec. 2020, 186, 320. [Google Scholar] [CrossRef]
- Webster, N.; Holloway, A. Use of contrast ultrasonography in the diagnosis of metastatic feline visceral hemangiosarcoma. J. Feline Med. Surg. 2008, 10, 388–394. [Google Scholar] [CrossRef]
- O’Brien, R.T.; Iani, M.; Matheson, J.; Delaney, F.; Young, K. Contrast harmonic ultrasound of spontaneous liver nodules in 32 dogs. Vet. Radiol. Ultrasound 2004, 45, 547–553. [Google Scholar] [CrossRef]
- Nakamura, K.; Takagi, S.; Sasaki, N.; Kumara, W.R.B.; Murakami, M.; Ohta, H.; Yamasaki, M.; Takiguchi, M. Contrast-enhanced ultrasonography for characterization of canine focal liver lesions. Vet. Radiol. Ultrasound 2010, 51, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Pey, P.; Rossi, F.; Vignoli, M.; Duchateau, L.; Marescaux, L.; Saunders, J.H. Use of contrast-enhanced ultrasonography to characterize adrenal gland tumors in dogs. Am. J. Vet. Res. 2014, 75, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Nagumo, T.; Ishigaki, K.; Yoshida, O.; Iizuka, K.; Tamura, K.; Sakurai, N.; Terai, K.; Seki, M.; Edamura, K.; Asano, K. Utility of contrast-enhanced ultrasound in differential diagnosis of adrenal tumors in dogs. J. Vet. Med. Sci. 2020, 82, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Bargellini, P.; Orlandi, R.; Dentini, A.; Paloni, C.; Rubioni, G.; Fonti, P.; Diana, A.; Peterson, M.E.; Boiti, C. Use of contrast-enhanced ultrasound in the diagnosis of adrenal tumors in dogs. J. Am. Anim. Hosp. Assoc. 2016, 52, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Burti, S.; Zotti, A.; Rubini, G.; Orlandi, R.; Bargellini, P.; Bonsembiante, F.; Contiero, B.; Bendazzoli, M.; Banzato, T. Contrast-enhanced ultrasound features of adrenal lesions in dogs. Vet. Rec. 2023, 193, e2949. [Google Scholar] [CrossRef]
- Burti, S.; Zotti, A.; Rubini, G.; Orlandi, R.; Bargellini, P.; Bonsembiante, F.; Contiero, B.; Banzato, T. Contrast-enhanced ultrasound features of focal pancreatic lesions in dogs. Vet. Rec. 2022, 191, e2080. [Google Scholar] [CrossRef]
- Vanderperren, K.; Haers, H.; Van der Vekens, E.; Stock, E.; Paepe, D.; Daminet, S.; Saunders, J.H. Description of the use of contrast-enhanced ultrasonography in four dogs with pancreatic tumors. J. Small Anim. Pract. 2014, 55, 164–169. [Google Scholar] [CrossRef]
- Nakamura, K.; Lim, S.Y.; Ochiai, K.; Yamasaki, M.; Ohta, H.; Morishita, K.; Takagi, S.; Takiguchi, M. Contrast-enhanced ultrasonographic findings in three dogs with pancreatic insulinoma. Vet. Radiol. Ultrasound 2015, 56, 55–62. [Google Scholar] [CrossRef]
- Cervone, M.; Harel, M.; Segard-Weisse, E.; Krafft, E. Use of contrast-enhanced ultrasonography for the detection of a feline insulinoma. J. Feline Med. Surg. Open Rep. 2019, 5, 2055116919876140. [Google Scholar] [CrossRef]
- Burti, S.; Zotti, A.; Rubini, G.; Orlandi, R.; Bargellini, P.; Bonsembiante, F.; Contiero, B.; Marcuzzi, M.; Banzato, T. Contrast-enhanced ultrasound features of focal pancreatic lesions in cats. Front. Vet. Sci. 2022, 28, 986948. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ercolin, A.C.M.; Uchôa, A.S.; Aires, L.P.N.; Gomes, D.R.; Tinto, S.T.; Feliciano, G.S.M.; Feliciano, M.A.R. Use of New Ultrasonography Methods for Detecting Neoplasms in Dogs and Cats: A Review. Animals 2024, 14, 312. https://doi.org/10.3390/ani14020312
Ercolin ACM, Uchôa AS, Aires LPN, Gomes DR, Tinto ST, Feliciano GSM, Feliciano MAR. Use of New Ultrasonography Methods for Detecting Neoplasms in Dogs and Cats: A Review. Animals. 2024; 14(2):312. https://doi.org/10.3390/ani14020312
Chicago/Turabian StyleErcolin, Anna Carolina Mazeto, Alex Silveira Uchôa, Luiz Paulo Nogueira Aires, Diego Rodrigues Gomes, Stefany Tagliatela Tinto, Giovanna Serpa Maciel Feliciano, and Marcus Antônio Rossi Feliciano. 2024. "Use of New Ultrasonography Methods for Detecting Neoplasms in Dogs and Cats: A Review" Animals 14, no. 2: 312. https://doi.org/10.3390/ani14020312
APA StyleErcolin, A. C. M., Uchôa, A. S., Aires, L. P. N., Gomes, D. R., Tinto, S. T., Feliciano, G. S. M., & Feliciano, M. A. R. (2024). Use of New Ultrasonography Methods for Detecting Neoplasms in Dogs and Cats: A Review. Animals, 14(2), 312. https://doi.org/10.3390/ani14020312






