Anatomical and Functional Study of the Ostrich (Struthio camelus) Lung through Macroscopic Analysis in Combination with Optical and Electron Microscopy Techniques
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Tissue Fixation
2.3. Macroscopic Observations
2.4. Light and Transmission Electron Microscopy
2.5. Silicon Rubber Casting
2.6. Scanning Electron Microscopy
3. Results
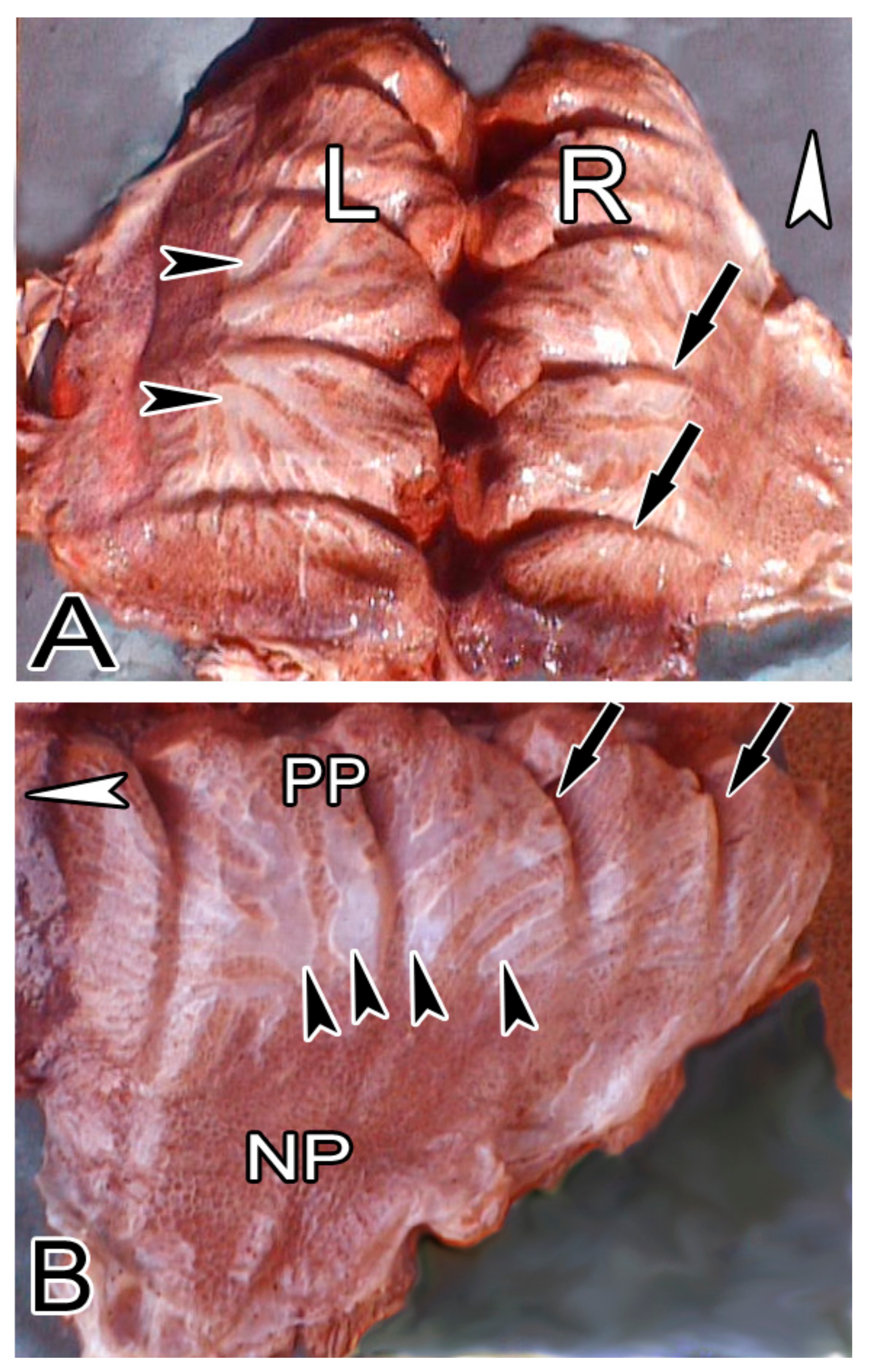
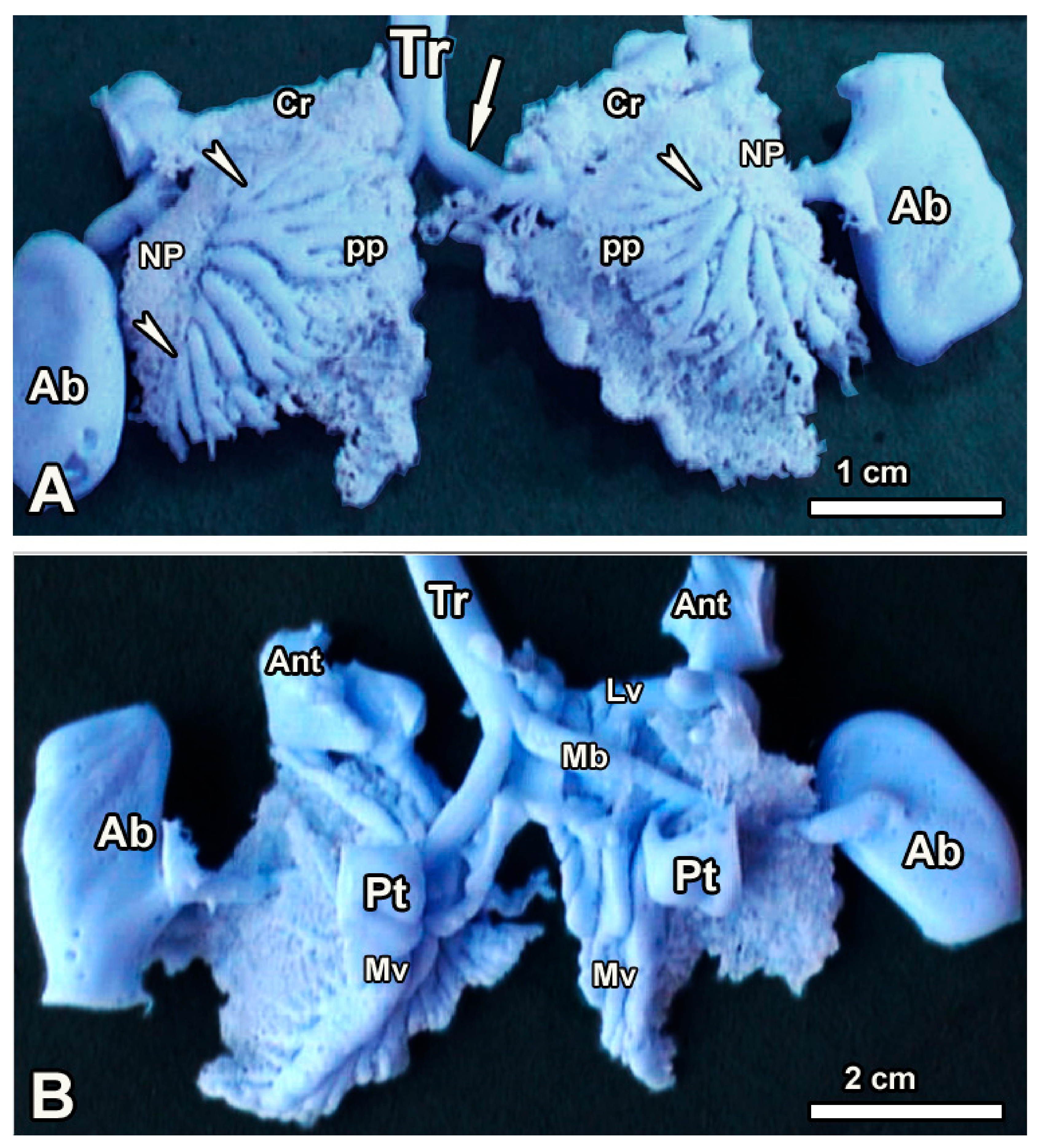
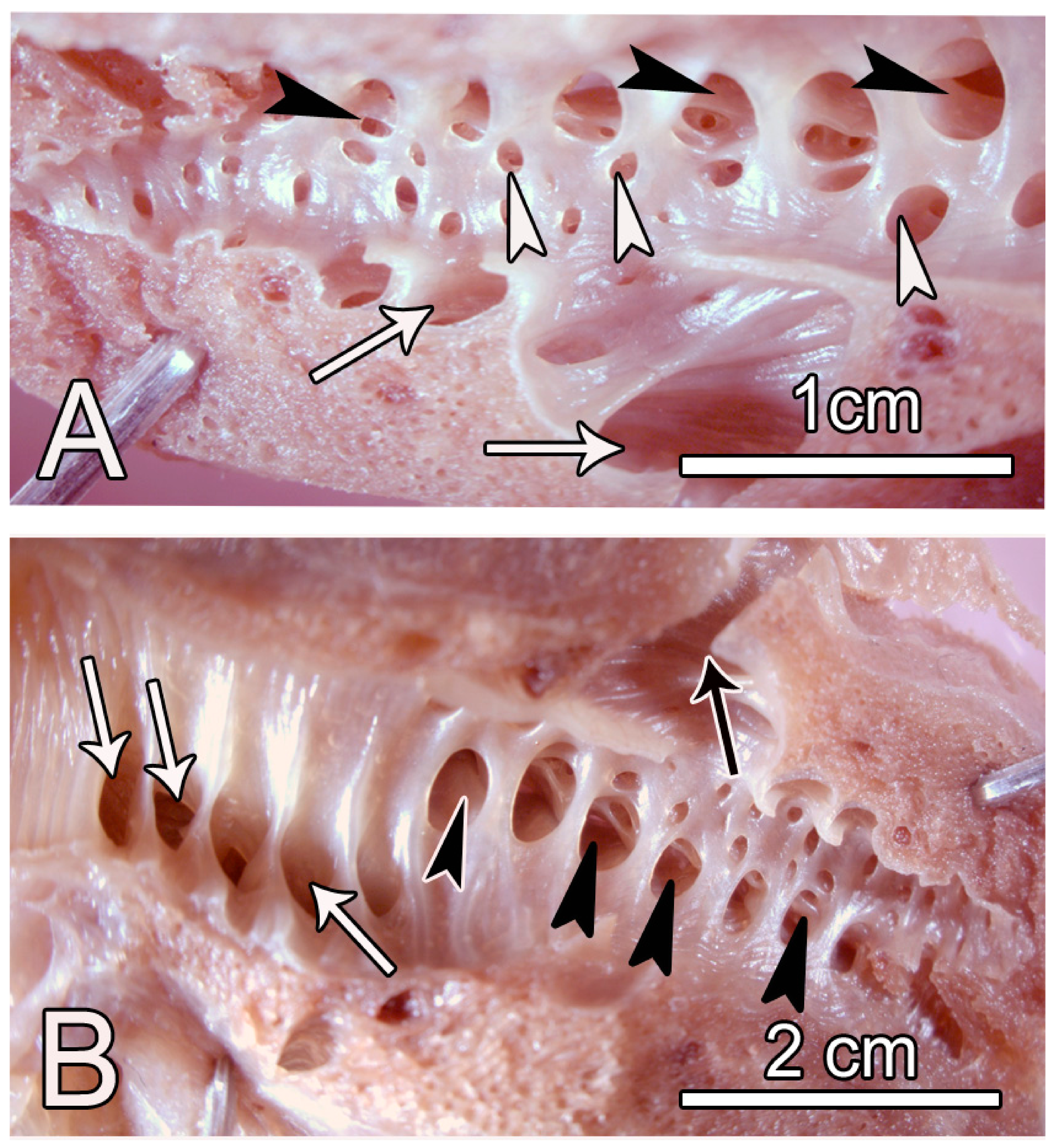
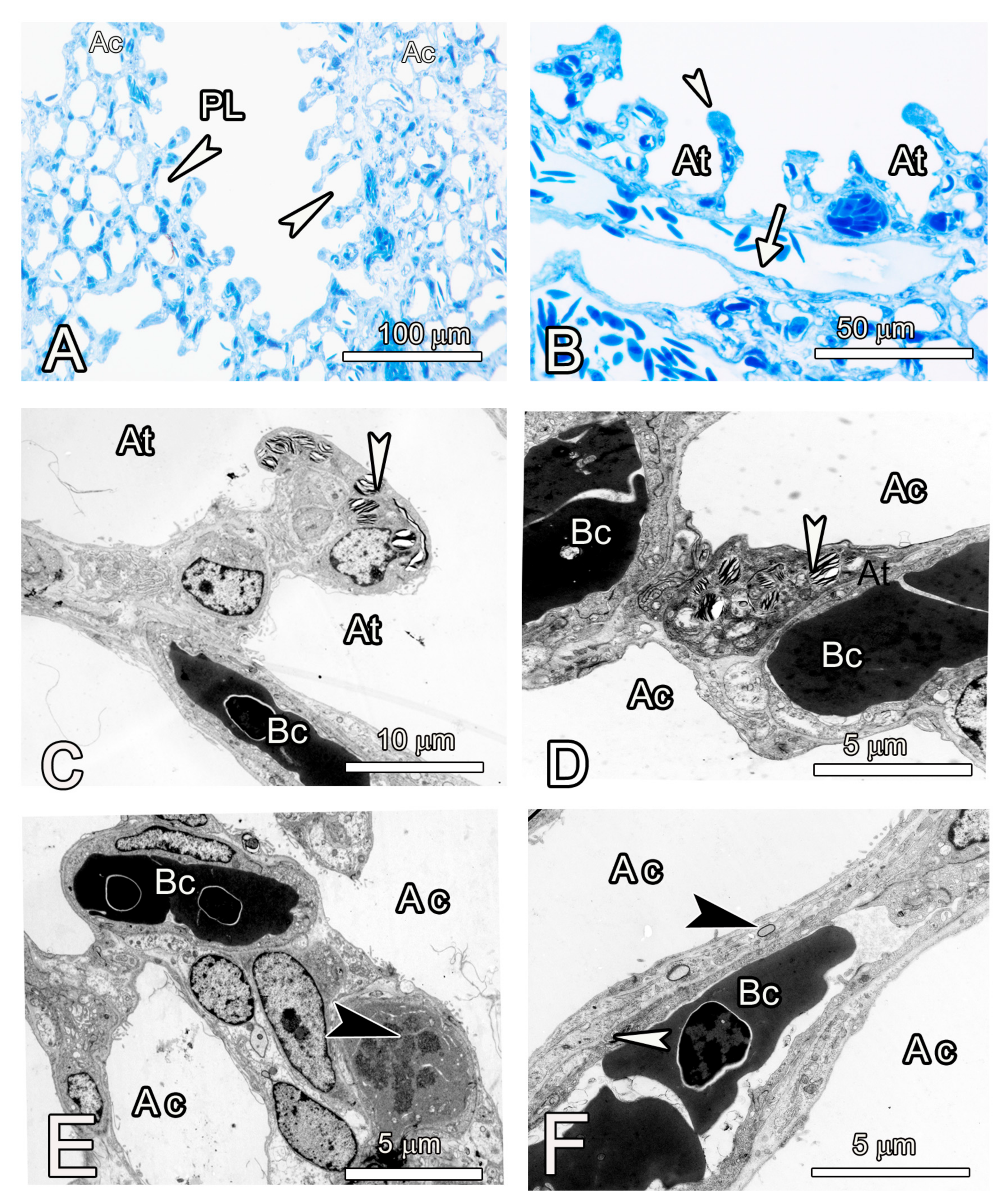
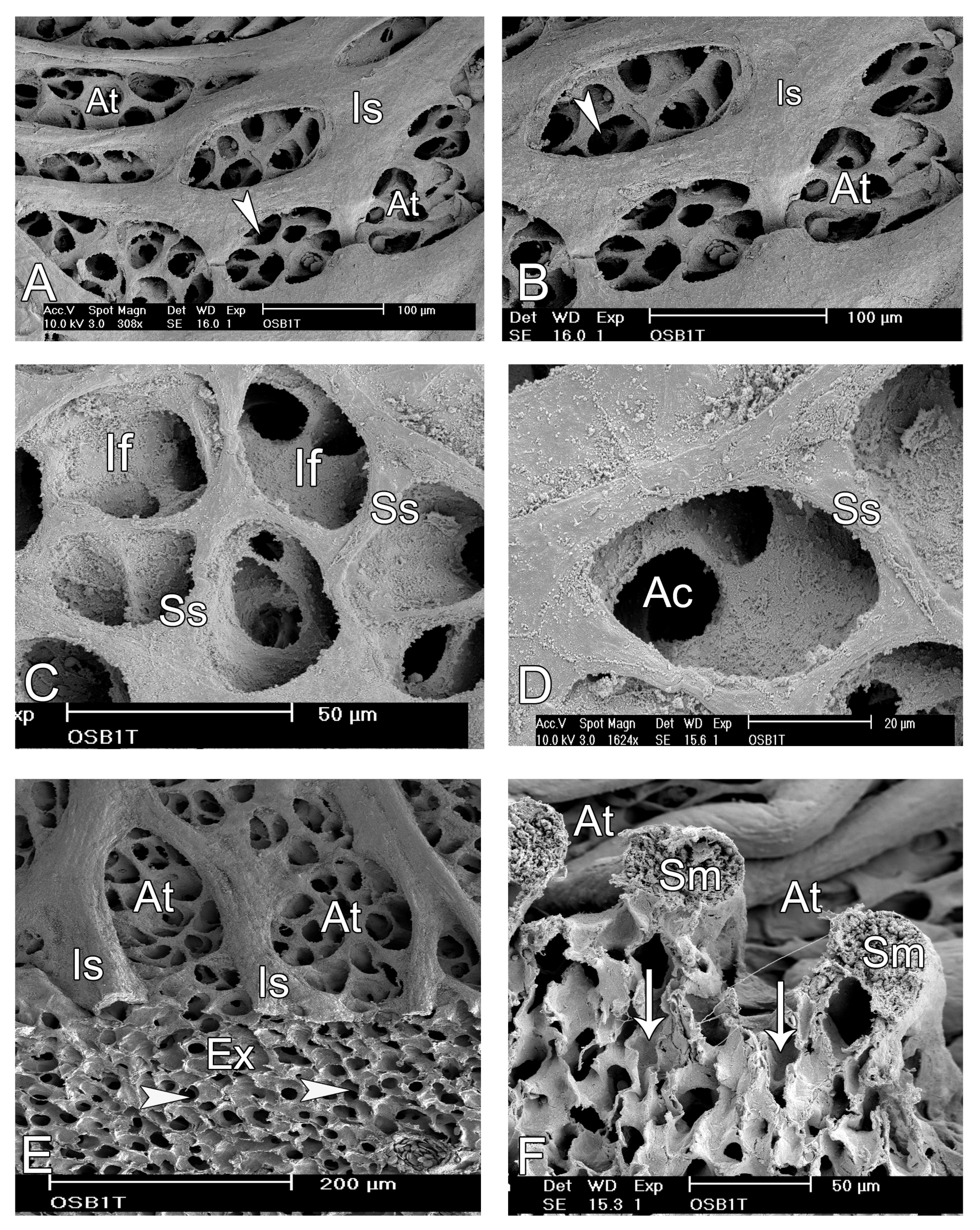
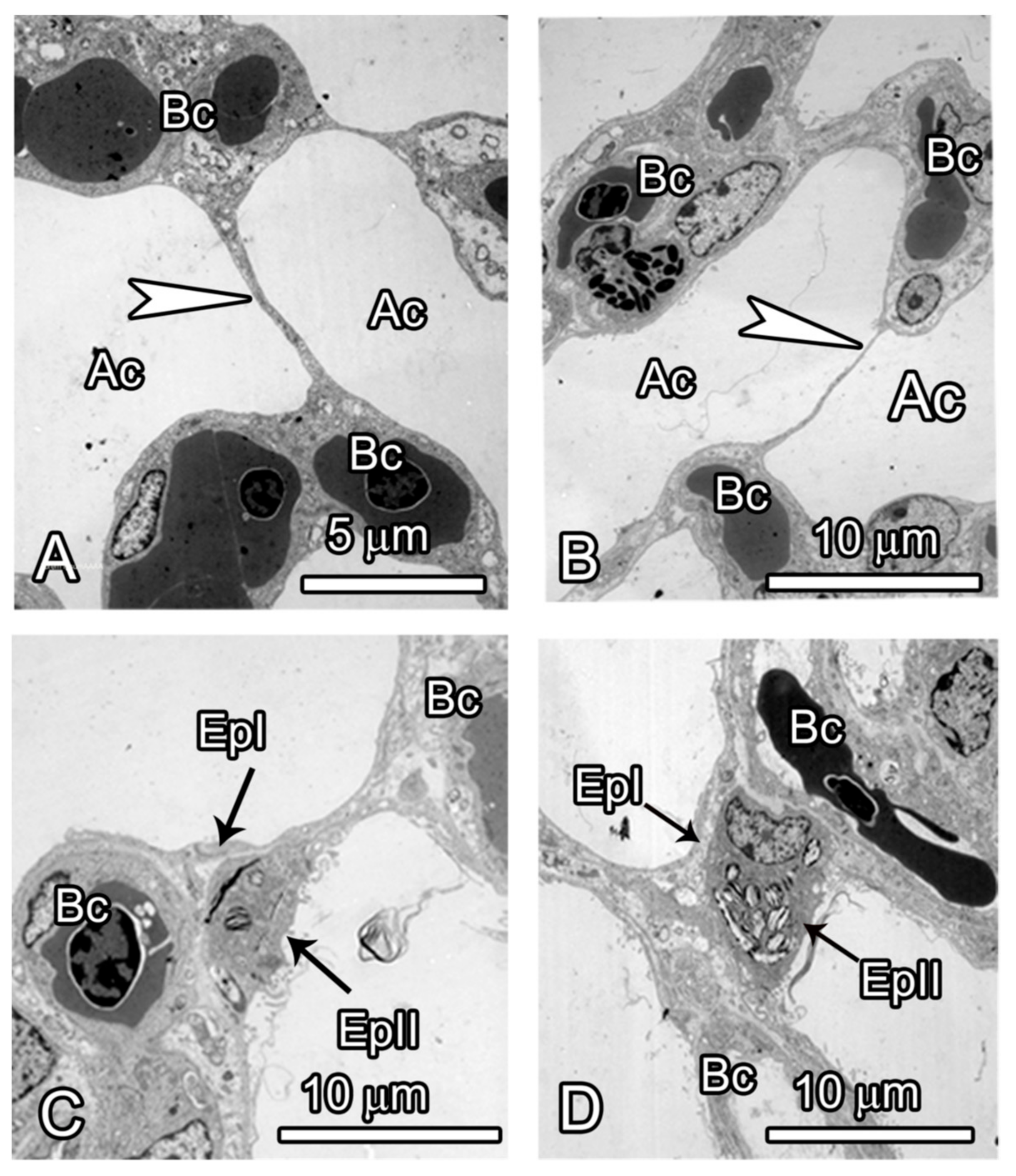
3.1. Macroscopic Observations
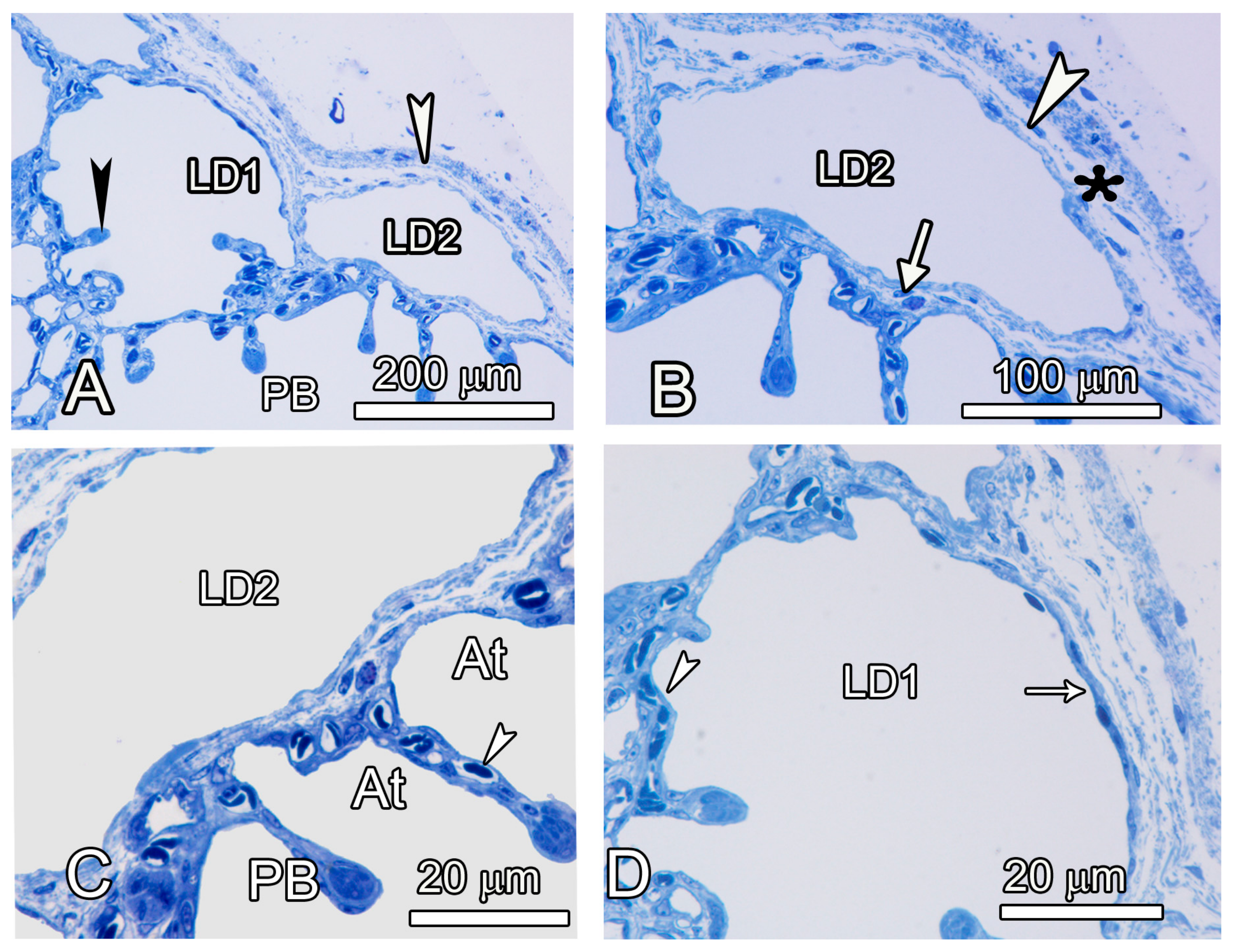
3.2. Microscopic Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, R.G.; Horbanczuk, J.O. Anatomical and physiological characteristics of ostrich (Struthio camelus var. domesticus) meat determine its nutritional importance for man. Anim. Sci. J. 2002, 73, 167–173. [Google Scholar]
- Storer, R. Adaptive radiation in birds. In Avian Biology; Farner, D., King, J., Eds.; Academic Press: New York, NY, USA, 1971; pp. 147–188. [Google Scholar]
- Freesmeyer, M.; Kuehnel, C.; Opfermann, T.; Niksch, T.; Wiegand, S.; Stolz, R.; Huonker, R.; Witte, O.W.; Winkens, T. The use of ostrich eggs for in ovo research: Making preclinical imaging research affordable and available. J. Nucl. Med. 2018, 59, 1901–1906. [Google Scholar] [CrossRef]
- Makanya, A.N.; Jimoh, S.A.; Maina, J.N. Methods of in ovo and ex ovo ostrich embryo culture with observations on the development and maturation of the chorioallantoic membrane. Microsc. Microanal. 2023, 29, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Pomraenke, M.; Bolney, R.; Winkens, T.; Perkas, O.; Pretzel, D.; Theis, B.; Greiser, J.; Freesmeyer, M. A Novel Breast Cancer Xenograft Model Using the Ostrich Chorioallantoic Membrane—A Proof of Concept. Vet. Sci. 2023, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Maina, J.N.; Nathaniel, C. A qualitative and quantitative study of the lung of an ostrich, Struthio camelus. J. Exp. Biol. 2001, 204 Pt 13, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Bezuidenhout, A.J.; Groenewald, H.B.; Soley, J.T. An anatomical study of the respiratory air sacs in ostriches. Onderstepoort J. Vet. Res. 1999, 66, 317–325. [Google Scholar] [PubMed]
- Maina, J.N. Morphometries of the avian lung: The structural-functional correlations in the design of the lungs of birds. Comp. Biochem. Physiol. 1993, 105, 397–410. [Google Scholar] [CrossRef]
- Makanya, A.N. Development of the Airways and the Vasculature in the Lungs of Birds. In The Biology of the Avian Respiratory System; Springer International Publishing: Cham, Switzerland, 2017; pp. 147–178. [Google Scholar]
- Makanya, A.; Djonov, V. Development and spatial organization of the air conduits in the lung of the Domestic Fowl, Gallus gallus variant domesticus. Microsc. Res. Tech. 2008, 71, 689–702. [Google Scholar] [CrossRef]
- Makanya, A.; Kavoi, B.; Djonov, V. Three-Dimensional Structure and Disposition of the Air Conducting and Gas Exchange Conduits of the Avian Lung: The Domestic Duck (Cairina moschata). ISRN Anat. 2014, 2014, 621982. [Google Scholar] [CrossRef]
- Maina, J.N.; Woodward, J.D. Three-dimensional serial section computer reconstruction of the arrangement of the structural components of the parabronchus of the Ostrich, Struthio camelus lung. Anat. Rec. 2009, 292, 1685–1698. [Google Scholar] [CrossRef]
- Maina, J.N. Pivotal debates and controversies on the structure and function of the avian respiratory system: Setting the record straight. Biol. Rev. 2017, 92, 1475–1504. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.D.; Maina, J.N. A 3D digital reconstruction of the components of the gas exchange tissue of the lung of the Muscovy Duck, Cairina moschata. J. Anat. 2005, 206, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Maina, J.N. Perspectives on the Structure and Function of the Avian Respiratory System: Functional Efficiency Built on Structural Complexity. Front. Anim. Sci. 2022, 3, 851574. [Google Scholar] [CrossRef]
- Maina, J.N.; Ramonisi, Y.; Mashiteng, R.; Mokae, L.; Woodward, J.D. 3D Computer Reconstruction of the Airway and the Vascular Systems of the Lung of the Domestic Fowl, Gallus gallus variant domesticus. J. Appl. Math. Comput. 2021, 5, 89–104. [Google Scholar] [CrossRef]
- Lawson, A.B.; Hedrick, B.P.; Echols, S.; Schachner, E.R. Anatomy, variation, and asymmetry of the bronchial tree in the African grey parrot (Psittacus erithacus). J. Morphol. 2021, 282, 701–719. [Google Scholar] [CrossRef] [PubMed]
- Makanya, A.; El-Darawish, Y.; Kavoi, B.; Djonov, V. Spatial and functional relationships between air conduits and blood capillaries in the pulmonary gas exchange tissue of adult and developing chickens. Microsc. Res. Tech. 2011, 74, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Makanya, A.; Kavoi, B.; Kihurani, D. Slight volume changes in the Duck lung do not imply a fundamental change in the structure of the parenchyma. Anat. Histol. Embryol. 2021, 50, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Maina, J.N. Critical appraisal of some factors pertinent to the functional designs of the gas exchangers. Cell Tissue Res. 2017, 367, 747–767. [Google Scholar] [CrossRef]
- Maina, J.N.; Singh, P.; Moss, E.A. Inspiratory aerodynamic valving occurs in the ostrich, Struthio camelus lung: A computational fluid dynamics study under resting unsteady state inhalation. Respir. Physiol. Neurobiol. 2009, 169, 262–270. [Google Scholar] [CrossRef]
- Locy, W.A.; Larsell, O. The embryology of the bird’s lung. Based on observations of the Domestic Fowl. Part 1. Am. J. Anat. 1916, 19, 447–504. [Google Scholar] [CrossRef]
- Makanya, A.; Koller, T.; Hlushchuk, R.; Djonov, V. Pre-hatch lung development in the ostrich. Respir. Physiol. Neurobiol. 2012, 180, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Makanya, A.; Djonov, V. Prenatal and postnatal development of the vertebrate blood-gas barrier. In The Vertebrate Blood-Gas Barrier in Health and Disease: Structure, Development and Remodeling; Makanya, A., Ed.; Springer: New York, NY, USA, 2015; pp. 39–64. [Google Scholar]
- Makanya, A.N.; Hlushchuk, R.; Duncker, H.R.; Draeger, A.; Djonov, V. Epithelial transformations in the establishment of the blood-gas barrier in the developing chick embryo lung. Dev. Dyn. 2006, 235, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Makanya, A.N. Membrane mediated development of the vertebrate blood-gas-barrier. Birth Defects Res. C Embryo Today 2016, 108, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Makanya, A.; Anagnostopoulou, A.; Djonov, V. Development and remodeling of the vertebrate blood-gas barrier. Biomed. Res. Int. 2013, 2013, 101597. [Google Scholar] [CrossRef] [PubMed]
- Schachner, E.R.; Hedrick, B.P.; Richbourg, H.A.; Hutchinson, J.R.; Farmer, C.G. Anatomy, ontogeny, and evolution of the archosaurian respiratory system: A case study on Alligator mississippiensis and Struthio camelus. J. Anat. 2021, 238, 845–873. [Google Scholar] [CrossRef] [PubMed]
- Maina, J.N.; King, A.S. The lung of the Emu, Dromaius novaehollandiae: A microscopic and morphometric study. J. Anat. 1989, 163, 67–73. [Google Scholar]
- Nickel, R.; Schummer, A.; Seiferle, E. Respiratory system. In Anatomy of the Domestic Birds; Nickel, A., Schummer, R., Seiferle, E., Eds.; Verlag Paul-Parey: Hamburg-Berlin, Germany, 1977; pp. 62–69. [Google Scholar]
- Haenssgen, K.; Makanya, A.N.; Djonov, V. Casting Materials and their Application in Research and Teaching. Microsc. Microanal. 2014, 20, 493–513. [Google Scholar] [CrossRef]
- Akester, A. The comparative anatomy of the respiratory pathways in the Domestic Fowl (Gallus domesticus), Pigeon (Columbia livia) and Domestic Duck (Anas platyrhynchos). J. Anat. 1960, 94, 487–505. [Google Scholar]
- Makanya, A.N.; Djonov, V. Parabronchial angioarchitecture in developing and adult chickens. J. Appl. Physiol. 2009, 106, 1959–1969. [Google Scholar] [CrossRef]
- West, J.B. Comparative Physiology of the Pulmonary Circulation. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2011; pp. 1525–1539. [Google Scholar]
- West, J.B. Comparative physiology of the pulmonary blood-gas barrier: The unique avian solution. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R1625–R1634. [Google Scholar] [CrossRef]
- West, J.B.; Fu, Z.; Deerinck, T.J.; Mackey, M.R.; Obayashi, J.T.; Ellisman, M.H. Structure–function studies of blood and air capillaries in chicken lung using 3D electron microscopy. Respir. Physiol. Neurobiol. 2010, 170, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Maina, J.N.; West, J.B.; Orgeig, S.; Foot, N.J.; Daniels, C.B.; Kiama, S.G.; Gehr, P.; Mühlfeld, C.; Blank, F.; Müller, L.; et al. Recent advances into understanding some aspects of the structure and function of mammalian and avian lungs. Physiol. Biochem. Zool. 2010, 83, 792–807. [Google Scholar] [CrossRef] [PubMed]
- West, J.B.; Watson, R.R.; Fu, Z. The honeycomb-like structure of the bird lung allows a uniquely thin blood-gas barrier. Respir. Physiol. Neurobiol. 2006, 152, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Maina, J.N. Spectacularly robust! Tensegrity principle explains the mechanical strength of the avian lung. Respir. Physiol. Neurobiol. 2007, 155, 1–10. [Google Scholar] [CrossRef]


| Technique | Animal No. | Age | Sex | Results Accomplished |
|---|---|---|---|---|
| Macroscopic and Microscopic examination | 1. | Adult | Male | Enumeration of the secondary bronchi; study of the relationship between the secondary bronchi, the intercostal muscles and the ribcage; semithin sections, SEM and TEM micrographs. |
| 2. | Adult | Female | ||
| 3 | Adult | Male | ||
| Macroscopic examination | 4 | Adult | Male | Enumeration of the secondary bronchi. |
| 5 * | Adult | Female | Study of the rib cage skeleton and its relationship to the topography of the lung | |
| Macroscopic and Microscopic examination | 6 | Chick | ND | Enumeration of the secondary bronchi; semithin sections, SEM and TEM micrographs. |
| 7 | Chick | ND | ||
| Silicon rubber casting | 8 | Chick | ND | Study of the 3D arrangement of secondary bronchi |
| 9 | Chick | ND | ||
| 10 | Chick | ND |
| Animal. No. | MVSB | LDSB | LVSB | POSB | ||||
|---|---|---|---|---|---|---|---|---|
| L | R | L | R | L | R | L | R | |
| 1 | 4 | 4 | 8 | 10 | 5 | 5 | 24 | 22 |
| 2 | 4 | 4 | 8 | 10 | 4 | 6 | 18 | 20 |
| 3 | 4 | 4 | 8 | 8 | 4 | 6 | 20 | 22 |
| 4 | 4 | 4 | 8 | 8 | 5 | 5 | 18 | 20 |
| 5 | 4 | 4 | 8 | 10 | 5 | 5 | 20 | 18 |
| 6 | 5 | 5 | 8 | 9 | 5 | 5 | 20 | 6 |
| Mean | 4.2 | 4.2 | 8 | 9.2 | 4.6 | 5.3 | 20 | 20.6 |
| SD | 0.37 | 0.37 | 8 | 0.9 | 0.47 | 0.47 | 2 | 1.5 |
| Range | 4–5 | 4–5 | 8–8 | 8–10 | 4–6 | 5–6 | 20–24 | 18–22 |
| Chicken | Duck | Ostrich | ||||
|---|---|---|---|---|---|---|
| CATEGORY | Range | Mean | Range | Mean | Range | Mean |
| MVSB | 4–4 | 4 | 4–4 | 4.4 | 4–5 | 4.2 |
| LDSB | 6–10 | 9.5 | 6–10 | 8.2 | 8–10 | 8.1 |
| LVSB | 1–3 | 1.4 | 2–4 | 3 | 4–6 | 4.7 |
| POSB | 20–60 | 37 | 38–44 | 40 | 18–24 | 20.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makanya, A.; Djonov, V. Anatomical and Functional Study of the Ostrich (Struthio camelus) Lung through Macroscopic Analysis in Combination with Optical and Electron Microscopy Techniques. Animals 2024, 14, 316. https://doi.org/10.3390/ani14020316
Makanya A, Djonov V. Anatomical and Functional Study of the Ostrich (Struthio camelus) Lung through Macroscopic Analysis in Combination with Optical and Electron Microscopy Techniques. Animals. 2024; 14(2):316. https://doi.org/10.3390/ani14020316
Chicago/Turabian StyleMakanya, Andrew, and Valentin Djonov. 2024. "Anatomical and Functional Study of the Ostrich (Struthio camelus) Lung through Macroscopic Analysis in Combination with Optical and Electron Microscopy Techniques" Animals 14, no. 2: 316. https://doi.org/10.3390/ani14020316





