Effects of Spinach Extract and Licorice Extract on Growth Performance, Antioxidant Capacity, and Gut Microbiota in Weaned Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals, Treatments, Housing, and Ethics Statement
2.3. Growth Performance and Diarrhea Rate
2.4. Sample Preparation and Analysis
2.5. Gut Microbiota Analysis
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Serum Biochemistry
3.3. Antioxidant Properties
3.4. Hemoglobin Concentration
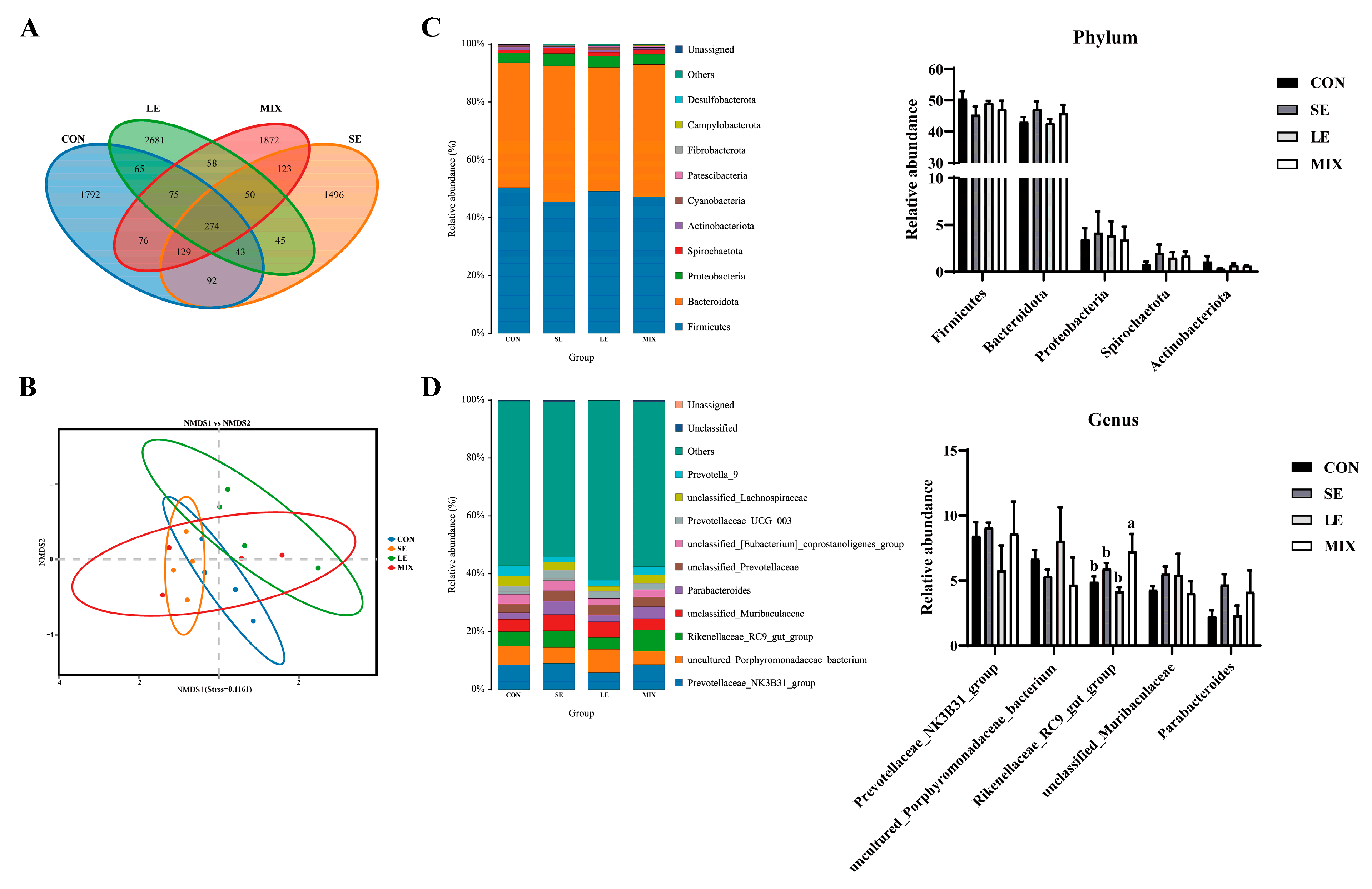
3.5. Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cougnon, M.; Carcy, R.; Melis, N.; Rubera, I.; Duranton, C.; Dumas, K.; Tanti, J.F.; Pons, C.; Soubeiran, N.; Shkreli, M.; et al. Inhibition of eIF5A hypusination reprogrammes metabolism and glucose handling in mouse kidney. Cell Death Dis. 2021, 12, 283. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Xiong, Q.; Kong, J.; Tian, C.; Miao, L.; Zhang, X.; Du, H. Intraperitoneal supplementation of iron alleviates dextran sodium sulfate-induced colitis by enhancing intestinal barrier function. Biomed. Pharmacother. 2021, 144, 112253. [Google Scholar] [CrossRef] [PubMed]
- de-Cara, A.; Saldaña, B.; Vázquez, P.; Rey, A.I. Dietary Protected Sodium Butyrate and/or Olive Leaf and Grape-Based By-Product Supplementation Modifies Productive Performance, Antioxidant Status and Meat Quality in Broilers. Antioxidants 2023, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Kang, K.S. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs. Sci. Rep. 2017, 7, 16530. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, Y.; Guan, P.; He, L.; Zhou, X. Serine Administration Improves Selenium Status, Oxidative Stress, and Mitochondrial Function in Longissimus Dorsi Muscle of Piglets with Intrauterine Growth Retardation. Biol. Trace Elem. Res. 2023, 201, 1740–1747. [Google Scholar] [CrossRef]
- Lv, M.; Yu, B.; Mao, X.B.; Zheng, P.; He, J.; Chen, D.W. Responses of growth performance and tryptophan metabolism to oxidative stress induced by diquat in weaned pigs. Animal 2012, 6, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Nan, G.X. Oxidative stress-induced angiogenesis. J. Clin. Neurosci. 2019, 63, 13–16. [Google Scholar] [CrossRef]
- Bissinger, R.; Bhuyan, A.; Qadri, S.M.; Lang, F. Oxidative stress, eryptosis and anemia: A pivotal mechanistic nexus in systemic diseases. FEBS J. 2019, 286, 826–854. [Google Scholar] [CrossRef]
- Sharma, P.; Puri, N. A new role for mast cells as scavengers for clearance of erythrocytes damaged due to oxidative stress. Immunol. Lett. 2018, 199, 23–35. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Cobley, J.N.; Paschalis, V.; Veskoukis, A.S.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Going retro: Oxidative stress biomarkers in modern redox biology. Free. Radic. Biol. Med. 2016, 98, 2–12. [Google Scholar] [CrossRef]
- Woolcock, A.D.; Serpa, P.B.S.; Santos, A.P.; Christian, J.A.; Moore, G.E. Reactive oxygen species, glutathione, and vitamin E concentrations in dogs with hemolytic or nonhemolytic anemia. J. Vet. Intern. Med. 2020, 34, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B(12), folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, F.; Wang, X.; Hu, X.; Liao, X.; Zhang, Y. Biological transformation of chlorophyll-rich spinach (Spinacia oleracea L.) extracts under in vitro gastrointestinal digestion and colonic fermentation. Food Res. Int. 2021, 139, 109941. [Google Scholar] [CrossRef] [PubMed]
- Grenier, D.; Marcoux, E.; Azelmat, J.; Ben, L.A.; Gauthier, P. Biocompatible combinations of nisin and licorice polyphenols exert synergistic bactericidal effects against Enterococcus faecalis and inhibit NF-kappaB activation in monocytes. AMB Express 2020, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Gani, A.; Masarat, D.M.; Bhat, N.A. Bioactive characterization of ultrasonicated ginger (Zingiber officinale) and licorice (Glycyrrhiza Glabra) freeze dried extracts. Ultrason. Sonochem 2022, 88, 106048. [Google Scholar] [CrossRef]
- Babu, V.; Kapkoti, D.S.; Binwal, M.; Bhakuni, R.S.; Shanker, K.; Singh, M.; Tandon, S.; Mugale, M.N.; Kumar, N.; Bawankule, D.U. Liquiritigenin, isoliquiritigenin rich extract of glycyrrhiza glabra roots attenuates inflammation in macrophages and collagen-induced arthritis in rats. Inflammopharmacology 2023, 31, 983–996. [Google Scholar] [CrossRef]
- Szudzik, M.; Lipinski, P.; Jonczy, A.; Mazgaj, R.; Pieszka, M.; Kamyczek, M.; Smuda, E.; Starzynski, R.R. Long-term Effect of Split Iron Dextran/Hemoglobin Supplementation on Erythrocyte and Iron Status, Growth Performance, Carcass Parameters, and Meat Quality of Polish Large White and 990 Line Pigs. Biol. Trace Elem. Res. 2020, 196, 472–480. [Google Scholar] [CrossRef]
- Seip, V.; Friendship, R.; Amezcua, R.; Farzan, A. The relationship between hemoglobin levels at weaning and growth performance and antibody response in nursery pigs. Can. Vet. J.-Rev. Vet. Can. 2020, 61, 1170–1174. [Google Scholar]
- Socha, D.S.; DeSouza, S.I.; Flagg, A.; Sekeres, M.; Rogers, H.J. Severe megaloblastic anemia: Vitamin deficiency and other causes. Clevel. Clin. J. Med. 2020, 87, 153–164. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, D.; Wu, X.; Yin, Y.; Wan, D. Ferrous Bisglycinate Supplementation Modulates Intestinal Antioxidant Capacity via the AMPK/FOXO Pathway and Reconstitutes Gut Microbiota and Bile Acid Profiles in Pigs. J. Agric. Food Chem. 2022, 70, 4942–4951. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhang, F.; Cao, R.; Ni, X.; Xin, Z.; Deng, J.; Wu, G.; Ren, W.; Yin, Y.; Deng, B. Cecropin A Alleviates Inflammation Through Modulating the Gut Microbiota of C57BL/6 Mice With DSS-Induced IBD. Front. Microbiol. 2019, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.P.; Mainau, E.; Ceron, J.J.; Contreras-Aguilar, M.D.; Martinez-Subiela, S.; Navarro, E.; Tecles, F.; Manteca, X.; Escribano, D. Biomarkers of oxidative stress in saliva in pigs: Analytical validation and changes in lactation. BMC Vet. Res. 2019, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.T.; Mun, H.S.; Islam, M.M.; Ko, S.Y.; Yang, C.J. Effects of dietary natural and fermented herb combination on growth performance, carcass traits and meat quality in grower-finisher pigs. Meat Sci. 2016, 122, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Toson, E.; Abd, E.L.M.; Mohamed, A.; Gazwi, H.; Saleh, M.; Kokoszynski, D.; Elnesr, S.S.; Hozzein, W.N.; Wadaan, M.; Elwan, H. Efficacy of licorice extract on the growth performance, carcass characteristics, blood indices and antioxidants capacity in broilers. Animal 2023, 17, 100696. [Google Scholar] [CrossRef] [PubMed]
- Katayama, M.; Fukuda, T.; Okamura, T.; Suzuki, E.; Tamura, K.; Shimizu, Y.; Suda, Y.; Suzuki, K. Effect of dietary addition of seaweed and licorice on the immune performance of pigs. Anim. Sci. J. 2011, 82, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Hollema, B.L.; Zwiers, S.; Hermesch, S. Genetic parameters for haemoglobin levels in sows and piglets as well as sow reproductive performance and piglet survival. Animal 2020, 14, 688–696. [Google Scholar] [CrossRef]
- Peters, J.C.; Mahan, D.C. Effects of neonatal iron status, iron injections at birth, and weaning in young pigs from sows fed either organic or inorganic trace minerals. J. Anim. Sci. 2008, 86, 2261–2269. [Google Scholar] [CrossRef]
- Knight, L.C.; Dilger, R.N. Longitudinal Effects of Iron Deficiency Anemia and Subsequent Repletion on Blood Parameters and the Rate and Composition of Growth in Pigs. Nutrients 2018, 10, 632. [Google Scholar] [CrossRef]
- Amoroso, C.; Perillo, F.; Strati, F.; Fantini, M.C.; Caprioli, F.; Facciotti, F. The Role of Gut Microbiota Biomodulators on Mucosal Immunity and Intestinal Inflammation. Cells 2020, 9, 1234. [Google Scholar] [CrossRef]
- Xiang, X.-D.; Deng, Z.-C.; Wang, Y.-W.; Sun, H.; Wang, L.; Han, Y.-M.; Wu, Y.-Y.; Liu, J.-G.; Sun, L.-H. Organic Acids Improve Growth Performance with Potential Regulation of Redox Homeostasis, Immunity, and Microflora in Intestines of Weaned Piglets. Antioxidants 2021, 10, 1665. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiang, S.; Zhang, H.; Ye, K.; Zhang, Y.; Ge, Y.; Feng, X.; Bao, X.; Chen, J.; Zhu, X. Vitamin B12 Enriched in Spinach and its Effects on Gut Microbiota. J. Agric. Food Chem. 2021, 69, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, Y.; Hu, X.; Liao, X.; Zhang, Y. Chlorophyll Supplementation in Early Life Prevents Diet-Induced Obesity and Modulates Gut Microbiota in Mice. Mol. Nutr. Food Res. 2019, 63, e1801219. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, C.; Che, Y.; Zhang, T.; Dai, C.; Nguyen, A.D.; Duan, K.; Huang, Y.; Li, N.; Zhou, H.; et al. Effects of Glycyrrhiza Polysaccharides on Chickens’ Intestinal Health and Homeostasis. Front. Vet. Sci. 2022, 9, 891429. [Google Scholar] [CrossRef]
- Zheng, J.; Liang, S.; Zhang, Y.; Sun, X.; Li, Y.; Diao, J.; Dong, L.; Ni, H.; Yin, Y.; Ren, J.; et al. Effects of Compound Chinese Herbal Medicine Additive on Growth Performance and Gut Microbiota Diversity of Zi Goose. Animals 2022, 12, 2942. [Google Scholar] [CrossRef] [PubMed]
- Su, X.L.; Tian, Q.; Zhang, J.; Yuan, X.Z.; Shi, X.S.; Guo, R.B.; Qiu, Y.L. Acetobacteroides hydrogenigenes gen. nov., sp. nov., an anaerobic hydrogen-producing bacterium in the family Rikenellaceae isolated from a reed swamp. Int. J. Syst. Evol. Microbiol. 2014, 64, 2986–2991. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | Content % | Nutrient Levels 2 | Content |
|---|---|---|---|
| Corn | 60.1 | Crude protein | 19.86 |
| Soybean meal | 19.6 | Ether extract | 4.03 |
| Extruded soybean | 8.0 | Calcium | 0.86 |
| Fish meal | 3.0 | Total phosphorus | 0.67 |
| Whey powder | 3.0 | Lysine | 1.43 |
| Soy oil | 2.0 | Methionine + Cystine | 0.78 |
| NaCl | 0.3 | Threonine | 0.86 |
| Premix 1 | 4.0 | Tryptophan | 0.24 |
| Total | 100 | Digestible energy, MJ/kg | 12.98 |
| Item 1 | Treatment 2 | p-Value | |||
|---|---|---|---|---|---|
| CON | SE | LE | MIX | ||
| Initial weight, kg | 11.31 ± 0.41 | 10.3 ± 0.58 | 10.74 ± 0.73 | 10.47 ± 0.45 | 0.610 |
| Final weight, kg | 20.74 ± 0.55 ab | 21.65 ± 0.99 ab | 19.94 ± 0.29 b | 22.67 ± 0.48 a | 0.040 |
| ADFI, kg/d | 0.29 ± 0.02 b | 0.41 ± 0.02 a | 0.33 ± 0.02 b | 0.44 ± 0.01 a | 0.006 |
| ADG, kg/d | 0.67 ± 0.02 | 0.70 ± 0.03 | 0.68 ± 0.01 | 0.71 ± 0.04 | 0.684 |
| F/G | 2.03 ± 0.16 ab | 1.74 ± 0.08 bc | 2.11 ± 0.11 a | 1.64 ± 0.09 c | 0.020 |
| Diarrhea rate, % | 1.48 ± 0.21 | 1.33 ± 0.23 | 1.43 ± 0.25 | 1.28 ± 0.19 | 0.913 |
| Item 1 | Treatment 2 | p-Value | |||
|---|---|---|---|---|---|
| CON | SE | LE | MIX | ||
| TP (g/L) | 53.18 ± 2.87 b | 57.14 ± 1.99 b | 57.60 ± 0.22 b | 66.57 ± 1.93 a | 0.009 |
| GLB (g/L) | 17.27 ± 1.09 b | 18.75 ± 0.83 b | 21.98 ± 1.19 a | 21.88 ± 0.87 a | 0.021 |
| ALB (g/L) | 35.91 ± 3.86 b | 38.39 ± 1.25 ab | 35.62 ± 1.89 b | 44.69 ± 1.27 a | 0.051 |
| BUN (mmol/L) | 3.22 ± 0.22 | 3.09 ± 0.05 | 3.47 ± 0.22 | 3.33 ± 0.25 | 0.689 |
| GLU (mmol/L) | 4.58 ± 0.45 c | 6.01 ± 0.64 bc | 6.78 ± 0.66 ab | 8.43 ± 0.37 a | 0.005 |
| TG (mmol/L) | 0.73 ± 0.08 b | 0.52 ± 0.06 b | 0.52 ± 0.07 b | 1.00 ± 0.05 a | 0.002 |
| TCH (mmol/L) | 1.74 ± 0.17 ab | 1.65 ± 0.09 ab | 1.55 ± 0.11 b | 2.06 ± 0.12 a | 0.089 |
| ALT (U/L) | 3.99 ± 0.25 ab | 2.80 ± 0.22 b | 3.02 ± 0.49 b | 5.06 ± 0.49 a | 0.011 |
| AST (U/L) | 4.28 ± 0.56 | 4.59 ± 0.58 | 6.71 ± 1.61 | 6.88 ± 0.60 | 0.117 |
| Item 1 | Treatment 2 | p-Value | |||
|---|---|---|---|---|---|
| CON | SE | LE | MIX | ||
| SOD (U/mL) | 211.66 ± 10.88 | 202.01 ± 11.86 | 222.51 ± 11.74 | 211.52 ± 8.58 | 0.650 |
| MDA (nmol/mL) | 4.11 ± 0.11 a | 2.84 ± 0.53 b | 2.35 ± 0.45 b | 3.48 ± 0.12 ab | 0.004 |
| GSH-Px (U/mL) | 952.18 ± 21.19 b | 1396.39 ± 34.62 a | 1248.12 ± 66.49 a | 1384.36 ± 78.57 a | 0.002 |
| CAT (U/mL) | 5.13 ± 0.19 | 4.85 ± 0.93 | 5.21 ± 0.34 | 4.53 ± 0.62 | 0.901 |
| Item | Treatment 1 | p-Value | |||
|---|---|---|---|---|---|
| CON | SE | LE | MIX | ||
| Initial concentration (g/L) | 83.40 ± 4.82 | 82.20 ± 5.07 | 85.00 ± 3.48 | 84.20 ± 2.92 | 0.969 |
| Final concentration (g/L) | 79.00 ± 1.48 b | 87.60 ± 1.21 a | 82.80 ± 2.20 b | 88.40 ± 1.03 a | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Lian, J.; Deng, H.; Luo, J.; Chen, T.; Sun, J.; Zhang, Y.; Yang, Y.; Liu, P.; Xi, Q. Effects of Spinach Extract and Licorice Extract on Growth Performance, Antioxidant Capacity, and Gut Microbiota in Weaned Piglets. Animals 2024, 14, 321. https://doi.org/10.3390/ani14020321
Zhu J, Lian J, Deng H, Luo J, Chen T, Sun J, Zhang Y, Yang Y, Liu P, Xi Q. Effects of Spinach Extract and Licorice Extract on Growth Performance, Antioxidant Capacity, and Gut Microbiota in Weaned Piglets. Animals. 2024; 14(2):321. https://doi.org/10.3390/ani14020321
Chicago/Turabian StyleZhu, Jiahao, Jincong Lian, Haibin Deng, Junyi Luo, Ting Chen, Jiajie Sun, Yongliang Zhang, Yongan Yang, Pingxiang Liu, and Qianyun Xi. 2024. "Effects of Spinach Extract and Licorice Extract on Growth Performance, Antioxidant Capacity, and Gut Microbiota in Weaned Piglets" Animals 14, no. 2: 321. https://doi.org/10.3390/ani14020321






