Detection and Molecular Diversity of Cryptosporidium spp. and Giardia duodenalis in the Endangered Iberian Lynx (Lynx pardinus), Spain
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
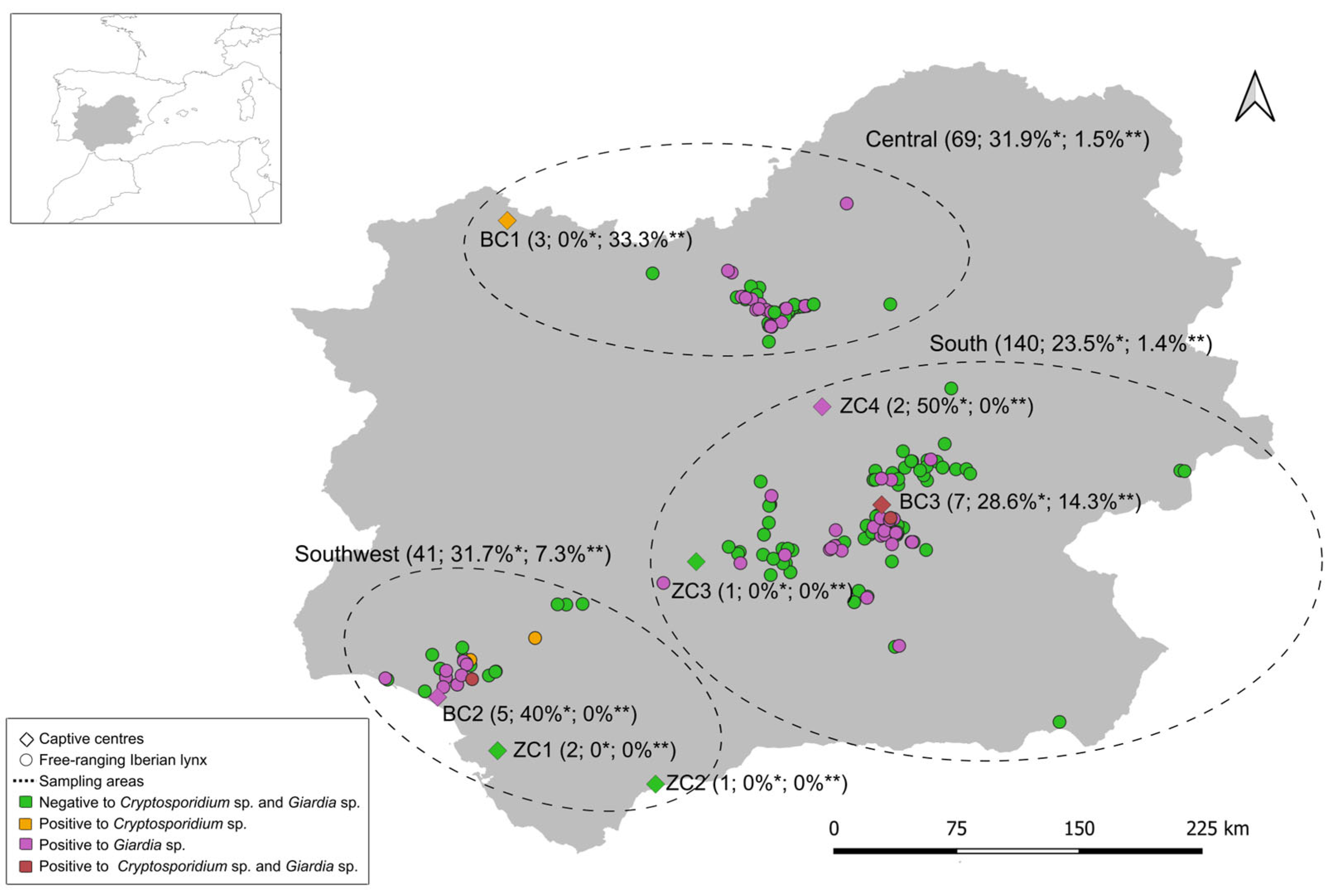
2.1. Study Area and Sampling
2.2. DNA Extraction and Purification of Faecal and Tissue Samples
2.3. Molecular Detection and Characterisation of Cryptosporidium spp.
2.4. Molecular Detection and Characterisation of Giardia duodenalis
2.5. General Procedures
2.6. Sequence and Phylogenetic Analysis
2.7. Statistics Analysis
3. Results
3.1. Occurrence of Cryptosporidium spp. and Giardia duodenalis
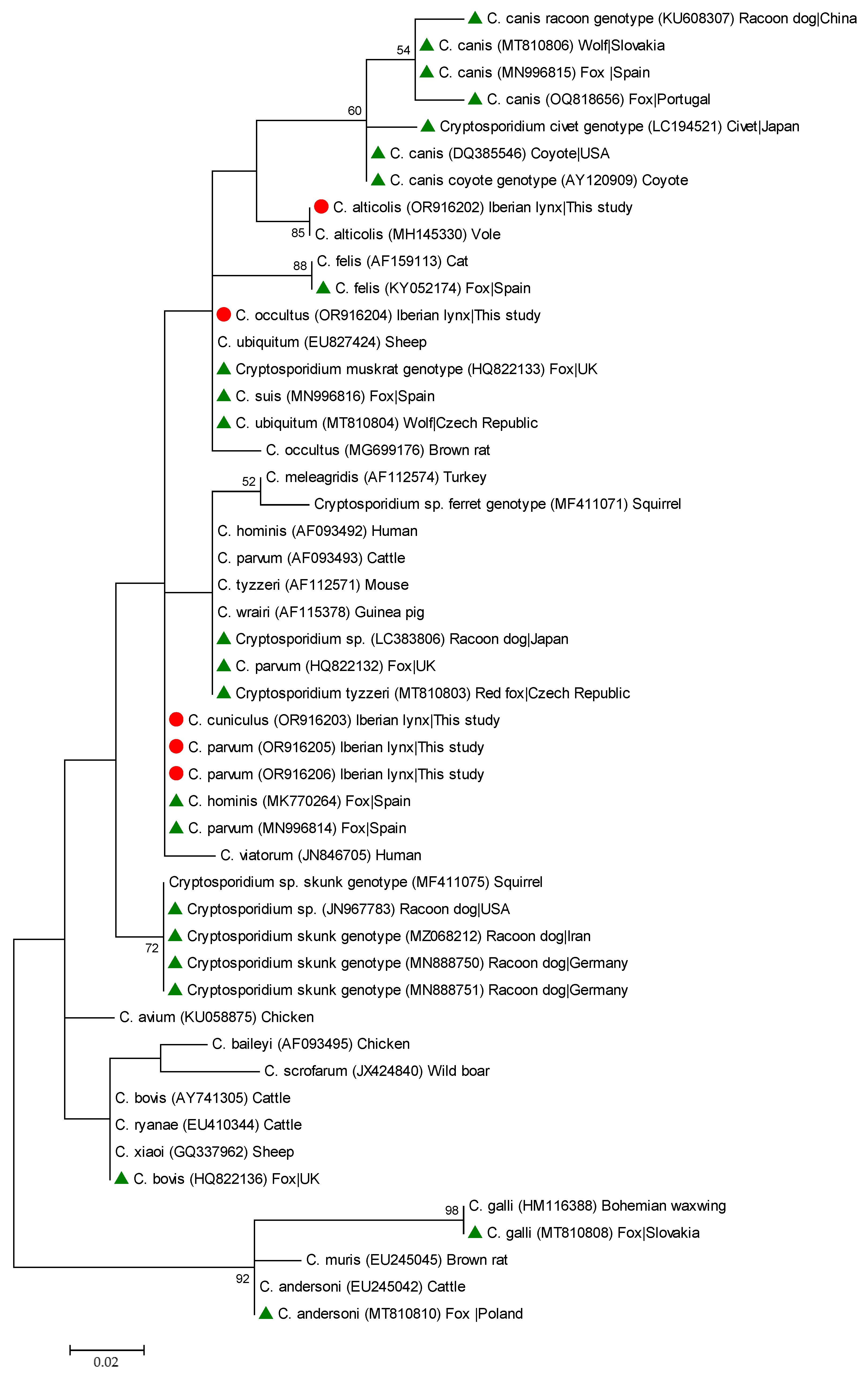
3.2. Molecular Characterisation of Cryptosporidium spp.
3.3. Molecular Characterisation of Giardia duodenalis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mmbaga, B.T.; Houpt, E.R. Cryptosporidium and Giardia infections in children: A review. Pediatr. Clin. N. Am. 2017, 64, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Santin, M. Cryptosporidium and Giardia in ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Donowitz, J.R.; Alam, M.; Kabir, M.; Ma, J.Z.; Nazib, F.; Platts-Mills, J.A.; Bartelt, L.A.; Haque, R.; Petri, W.A., Jr. A prospective longitudinal cohort to investigate the effects of early life giardiasis on growth and all cause diarrhea. Clin. Infect. Dis. 2016, 63, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, M.; Bartelt, L.A. Giardia and growth impairment in children in high-prevalence settings: Consequence or co-incidence? Curr. Opin. Infect. Dis. 2022, 35, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Santín, M. Clinical and subclinical infections with Cryptosporidium in animals. N. Z. Vet. J. 2013, 61, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hatam-Nahavandi, K.; Ahmadpour, E.; Carmena, D.; Spotin, A.; Bangoura, B.; Xiao, L. Cryptosporidium infections in terrestrial ungulates with focus on livestock: A systematic review and meta-analysis. Parasit Vectors 2019, 12, 453. [Google Scholar] [CrossRef] [PubMed]
- Utaaker, K.S.; Chaudhary, S.; Kifleyohannes, T.; Robertson, L.J. Global goat! Is the expanding goat population an important reservoir of Cryptosporidium? Front. Vet. Sci. 2021, 8, 648500. [Google Scholar] [CrossRef]
- Taghipour, A.; Sharbatkhori, M.; Tohidi, F.; Ghanbari, M.R.; Karanis, P.; Olfatifar, M.; Majidiani, H.; Khazaei, S.; Bahadory, S.; Javanmard, E. Global prevalence of Giardia duodenalis in cattle: A systematic review and meta-analysis. Prev. Vet. Med. 2022, 203, 105632. [Google Scholar] [CrossRef]
- Aloisio, F.; Filippini, G.; Antenucci, P.; Lepri, E.; Pezzotti, G.; Cacciò, S.M.; Pozio, E. Severe weight loss in lambs infected with Giardia duodenalis assemblage B. Vet. Parasitol. 2006, 142, 154–158. [Google Scholar] [CrossRef]
- Roblin, M.; Canniere, E.; Barbier, A.; Daandels, Y.; Dellevoet-Groenewegen, M.; Pinto, P.; Tsaousis, A.; Leruste, H.; Brainard, J.; Hunter, P.R.; et al. Study of the economic impact of cryptosporidiosis in calves after implementing good practices to manage the disease on dairy farms in Belgium, France, and the Netherlands. Curr. Res. Parasitol. Vector Borne Dis. 2023, 4, 100149. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.R. Giardia duodenalis in humans and animals—Transmission and disease. Res. Vet. Sci. 2021, 135, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Zahedi, A.; Feng, Y.; Xiao, L. An update on zoonotic Cryptosporidium species and genotypes in humans. Animals 2021, 11, 3307. [Google Scholar] [CrossRef] [PubMed]
- Myšková, E.; Brož, M.; Fuglei, E.; Kvičerová, J.; Mácová, A.; Sak, B.; Kváč, M.; Ditrich, O. Gastrointestinal parasites of arctic foxes (Vulpes lagopus) and sibling voles (Microtus levis) in Spitsbergen, Svalbard. Parasitol. Res. 2019, 118, 3409–3418. [Google Scholar] [CrossRef] [PubMed]
- Paziewska, A.; Bednarska, M.; Niewegłowski, H.; Karbowiak, G.; Bajer, A. Distribution of Cryptosporidium and Giardia spp. in selected species of protected and game mammals from North-Eastern Poland. Ann. Agric. Environ. Med. 2007, 14, 265–270. [Google Scholar] [PubMed]
- Figueiredo, A.M.; Köster, P.C.; Dashti, A.; Torres, R.T.; Fonseca, C.; Mysterud, A.; Bailo, B.; Carvalho, J.; Ferreira, E.; Hipólito, D.; et al. Molecular detection and distribution of Giardia duodenalis and Cryptosporidium spp. infections in wild and domestic animals in Portugal. Transbound. Emerg. Dis. 2023, 2023, 5849842. [Google Scholar] [CrossRef]
- Perec-Matysiak, A.; Hildebrand, J.; Popiołek, M.; Buńkowska-Gawlik, K. The occurrence of Cryptosporidium spp. in wild-living carnivores in Poland—A question concerning its host specificity. Pathogens 2023, 12, 198. [Google Scholar] [CrossRef] [PubMed]
- Stuart, P.; Golden, O.; Zintl, A.; de Waal, T.; Mulcahy, G.; McCarthy, E.; Lawton, C. A coprological survey of parasites of wild carnivores in Ireland. Parasitol. Res. 2013, 112, 3587–3593. [Google Scholar] [CrossRef]
- Hamnes, I.S.; Gjerde, B.K.; Forberg, T.; Robertson, L.J. Occurrence of Giardia and Cryptosporidium in Norwegian red foxes (Vulpes vulpes). Vet. Parasitol. 2007, 143, 347–353. [Google Scholar] [CrossRef]
- Mateo, M.; de Mingo, M.H.; de Lucio, A.; Morales, L.; Balseiro, A.; Espí, A.; Barral, M.; Lima Barbero, J.F.; Habela, M.Á.; Fernández-García, J.L.; et al. Occurrence and molecular genotyping of Giardia duodenalis and Cryptosporidium spp. in wild mesocarnivores in Spain. Vet. Parasitol. 2017, 235, 86–93. [Google Scholar] [CrossRef]
- Barrera, J.P.; Carmena, D.; Rodríguez, E.; Checa, R.; López, A.M.; Fidalgo, L.E.; Gálvez, R.; Marino, V.; Fuentes, I.; Miró, G.; et al. The red fox (Vulpes vulpes) as a potential natural reservoir of human cryptosporidiosis by Cryptosporidium hominis in Northwest Spain. Transbound. Emerg. Dis. 2020, 67, 2172–2182. [Google Scholar] [CrossRef] [PubMed]
- Nagano, Y.; Finn, M.B.; Lowery, C.J.; Murphy, T.; Moriarty, J.; Power, E.; Toolan, D.; O’Loughlin, A.; Watabe, M.; McCorry, K.A.; et al. Occurrence of Cryptosporidium parvum and bacterial pathogens in faecal material in the red fox (Vulpes vulpes) population. Vet. Res. Commun. 2007, 31, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.; Chalmers, R.M.; Stapleton, C.; Palmer, S.R.; Watkins, J.; Francis, C.; Kay, D. A whole water catchment approach to investigating the origin and distribution of Cryptosporidium species. J. Appl. Microbiol. 2011, 111, 717–730. [Google Scholar] [CrossRef]
- Segeritz, L.; Anders, O.; Middelhoff, T.L.; Winterfeld, D.T.; Maksimov, P.; Schares, G.; Conraths, F.J.; Taubert, A.; Hermosilla, C. New insights into gastrointestinal and pulmonary parasitofauna of wild Eurasian lynx (Lynx lynx) in the Harz Mountains of Germany. Pathogens 2021, 10, 1650. [Google Scholar] [CrossRef] [PubMed]
- Leśniańska, K.; Perec-Matysiak, A.; Hildebrand, J.; Buńkowska-Gawlik, K.; Piróg, A.; Popiołek, M. Cryptosporidium spp. and Enterocytozoon bieneusi in introduced raccoons (Procyon lotor)—First evidence from Poland and Germany. Parasitol. Res. 2016, 115, 4535–4541. [Google Scholar] [CrossRef]
- Rentería-Solís, Z.; Meyer-Kayser, E.; Obiegala, A.; Ackermann, F.; Król, N.; Birka, S. Cryptosporidium sp. skunk genotype in wild raccoons (Procyon lotor) naturally infected with Baylisascaris procyonis from Central Germany. Parasitol. Int. 2020, 79, 102159. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.H.; Adams, M.; Boreham, P.F.; Mayrhofer, G.; Meloni, B.P. Giardia intestinalis: Electrophoretic evidence for a species complex. Int. J. Parasitol. 1989, 19, 183–190. [Google Scholar] [CrossRef]
- Di Francesco, C.E.; Smoglica, C.; Paoletti, B.; Angelucci, S.; Innocenti, M.; Antonucci, A.; Di Domenico, G.; Marsilio, F. Detection of selected pathogens in Apennine wolf (Canis lupus italicus) by a non-invasive GPS-based telemetry sampling of two packs from Majella National Park, Italy. Eur. J. Wildl. Res. 2019, 65, 84. [Google Scholar] [CrossRef]
- Guadano Procesi, I.; Montalbano Di Filippo, M.; De Liberato, C.; Lombardo, A.; Brocherel, G.; Perrucci, S.; Di Cave, D.; Berrilli, F. Giardia duodenalis in wildlife: Exploring genotype diversity in Italy and across Europe. Pathogens 2022, 16, 105. [Google Scholar] [CrossRef]
- Beck, R.; Sprong, H.; Lucinger, S.; Pozio, E.; Cacciò, S.M. A large survey of Croatian wild mammals for Giardia duodenalis reveals a low prevalence and limited zoonotic potential. Vector Borne Zoonotic Dis. 2011, 11, 1049–1055. [Google Scholar] [CrossRef]
- Stojecki, K.; Sroka, J.; Caccio, S.M.; Cencek, T.; Dutkiewicz, J.; Kusyk, P. Prevalence and molecular typing of Giardia duodenalis in wildlife from eastern Poland. Folia Parasitol. 2015, 62, 2015.042. [Google Scholar] [CrossRef]
- Györke, A.; Kalmár, Z.; Dumitrache, M.O.; Gherman Călin, M.; Mircean, V. Giardia duodenalis genotypes in domestic and wild animals from Romania identified by PCR-RFLP targeting the gdh gene. Vet. Parasitol. 2016, 217, 71–75. [Google Scholar] [CrossRef]
- Papini, R.A.; Verin, R. Giardia and Cryptosporidium in red foxes (Vulpes vulpes): Screening for coproantigens in a population of central Italy and mini-review of the literature. Maced Vet. Rev. 2019, 42, 101–106. [Google Scholar] [CrossRef]
- Onac, D.; Oltean, M.; Mircean, V.; Jarca, A.; Cozma, V. Occurrence of Giardia duodenalis zoonotic assemblages in red foxes from Romania. Sci. Parasitol. 2015, 16, 177–180. [Google Scholar]
- Debenham, J.; Landuyt, H.; Troell, K.; Tysnes, K.; Robertson, L.J. Occurrence of Giardia in Swedish red foxes (Vulpes vulpes). J. Wildl. Dis. 2017, 53, 649–652. [Google Scholar] [CrossRef]
- Solarczyk, P.; Osten-Sacken, N.; Frantz, A.C.; Schneider, S.; Pir, J.B.; Heddergott, M. First molecular detection of Giardia duodenalis assemblage B in a free-living European wildcat (Felis s. silvestris) from Luxembourg. Acta Protozool. 2019, 58, 1–5. [Google Scholar] [CrossRef]
- Takeuchi-Storm, N.; Al-Sabi, M.N.S.; Chriel, M.; Enemark, H.L. Systematic examination of the cardiopulmonary, urogenital, muscular and gastrointestinal parasites of the Eurasian otters (Lutra lutra) in Denmark, a protected species recovering from a dramatic decline. Parasitol. Int. 2021, 84, 102418. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Hermida, F.; Gómez-Couso, H.; Romero-Suances, R.; Ares-Mazás, E. Cryptosporidium and Giardia in wild otters (Lutra lutra). Vet. Parasitol. 2007, 144, 153–156. [Google Scholar] [CrossRef]
- Maestrini, M.; Berrilli, F.; Di Rosso, A.; Coppola, F.; Guadano Procesi, I.; Mariacher, A.; Felicioli, A.; Perrucci, S. Zoonotic Giardia duodenalis genotypes and other gastrointestinal parasites in a badger population living in an anthropized area of central Italy. Pathogens 2022, 11, 906. [Google Scholar] [CrossRef]
- Barlow, A.M.; Mullineaux, E.; Wood, R.; Taweenan, W.; Wastling, J.M. Giardiosis in Eurasian badgers (Meles meles). Vet. Rec. 2010, 167, 1017. [Google Scholar] [CrossRef]
- Solarczyk, P.; Dabert, M.; Frantz, A.C.; Osten-Sacken, N.; Trzebny, A.; Wojtkowiak-Giera, A.; Heddergott, M. Zoonotic Giardia duodenalis sub-assemblage BIV in wild raccoons (Procyon lotor) from Germany and Luxembourg. Zoonoses Public Health 2021, 68, 538–543. [Google Scholar] [CrossRef]
- International Union for Conservation of Nature. The IUCN Red List of Threatened Species. 2023. Available online: https://www.iucnredlist.org/species/12520/174111773 (accessed on 8 December 2023).
- Simón, M.A.; Gil-Sánchez, J.M.; Ruiz, G.; Garrote, G.; McCain, E.B.; Fernández, L.; López-Parra, M.; Rojas, E.; Arenas-Rojas, R.; Rey, T.D.; et al. Reverse of the decline of the endangered Iberian lynx. Conserv. Biol. 2012, 26, 731–736. [Google Scholar] [CrossRef] [PubMed]
- López, G.; López-Parra, M.; Garrote, G.; Fernández, L.; del Rey-Wamba, T.; Arenas-Rojas, R.; García-Tardío, M.; Ruiz, G.; Zorrilla, I.; Moral, M.; et al. Evaluating mortality rates and causalities in a critically endangered felid across its whole distribution range. Eur. J. Wildl. Res. 2014, 60, 359–366. [Google Scholar] [CrossRef]
- Briones, V.; de Juan, L.; Sánchez, C.; Vela, A.I.; Galka, M.; Montero Goyache, J.; Aranaz, A.; Dominguez, L. Bovine tuberculosis and the endangered Iberian lynx. Emerg. Infect. Dis. 2000, 6, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Nájera, F.; Sánchez-Cuerda, S.; Gil-Molino, M.; Varela, E.; Serra, R.; Soler, F.; Vallverdú-Coll, N.; Panadero, J.; Zorrilla, I.; García, A.; et al. Fatal Streptococcus canis necrotizing fasciitis and myositis in a free-ranging Iberian Lynx (Lynx pardinus). J. Wildl. Dis. 2019, 55, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Meli, M.L.; Cattori, V.; Martínez, F.; López, G.; Vargas, A.; Palomares, F.; López-Bao, J.V.; Hofmann-Lehmann, R.; Lutz, H. Feline leukemia virus infection: A threat for the survival of the critically endangered Iberian lynx (Lynx pardinus). Vet. Immunol. Immunopathol. 2010, 134, 61–67. [Google Scholar] [CrossRef]
- Masot, A.J.; Gil, M.; Risco, D.; Jiménez, O.M.; Núñez, J.I.; Redondo, E. Pseudorabies virus infection (Aujeszky’s disease) in an Iberian lynx (Lynx pardinus) in Spain: A case report. BMC Vet. Res. 2017, 13, 6. [Google Scholar] [CrossRef]
- Millán, J.; Candela, M.G.; Palomares, F.; Cubero, M.J.; Rodríguez, A.; Barral, M.; de la Fuente, J.; Almeria, S.; León-Vizcaíno, L. Disease threats to the endangered Iberian lynx (Lynx pardinus). Vet. J. 2009, 182, 114–124. [Google Scholar] [CrossRef]
- García-Bocanegra, I.; Dubey, J.P.; Martínez, F.; Vargas, A.; Cabezón, O.; Zorrilla, I.; Arenas, A.; Almería, S. Factors affecting seroprevalence of Toxoplasma gondii in the endangered Iberian lynx (Lynx pardinus). Vet. Parasitol. 2010, 167, 36–42. [Google Scholar] [CrossRef]
- Figueiredo, A.M.; de Carvalho, L.M.; González, M.J.P.; Torres, R.T.; Pla, S.; Núñez-Arjona, J.C.; Rueda, C.; Vallverdú-Coll, N.; Silvestre, F.; Peña, J.; et al. Parasites of the reintroduced Iberian lynx (Lynx pardinus) and sympatric mesocarnivores in Extremadura, Spain. Pathogens 2021, 10, 274. [Google Scholar] [CrossRef]
- Matas Méndez, P.; Fuentes Corripio, I.; Montoya Matute, A.; Bailo Barroso, B.; Grande Gómez, R.; Apruzzese Rubio, A.; Ponce Gordo, F.; Mateo Barrientos, M. Prevalence of Toxoplasma gondii in endangered wild felines (Felis silvestris and Lynx pardinus) in Spain. Animals 2023, 13, 2488. [Google Scholar] [CrossRef]
- Ministerio de Transición Ecológica. Censo del Lince Ibérico (España y Portugal). 2022. Available online: https://www.google.com/url?sa=i&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=0CDcQw7AJahcKEwj4_IanwMCBAxUAAAAAHQAAAAAQAg&url=https%3A%2F%2Fwww.miteco.gob.es%2Fcontent%2Fdam%2Fmiteco%2Fes%2Fbiodiversidad%2Ftemas%2Finventarios-nacionales%2Finformecensodelinceiberico2022_tcm30-569643.pdf&psig=AOvVaw1qiILTvdLMqepDrQ3kRuDR&ust=1695550552083073&opi=89978449 (accessed on 8 December 2023).
- Rivas, A. Manual del Manejo del Lince Ibérico en Cautividad. Programa de Conservación Ex-Situ del Lince Ibérico. Available online: https://www.lynxexsitu.es/ficheros/documentos_pdf/84/Manual_Manejo_Lince_Iberico_2016.pdf (accessed on 8 December 2023).
- Nájera, F.; Grande-Gómez, R.; Peña, J.; Vázquez, A.; Palacios, M.J.; Rueda, C.; Corona-Bravo, A.I.; Zorrilla, I.; Revuelta, L.; Gil-Molino, M.; et al. Disease surveillance during the reintroduction of the Iberian lynx (Lynx pardinus) in southwestern Spain. Animals 2021, 11, 547. [Google Scholar] [CrossRef] [PubMed]
- Ávalos, G.; Caballero-Gómez, J.; Matas-Méndez, P.; Castro-Scholten, S.; Jiménez-Martín, D.; Köster, P.C.; Santín, M.; Bailo, B.; Cano-Terriza, D.; González-Barrio, D.; et al. Molecular identification of zoonotic Microsporidia in the endangered Iberian lynx (Lynx pardinus). Med. Mycol. 2024; under review. [Google Scholar]
- Tiangtip, R.; Jongwutiwes, S. Molecular analysis of Cryptosporidium species isolated from HIV-infected patients in Thailand. Trop. Med. Int. Health 2002, 7, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Feltus, D.C.; Giddings, C.W.; Schneck, B.L.; Monson, T.; Warshauer, D.; McEvoy, J.M. Evidence supporting zoonotic transmission of Cryptosporidium spp. in Wisconsin. J. Clin. Microbiol. 2006, 44, 4303–4308. [Google Scholar] [CrossRef] [PubMed]
- Verweij, J.J.; Schinkel, J.; Laeijendecker, D.; van Rooyen, M.A.; van Lieshout, L.; Polderman, A.M. Real-time PCR for the detection of Giardia lamblia. Mol. Cell Probes 2003, 17, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Appelbee, A.J.; Frederick, L.M.; Heitman, T.L.; Olson, M.E. Prevalence and genotyping of Giardia duodenalis from beef calves in Alberta, Canada. Vet. Parasitol. 2003, 112, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.M.; Meloni, B.P.; Groth, D.M.; Wetherall, J.D.; Reynoldson, J.A.; Thompson, R.C. Ribosomal RNA sequencing reveals differences between the genotypes of Giardia isolates recovered from humans and dogs living in the same locality. J. Parasitol. 1997, 83, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Read, C.M.; Monis, P.T.; Thompson, R.C. Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCR-RFLP. Infect. Genet. Evol. 2004, 4, 125–130. [Google Scholar] [CrossRef]
- Lalle, M.; Pozio, E.; Capelli, G.; Bruschi, F.; Crotti, D.; Cacciò, S.M. Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int. J. Parasitol. 2005, 35, 207–213. [Google Scholar] [CrossRef]
- Sulaiman, I.M.; Fayer, R.; Bern, C.; Gilman, R.H.; Trout, J.M.; Schantz, P.M.; Das, P.; Lal, A.A.; Xiao, L. Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg. Infect. Dis. 2003, 9, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org (accessed on 24 November 2023).
- Gil-Sánchez, J.M.; Ballesteros-Duperón, E.; Bueno-Segura, J.F. Feeding ecology of the Iberian lynx Lynx pardinus in eastern Sierra Morena (Southern Spain). Acta Theriol. 2006, 51, 85–90. [Google Scholar] [CrossRef]
- Horčičková, M.; Čondlová, Š.; Holubová, N.; Sak, B.; Květoňová, D.; Hlásková, L.; Konečný, R.; Sedláček, F.; Clark, M.; Giddings, C.; et al. Diversity of Cryptosporidium in common voles and description of Cryptosporidium alticolis sp. n. and Cryptosporidium microti sp. n. (Apicomplexa: Cryptosporidiidae). Parasitology 2019, 146, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Kváč, M.; Vlnatá, G.; Ježková, J.; Horčičková, M.; Konečný, R.; Hlásková, L.; McEvoy, J.; Sak, B. Cryptosporidium occultus sp. n. (Apicomplexa: Cryptosporidiidae) in rats. Eur. J. Protistol. 2018, 63, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.; Wright, S.; Elwin, K.; Hadfield, S.; Katzer, F.; Bartley, P.M.; Hunter, P.R.; Nath, M.; Innes, E.A.; Chalmers, R.M. Re-description of Cryptosporidium cuniculus Inman and Takeuchi, 1979 (Apicomplexa: Cryptosporidiidae): Morphology, biology and phylogeny. Int. J. Parasitol. 2010, 40, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ryan, U.M.; Xiao, L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. 2018, 34, 997–1011. [Google Scholar] [CrossRef]
- Baz-González, E.; Martín-Carrillo, N.; García-Livia, K.; Foronda, P. Molecular detection of Cryptosporidium cuniculus in rabbits (Oryctolagus cuniculus) from Tenerife, Canary Islands, Spain. Vet. Sci. 2022, 9, 91. [Google Scholar] [CrossRef]
- Rego, L.; Castro-Scholten, S.; Cano, C.; Jiménez-Martín, D.; Köster, P.C.; Caballero-Gómez, J.; Bailo, B.; Dashti, A.; Hernández-Castro, C.; Cano-Terriza, D.; et al. Iberian wild leporidae as hosts of zoonotic enteroparasites in Mediterranean ecosystems of Southern Spain. Zoonoses Public Health 2023, 70, 223–237. [Google Scholar] [CrossRef]
- Xu, N.; Liu, H.; Jiang, Y.; Yin, J.; Yuan, Z.; Shen, Y.; Cao, J. First report of Cryptosporidium viatorum and Cryptosporidium occultus in humans in China, and of the unique novel C. viatorum subtype XVaA3h. BMC Infect. Dis. 2020, 20, 16. [Google Scholar] [CrossRef]
- Martínez-Ruiz, R.; de Lucio, A.; Fuentes, I.; Carmena, D. Autochthonous Cryptosporidium cuniculus infection in Spain: First report in a symptomatic paediatric patient from Madrid. Enferm. Infecc. Microbiol. Clin. 2016, 34, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Lebbad, M.; Winiecka-Krusnell, J.; Stensvold, C.R.; Beser, J. High diversity of Cryptosporidium species and subtypes identified in cryptosporidiosis acquired in Sweden and abroad. Pathogens 2021, 10, 523. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, R.M.; Elwin, K.; Hadfield, S.J.; Robinson, G. Sporadic human cryptosporidiosis caused by Cryptosporidium cuniculus, United Kingdom, 2007–2008. Emerg. Infect Dis. 2011, 17, 536–538. [Google Scholar] [CrossRef] [PubMed]
- Elwin, K.; Hadfield, S.J.; Robinson, G.; Chalmers, R.M. The epidemiology of sporadic human infections with unusual cryptosporidia detected during routine typing in England and Wales, 2000–2008. Epidemiol. Infect. 2012, 140, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Puleston, R.L.; Mallaghan, C.M.; Modha, D.E.; Hunter, P.R.; Nguyen-Van-Tam, J.S.; Regan, C.M.; Nichols, G.L.; Chalmers, R.M. The first recorded outbreak of cryptosporidiosis due to Cryptosporidium cuniculus (formerly rabbit genotype), following a water quality incident. J. Water Health 2014, 12, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Segura, R.; Prim, N.; Montemayor, M.; Valls, M.E.; Muñoz, C.; Segura, R.; Prim, N.; Montemayor, M.; Valls, M.E.; Muñoz, C. Predominant virulent IbA10G2 subtype of Cryptosporidium hominis in human isolates in Barcelona: A five-year study. PLoS ONE 2015, 10, e0121753. [Google Scholar] [CrossRef] [PubMed]
- Abal-Fabeiro, J.L.; Maside, X.; Llovo, J.; Bartolomé, C. Aetiology and epidemiology of human cryptosporidiosis cases in Galicia (NW Spain), 2000–2008. Epidemiol. Infect. 2015, 143, 3022–3035. [Google Scholar] [CrossRef] [PubMed]
- Azcona-Gutiérrez, J.M.; de Lucio, A.; Hernández-de-Mingo, M.; García-García, C.; Soria-Blanco, L.M.; Morales, L.; Aguilera, M.; Fuentes, I.; Carmena, D. Molecular diversity and frequency of the diarrheagenic enteric protozoan Giardia duodenalis and Cryptosporidium spp. in a hospital setting in Northern Spain. PLoS ONE 2017, 12, e0178575. [Google Scholar] [CrossRef]
- De Lucio, A.; Merino, F.J.; Martínez-Ruiz, R.; Bailo, B.; Aguilera, M.; Fuentes, I.; Carmena, D. Molecular genotyping and sub-genotyping of Cryptosporidium spp. isolates from symptomatic individuals attending two major public hospitals in Madrid, Spain. Infect. Genet. Evol. 2016, 37, 49–56. [Google Scholar] [CrossRef]
- Llorente, M.T.; Clavel, A.; Goñi, M.P.; Varea, M.; Seral, C.; Becerril, R.; Suarez, L.; Gómez-Lus, R. Genetic characterization of Cryptosporidium species from humans in Spain. Parasitol. Int. 2007, 56, 201–205. [Google Scholar] [CrossRef]
- Ramo, A.; Quílez, J.; Vergara-Castiblanco, C.; Monteagudo, L.; Del Cacho, E.; Clavel, A. Multilocus typing and population structure of Cryptosporidium from children in Zaragoza, Spain. Infect. Genet. Evol. 2015, 31, 190–197. [Google Scholar] [CrossRef]
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Ryan, U.; Zahedi, A. Molecular epidemiology of giardiasis from a veterinary perspective. Adv. Parasitol. 2019, 106, 209–254. [Google Scholar] [CrossRef] [PubMed]
- Traub, R.J.; Monis, P.T.; Robertson, I.; Irwin, P.; Mencke, N.; Thompson, R.C. Epidemiological and molecular evidence supports the zoonotic transmission of Giardia among humans and dogs living in the same community. Parasitology 2004, 128, 253–362. [Google Scholar] [CrossRef]
- Sahagún, J.; Clavel, A.; Goñi, P.; Seral, C.; Llorente, M.T.; Castillo, F.J.; Capilla, S.; Arias, A.; Gómez-Lus, R. Correlation between the presence of symptoms and the Giardia duodenalis genotype. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 81–83. [Google Scholar] [CrossRef] [PubMed]
- de Lucio, A.; Martínez-Ruiz, R.; Merino, F.J.; Bailo, B.; Aguilera, M.; Fuentes, I.; Carmena, D. Molecular genotyping of Giardia duodenalis isolates from symptomatic individuals attending two major public hospitals in Madrid, Spain. PLoS ONE 2015, 10, e0143981. [Google Scholar] [CrossRef] [PubMed]
- Gabín-García, L.B.; Bartolomé, C.; Abal-Fabeiro, J.L.; Méndez, S.; Llovo, J.; Maside, X. Strong genetic structure revealed by multilocus patterns of variation in Giardia duodenalis isolates of patients from Galicia (NW–Iberian Peninsula). Infect. Genet. Evol. 2017, 48, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Muadica, A.S.; Köster, P.C.; Dashti, A.; Bailo, B.; Hernández-de-Mingo, M.; Reh, L.; Balasegaram, S.; Verlander, N.Q.; Ruiz Chércoles, E.; Carmena, D. Molecular diversity of Giardia duodenalis, Cryptosporidium spp. and Blastocystis sp. in asymptomatic school children in Leganés, Madrid (Spain). Microorganisms 2020, 8, 466. [Google Scholar] [CrossRef] [PubMed]
| Family | Host (Common Name) | Host (Scientific Name) | Country | Frequency (%) | No. pos./Total | Genotype(s) (n) | Reference |
|---|---|---|---|---|---|---|---|
| Canidae | Arctic fox | Vulpes lagopus | Norway | 0.0 | 0/62 | – | [14] |
| Grey wolf | Canis lupus | Poland | 35.7 | 5/14 | C. parvum genotype 2 (5) | [15] | |
| Iberian wolf | Canis lupus signatus | Portugal | 2.5 | 3/121 | C. canis (3) | [16] | |
| Raccoon dog | Nyctereutes procyonoides | Poland | 24.1 | 21/87 | C. canis (dog genotype) (16) | [17] | |
| Red fox | Vulpes vulpes | Ireland | 0.0 | 0/13 | – | [18] | |
| Norway | 0.0 | 0/269 | – | [19] | |||
| Poland | 12.0 | 6/50 | C. canis (fox genotype) (3), C. alticolis (2), C. vole genotype II (1) | [17] | |||
| Portugal | 3.3 | 4/121 | C. canis (4) | [16] | |||
| Spain | 8.0 | 7/87 | C. canis (2), C. felis (1), C. parvum (3), C. ubiquitum (1) | [20] | |||
| 6.1% | 12/197 | C. hominis (4), C. canis (3), C. parvum (2), C. ubiquitum (1), C. suis (1) | [21] | ||||
| UK | 8.0 | 10/124 | C. parvum (2) | [22] | |||
| UK | 13.3 | 4/30 | C. bovis (1), C. parvum (1), C. muskrat genotype II (1) | [23] | |||
| Felidae | Eurasian lynx | Lynx lynx | Germany | 4.2 | 1/24 | C. felis (1) | [24] |
| Iberian lynx | Lynx pardinus | Portugal | 3.3 | 1/30 | C. felis (1) | [16] | |
| Spain | 0.0 | 0/6 | – | [20] | |||
| Wildcat | Felis silvestris | Spain | 0.0 | 0/2 | – | [20] | |
| Herpestidae | Mongoose | Herpestes ichneumon | Spain | 50.0 | 1/2 | C. canis (1) | [20] |
| Mustelidae | American mink | Mustela vison | Ireland | 6.2 | 5/81 | C. mink genotype (1), C. andersoni (3), Cryptosporidium spp. (1) | [18] |
| Beech marten | Martes foina | Poland | 29.4 | 15/51 | C. ditrichi (15) | [17] | |
| Spain | 0.0 | 0/8 | – | [20] | |||
| Eurasian badger | Meles meles | Ireland | 0.0 | 0/7 | – | [18] | |
| Poland | 20.0 | 9/45 | C. skunk genotype (5), C. erinacei (4) | [17] | |||
| Spain | 2.8 | 2/70 | C. hominis (1), Cryptosporidium spp. (1) | [20] | |||
| Eurasian otter | Lutra lutra | Ireland | 4.0 | 1/25 | Cryptosporidium spp. (1) | [18] | |
| Spain | 0.0 | 0/2 | – | [20] | |||
| Ferret | Mustela patois furo | Spain | 0.0 | 0/2 | – | [20] | |
| Genet | Genetta genetta | Spain | 16.6 | 1/6 | Cryptosporidium spp. (1) | [20] | |
| Irish stoats | Mustela ermine hibernica | Ireland | 0.0 | 0/30 | – | [18] | |
| Pine marten | Martes martes | Poland | 29.2 | 7/24 | C. ditrichi (7) | [17] | |
| Ireland | 0.0 | 0/7 | – | [18] | |||
| Polecat | Mustela putorius | Spain | 0.0 | 0/2 | – | [20] | |
| Procyonidae | Raccoon | Procyon lotor | Poland | 24.6 | 16/65 | C. skunk genotype (16) | [17] |
| Poland | 43.7 | 14/32 | C. skunk genotype (9), Cryptosporidium spp. (5) | [25] | |||
| Germany | 3.9 | 2/51 | C. skunk genotype (2) | [26] | |||
| Germany | 17.6 | 3/17 | Cryptosporidium spp. (3), C. erinacei (3), C. suis (2) | [25] |
| Family | Host (Common Name) | Host (Scientific Name) | Country | Frequency (%) | No. pos./Total | Genotype(s) (n) | Reference |
|---|---|---|---|---|---|---|---|
| Canidae | Apennine wolf | Canis lupus italicus | Italy | 5.0 | 1/20 | C (1) | [28] |
| 100 | 1/1 | D (1) | [29] | ||||
| Grey wolf | Canis lupus | Croatia | 10.2 | 13/127 | A (1), A1 (5), C (2), D (1), AI+B+D (1), A+C+D (1), C+D (1) | [30] | |
| Poland | 28.6 | 2/7 | D (2) | [31] | |||
| Romania | 100 | 3/3 | D (3) | [32] | |||
| Iberian wolf | Canis lupus signatus | Portugal | 25.6 | 31/121 | D (4), C+D (2) | [16] | |
| Spain | 15.9 | 1/6 | Unknown | [20] | |||
| Jackal | Canis aureus | Croatia | 12.5 | 1/8 | A+B (1) | [30] | |
| Raccoon dog | Nyctereutes procyonoides | Romania | 100 | 1/1 | D (1) | [32] | |
| Red fox | Vulpes vulpes | Croatia | 4.6 | 3/66 | A (1) | [30] | |
| Italy | 7.0 | 5/71 | Unknown | [33] | |||
| Norway | 2.2 | 6/269 | A (3), AI (2), B3 (1) | [19] | |||
| Portugal | 18.6 | 22/118 | C+D (1) | [16] | |||
| Romania | 4.6 | 10/217 | A (2), B (1) | [34] | |||
| Spain | 8.1 | 7/87 | Unknown | [20] | |||
| 9.6 | 19/197 | Unknown | [21] | ||||
| Sweden | 44.2 | 46/104 | B (4) | [35] | |||
| Felidae | Eurasian lynx | Lynx lynx | Germany | 16.7 | 4/24 | Unknown | [24] |
| Iberian lynx | Lynx pardinus | Portugal | 26.7 | 8/30 | Unknown | [16] | |
| Spain | 0.0 | 0/6 | – | [20] | |||
| Wildcat | Felis silvestris | Luxembourg | 10.0 | 1/10 | B (1) | [36] | |
| 0.0 | 0/2 | – | [20] | ||||
| Herpestidae | Mangoose | Herpestes ichneumon | Spain | 0.0 | 0/2 | – | [20] |
| Mustelidae | Badger | Meles meles | Italy | 25.6 | 11/43 | AII (6) | [39] |
| Poland | 0.0 | 0/1 | – | [31] | |||
| Spain | 0.0 | 0/70 | – | [20] | |||
| UK | 100 | 1/1 | E (1) | [40] | |||
| Ferret | Mustela putorius furo | Spain | 0.0 | 0/2 | – | [20] | |
| Marten | Martes sp. | Poland | 0.0 | 0/1 | – | [31] | |
| Eurasian otter | Lutra lutra | Denmark | 3.1 | 1/33 | Unknown | [37] | |
| Poland | 0.0 | 0/1 | – | [31] | |||
| Spain | 6.8 | 30/437 | Unknown | [38] | |||
| 0.0 | 0/2 | – | [20] | ||||
| Polecat | Mustela putorius | Spain | 0.0 | 0/2 | – | [20] | |
| Stone marten | Martes foina | Portugal | 15.8 | 3/19 | Unknown | [32] | |
| Spain | 12.5 | 1/8 | Unknown | [20] | |||
| Weasel | Mustela sp. | Poland | 0.0 | 0/1 | – | [31] | |
| Procyonidae | Racoon | Procyon lotor | Luxembourg | 33.3 | 3/9 | B (3) | [41] |
| Germany | 29.2 | 14/48 | B (13) | [41] | |||
| Ursidae | Brown bear | Ursus arctos | Croatia | 0.0 | 0/19 | – | [30] |
| Viverridae | Genet | Genetta genetta | Spain | 0.0 | 0/6 | – | [20] |
| Cryptosporidium spp. (n = 6) | Giardia duodenalis (n = 70) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Animals (n) | Positive (n) | % (95% CI) | p-Value | Positive (n) | % (95% CI) | p-Value |
| Sampling area (6) a | |||||||
| Central | 66 | 1 | 1.5 (0.04–8.2) | 0.101 | 22 | 33.3 (22.2–46.0) | 0.307 |
| South | 138 | 2 | 1.5 (0.2–5.1) | 33 | 23.9 (17.1–31.9) | ||
| Southwest | 41 | 3 | 7.3 (1.5–19.9) | 13 | 31.7 (18.1–48.1) | ||
| Sex (87) a | |||||||
| Male | 95 | 2 | 2.1 (0.3–7.4) | 0.619 | 21 | 22.1 (14.2–31.8) | 0.424 |
| Female | 69 | 1 | 1.5 (0.04–7.8) | 19 | 27.5 (17.5–39.6) | ||
| Age (67) a.b | |||||||
| Yearling | 54 | 2 | 3.7 (0.5–12.8) | 0.624 | 13 | 24.1 (13.5–37.6) | 0.856 |
| Sub-adult | 77 | 1 | 1.3 (0.03–7.0) | 21 | 27.3 (17.7–38.6) | ||
| Adult | 42 | 2 | 4.8 (0.6–16.2) | 12 | 28.6 (15.7–44.6) | ||
| Senile | 11 | 0 | 0.0 (0.0–0.0) | 4 | 36.4 (10.9–69.2) | ||
| Status (8) a | |||||||
| Free-living | 223 | 4 | 1.8 (0.5–4.5) | 0.079 | 64 | 28.7 (22.9–35.1) | 0.476 |
| Captive | 20 | 2 | 10.0 (1.2–31.7) | 5 | 25.0 (8.7–49.1) | ||
| Sampling year (14) a | |||||||
| 2017–2020 | 59 | 4 | 6.8 (1.9–16.5) | 0.042 | 17 | 28.8 (17.8–42.1) | 0.777 |
| 2021 | 69 | 0 | 0.0 (0.0–0.0) | 17 | 24.6 (15.1–36.5) | ||
| 2022–2023 | 109 | 2 | 1.8 (0.2–6.5) | 32 | 29.4 (21.0–38.9) | ||
| Species | Genotype | Isolates (n) | Locus | Reference Sequence | Stretch | Single Nucleotide Polymorphisms | GenBank ID |
|---|---|---|---|---|---|---|---|
| C. alticolis | – | 1 | ssu rRNA | MH145330 | 311–781 | A411T, 425_426DelTA, Ins464_467TAAT, 569DelT, 782InsG | OR916202 |
| C. cuniculus | – | 2 | ssu rRNA | AY120901 | 319–784 | None | OR916203 |
| VaA19 | 1 | gp60 | KU852733 | 5–750 | None | OR921171 | |
| C. occultus | – | 1 | ssu rRNA | MG699176 | 482–695 | None | OR916204 |
| C. parvum | – | 1 | ssu rRNA | AF112571 | 528–1025 | A646G, T649G, 686_689DelTAAT, A691T, A854R, A892G | OR916205 |
| – | 1 | ssu rRNA | AF112571 | 528–1030 | 646G, T649G, 686_688DelTAA, A691T, C795T, A891G, A933G | OR916206 |
| Sample ID | Age (yrs.) | Sex | CT Value in qPCR | ssu rRNA | gdh | bg | tpi | Assigned Genotype |
|---|---|---|---|---|---|---|---|---|
| 1091 | Sub-adult | Female | 33.1 | B | – | – | – | B |
| 962 | Unknown | Unknown | 20.0 | B | BIV | B | BIII | BIII/BIV |
| 1034 | Sub-adult | Female | 32.7 | A | – | – | – | A |
| 1079 | Yearling | Unknown | 24.2 | B | – | – | – | B |
| 486D | Adult | Female | 24.2 | A | – | – | – | A |
| 1004 | Sub-adult | Unknown | 30.1 | A | – | – | – | A |
| 948 | Unknown | Unknown | 24.7 | A | – | – | – | A |
| 83H | Sub-adult | Female | 20.3 | A | AI | AI | AI | AI |
| Assemblage | Sub-Assemblage | Isolates (n) | Locus | Reference Sequence | Stretch | Single Nucleotide Polymorphisms | GenBank ID |
|---|---|---|---|---|---|---|---|
| A | – | 4 | ssu rRNA | M54878 | 1–289 | None | OR916207 |
| – | 1 | ssu rRNA | M54878 | 1–289 | A87W, G153R, C207Y | OR916208 | |
| AI | 1 | gdh | L40509 | 73–491 | None | OR921172 | |
| AI | 1 | bg | AY655702 | 27–521 | None | OR921173 | |
| AI | 1 | tpi | L02120 | 559–1072 | None | OR921174 | |
| B | – | 3 | ssu rRNA | AF113898 | 1–275 | None | OR916209 |
| BIV | gdh | L40508 | 89–490 | T183C, C252T | OR921175 | ||
| – | bg | AY072727 | 98–593 | None | OR921176 | ||
| BIII | tpi | AF069560 | 1–479 | T134C, A176G, A395G | OR921177 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matas-Méndez, P.; Ávalos, G.; Caballero-Gómez, J.; Dashti, A.; Castro-Scholten, S.; Jiménez-Martín, D.; González-Barrio, D.; Muñoz-de-Mier, G.J.; Bailo, B.; Cano-Terriza, D.; et al. Detection and Molecular Diversity of Cryptosporidium spp. and Giardia duodenalis in the Endangered Iberian Lynx (Lynx pardinus), Spain. Animals 2024, 14, 340. https://doi.org/10.3390/ani14020340
Matas-Méndez P, Ávalos G, Caballero-Gómez J, Dashti A, Castro-Scholten S, Jiménez-Martín D, González-Barrio D, Muñoz-de-Mier GJ, Bailo B, Cano-Terriza D, et al. Detection and Molecular Diversity of Cryptosporidium spp. and Giardia duodenalis in the Endangered Iberian Lynx (Lynx pardinus), Spain. Animals. 2024; 14(2):340. https://doi.org/10.3390/ani14020340
Chicago/Turabian StyleMatas-Méndez, Pablo, Gabriel Ávalos, Javier Caballero-Gómez, Alejandro Dashti, Sabrina Castro-Scholten, Débora Jiménez-Martín, David González-Barrio, Gemma J. Muñoz-de-Mier, Begoña Bailo, David Cano-Terriza, and et al. 2024. "Detection and Molecular Diversity of Cryptosporidium spp. and Giardia duodenalis in the Endangered Iberian Lynx (Lynx pardinus), Spain" Animals 14, no. 2: 340. https://doi.org/10.3390/ani14020340
APA StyleMatas-Méndez, P., Ávalos, G., Caballero-Gómez, J., Dashti, A., Castro-Scholten, S., Jiménez-Martín, D., González-Barrio, D., Muñoz-de-Mier, G. J., Bailo, B., Cano-Terriza, D., Mateo, M., Nájera, F., Xiao, L., Köster, P. C., García-Bocanegra, I., & Carmena, D. (2024). Detection and Molecular Diversity of Cryptosporidium spp. and Giardia duodenalis in the Endangered Iberian Lynx (Lynx pardinus), Spain. Animals, 14(2), 340. https://doi.org/10.3390/ani14020340










