Mitochondria-Targeted Antioxidant MitoQ Improves In Vitro Maturation and Subsequent Embryonic Development from Culled Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
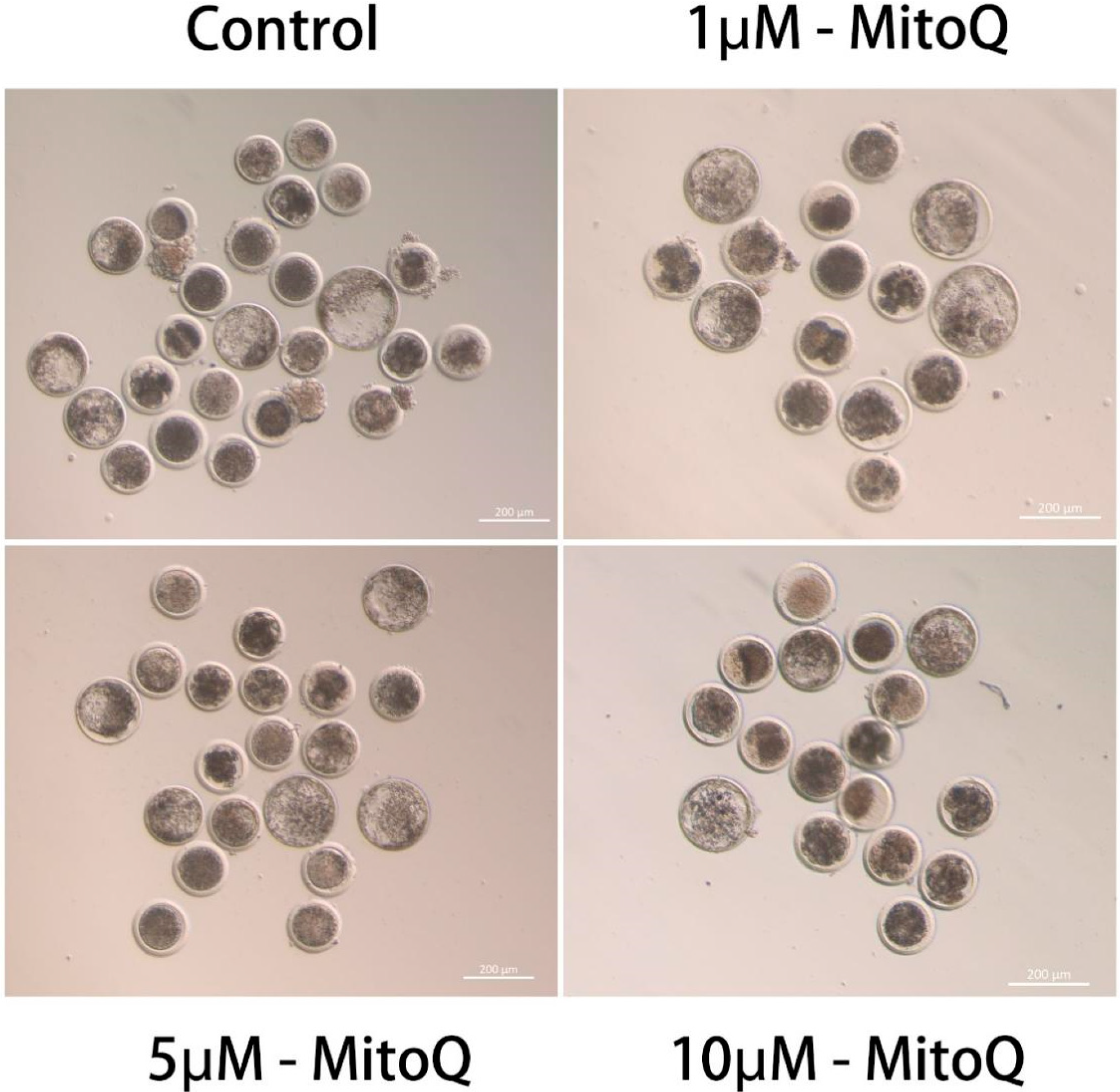
2.1. COCs Collection and In Vitro Maturation (IVM)
2.2. Parthenogenetic Activation (PA) and Embryo In Vitro Culture (IVC)
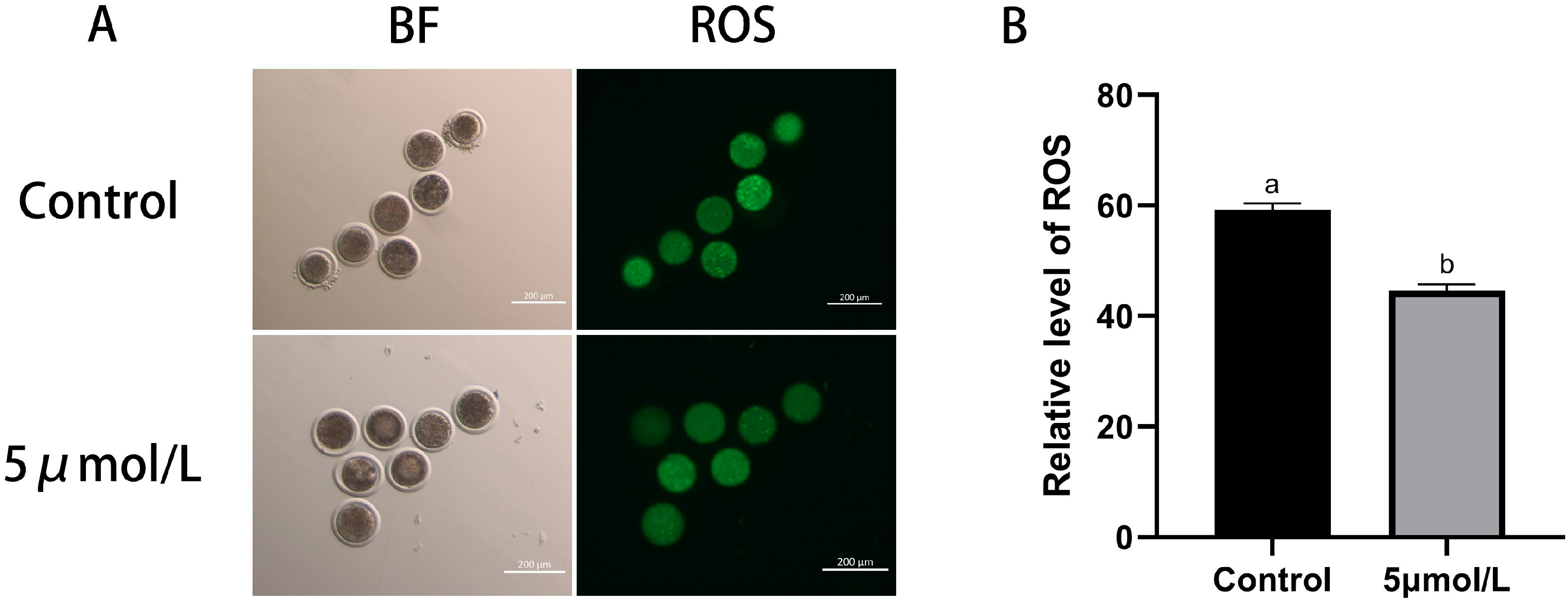
2.3. Assessment of ROS Level
2.4. Assessment of GSH Level
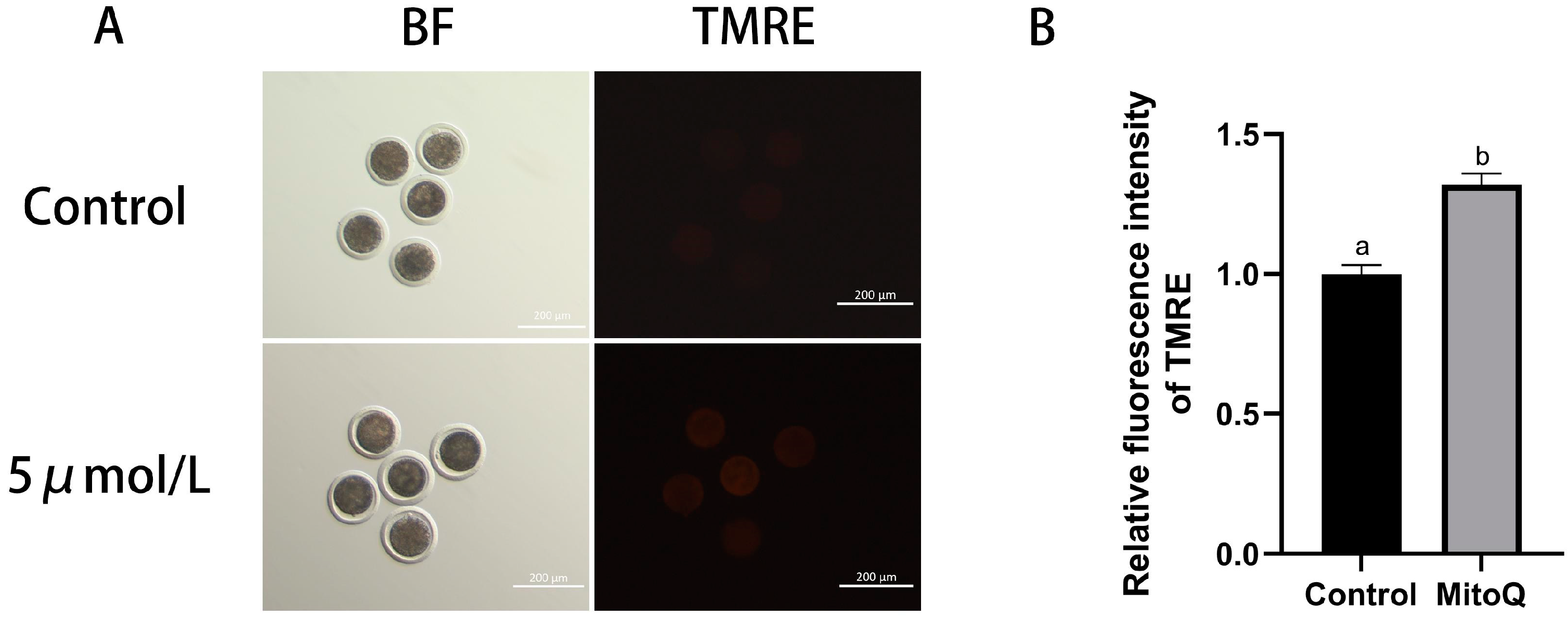
2.5. Assessment of MMP Level
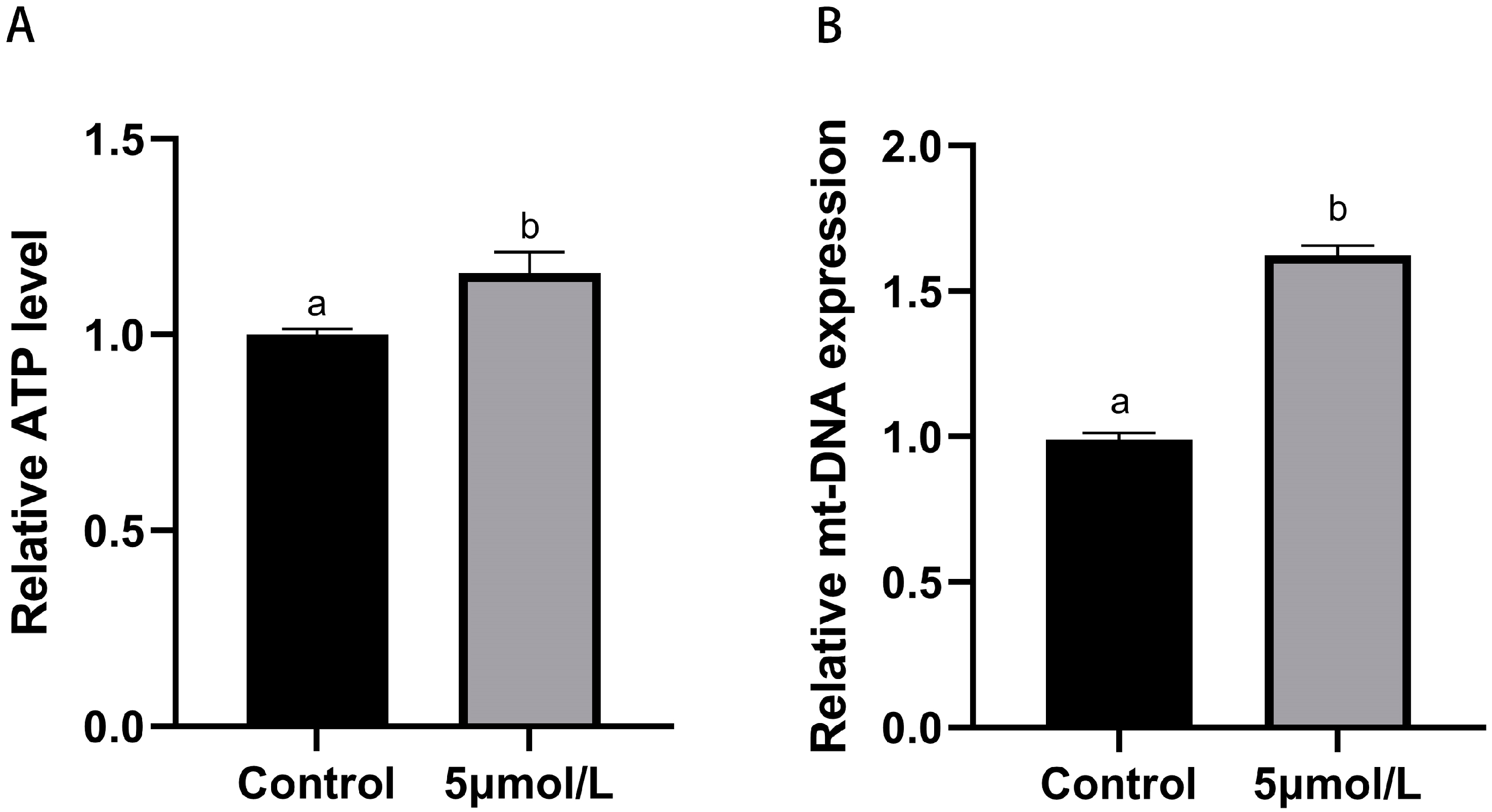
2.6. Assessment of ATP Level
2.7. DNA Extraction and Analysis of Mitochondrial DNA (mt-DNA) Copies Number by qPCR
2.8. Analysis of Gene Expression by qPCR
2.9. Statistical Analysis
3. Results
3.1. Effect of MitoQ on IVM and Embryonic Developmental Capacity
3.2. Influences of MitoQ on ROS and GSH Level in Bovine Oocytes
3.3. Influences of MitoQ on MMP Level in Bovine Oocytes
3.4. Influences of MitoQ on ATP and mt-DNA Copies Number Levels in Bovine Oocytes
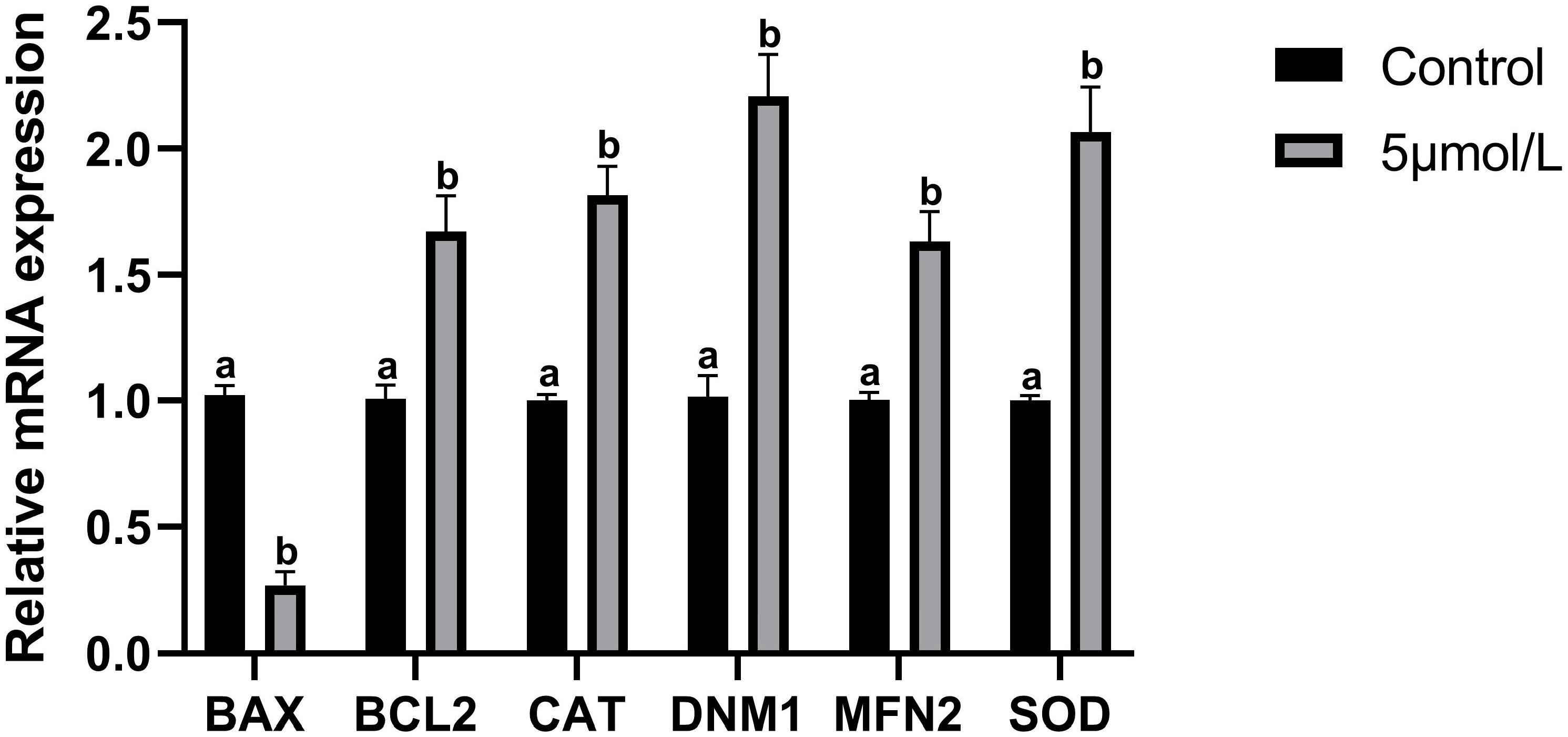
3.5. Influences of MitoQ on Related Genes Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, R.D.; Weigel, K.A.; Fricke, P.M.; Rutledge, J.J.; Leibfried-Rutledge, M.L.; Matthews, D.L.; Schutzkus, V.R. In vitro production of Holstein embryos using sex-sorted sperm and oocytes from selected cull cows. J. Dairy Sci. 2005, 88, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Wooldridge, L.K.; Keane, J.A.; Rhoads, M.L.; Ealy, A.D. Bioactive supplements influencing bovine in vitro embryo development. J. Anim. Sci. 2022, 100, skac091. [Google Scholar] [CrossRef] [PubMed]
- Butkiewicz, A.F.; Amaral, A.; Cerveira-Pinto, M.; Kordowitzki, P. Assessing the Influence of Maternal Age in Bovine Embryos and Oocytes: A Model for Human Reproductive Aging. Aging Dis. 2024, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Leibfried-Rutledge, M.L. Factors determining competence of in vitro produced cattle embryos. Theriogenology 1999, 51, 473–485. [Google Scholar] [CrossRef]
- Amoushahi, M.; Salehnia, M.; Ghorbanmehr, N. The mitochondrial DNA copy number, cytochrome c oxidase activity and reactive oxygen species level in metaphase II oocytes obtained from in vitro culture of cryopreserved ovarian tissue in comparison with in vivo-obtained oocyte. J. Obstet. Gynaecol. Res. 2018, 44, 1937–1946. [Google Scholar] [CrossRef]
- Combelles, C.M.; Gupta, S.; Agarwal, A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod. Biomed. Online 2009, 18, 864–880. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sikka, S. The role of free radicals and antioxidants in reproduction. Curr. Opin. Obstet. Gynecol. 2006, 18, 325–332. [Google Scholar] [CrossRef]
- Prasad, S.; Tiwari, M.; Pandey, A.N.; Shrivastav, T.G.; Chaube, S.K. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 2016, 23, 36. [Google Scholar] [CrossRef]
- Soto-Heras, S.; Paramio, M.-T. Impact of oxidative stress on oocyte competence for in vitro embryo production programs. Res. Vet. Sci. 2020, 132, 342–350. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Takeo, S.; Sato, D.; Kimura, K.; Monji, Y.; Kuwayama, T.; Kawahara-Miki, R.; Iwata, H. Resveratrol improves the mitochondrial function and fertilization outcome of bovine oocytes. J. Reprod. Dev. 2014, 60, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Pasquariello, R.; Verdile, N.; Brevini, T.A.L.; Gandolfi, F.; Boiti, C.; Zerani, M.; Maranesi, M. The Role of Resveratrol in Mammalian Reproduction. Molecules 2020, 25, 4554. [Google Scholar] [CrossRef] [PubMed]
- Marques, T.C.; da Silva Santos, E.C.; Diesel, T.O.; Leme, L.O.; Martins, C.F.; Dode, M.; Alves, B.G.; Costa, F.; de Oliveira, E.B.; Gambarini, M.L. Melatonin reduces apoptotic cells, SOD2 and HSPB1 and improves the in vitro production and quality of bovine blastocysts. Reprod. Domest. Anim. = Zuchthyg. 2018, 53, 226–236. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Peng, W.; Cheng, Y.; Lu, Z.; Zhou, C.; Zhang, Y.; Su, J. Melatonin supplementation during in vitro maturation of oocyte enhances subsequent development of bovine cloned embryos. J. Cell. Physiol. 2019, 234, 17370–17381. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jia, C.; Cheng, W.; Zhang, T.; Tao, R.; Ma, Y.; Si, L.; Xu, Y.; Li, J. The Effect of L-Carnitine Additive During In Vitro Maturation on the Vitrification of Pig Oocytes. Cell. Reprogramming 2020, 22, 198–207. [Google Scholar] [CrossRef]
- Tauskela, J.S. MitoQ—A mitochondria-targeted antioxidant. IDrugs Investig. Drugs J. 2007, 10, 399–412. [Google Scholar]
- Moreira, P.I.; Zhu, X.; Wang, X.; Lee, H.G.; Nunomura, A.; Petersen, R.B.; Perry, G.; Smith, M.A. Mitochondria: A therapeutic target in neurodegeneration. Biochim. Biophys. Acta 2010, 1802, 212–220. [Google Scholar] [CrossRef]
- Coulter, C.V.; Kelso, G.F.; Lin, T.K.; Smith, R.A.; Murphy, M.P. Mitochondrially targeted antioxidants and thiol reagents. Free Radic. Biol. Med. 2000, 28, 1547–1554. [Google Scholar] [CrossRef]
- Smith, R.A.; Murphy, M.P. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann. N. Y. Acad. Sci. 2010, 1201, 96–103. [Google Scholar] [CrossRef]
- Smith, R.A.; Porteous, C.M.; Coulter, C.V.; Murphy, M.P. Selective targeting of an antioxidant to mitochondria. Eur. J. Biochem. 1999, 263, 709–716. [Google Scholar] [CrossRef]
- McManus, M.J.; Murphy, M.P.; Franklin, J.L. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 15703–15715. [Google Scholar] [CrossRef] [PubMed]
- Solesio, M.E.; Prime, T.A.; Logan, A.; Murphy, M.P.; Del Mar Arroyo-Jimenez, M.; Jordán, J.; Galindo, M.F. The mitochondria-targeted anti-oxidant MitoQ reduces aspects of mitochondrial fission in the 6-OHDA cell model of Parkinson’s disease. Biochim. Biophys. Acta 2013, 1832, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. Understanding and preventing mitochondrial oxidative damage. Biochem. Soc. Trans. 2016, 44, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Wang, L.; Song, X.Y. Mitoquinone alleviates vincristine-induced neuropathic pain through inhibiting oxidative stress and apoptosis via the improvement of mitochondrial dysfunction. Biomed. Pharmacother. = Biomed. Pharmacother. 2020, 125, 110003. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, X.; Zhang, F.; Wang, M.; Xu, Y.; Tang, D.; Wang, J.; Qin, Y.; Liu, Y.; Tang, C.; et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017, 11, 297–311. [Google Scholar] [CrossRef]
- Goh, K.Y.; He, L.; Song, J.; Jinno, M.; Rogers, A.J.; Sethu, P.; Halade, G.V.; Rajasekaran, N.S.; Liu, X.; Prabhu, S.D.; et al. Mitoquinone ameliorates pressure overload-induced cardiac fibrosis and left ventricular dysfunction in mice. Redox Biol. 2019, 21, 101100. [Google Scholar] [CrossRef]
- Kao, S.H.; Chao, H.T.; Liu, H.W.; Liao, T.L.; Wei, Y.H. Sperm mitochondrial DNA depletion in men with asthenospermia. Fertil. Steril. 2004, 82, 66–73. [Google Scholar] [CrossRef]
- Kala, M.; Shaikh, M.V.; Nivsarkar, M. Equilibrium between anti-oxidants and reactive oxygen species: A requisite for oocyte development and maturation. Reprod. Med. Biol. 2017, 16, 28–35. [Google Scholar] [CrossRef]
- Hosseinzadeh Shirzeyli, M.; Amidi, F.; Shamsara, M.; Nazarian, H.; Eini, F.; Hosseinzadeh Shirzeyli, F.; Majidi Zolbin, M.; Ghaffari Novin, M.; Daliri Joupari, M. Exposing Mouse Oocytes to MitoQ during In Vitro Maturation Improves Maturation and Developmental Competence. Iran. J. Biotechnol. 2020, 18, e2454. [Google Scholar] [CrossRef]
- Marei, W.F.A.; Mohey-Elsaeed, O.; Pintelon, I.; Leroy, J. Risks of using mitoquinone during in vitro maturation and its potential protective effects against lipotoxicity-induced oocyte mitochondrial stress. J. Assist. Reprod. Genet. 2024, 41, 371–383. [Google Scholar] [CrossRef]
- Bootman, M.D.; Chehab, T.; Bultynck, G.; Parys, J.B.; Rietdorf, K. The regulation of autophagy by calcium signals: Do we have a consensus? Cell Calcium 2018, 70, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Leo, S.; Szabadkai, G.; Rizzuto, R. The mitochondrial antioxidants MitoE(2) and MitoQ(10) increase mitochondrial Ca(2+) load upon cell stimulation by inhibiting Ca(2+) efflux from the organelle. Ann. N. Y. Acad. Sci. 2008, 1147, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Esfandyari, S.; Aleyasin, A.; Noroozi, Z.; Taheri, M.; Khodarahmian, M.; Eslami, M.; Rashidi, Z.; Amidi, F. The Protective Effect of Sulforaphane against Oxidative Stress through Activation of NRF2/ARE Pathway in Human Granulosa Cells. Cell J. 2021, 23, 692–700. [Google Scholar] [CrossRef]
- Yang, Z.; Wei, M.L.; Dong, X.Y. Effects of Yu Linzhu on ovarian function and oocyte mitochondria in natural aging mice. Aging 2021, 13, 23328–23337. [Google Scholar] [CrossRef]
- Ferrucci, L.; Zampino, M. A mitochondrial root to accelerated ageing and frailty. Nat. Rev. Endocrinol. 2020, 16, 133–134. [Google Scholar] [CrossRef]
- Rehman, H.; Liu, Q.; Krishnasamy, Y.; Shi, Z.; Ramshesh, V.K.; Haque, K.; Schnellmann, R.G.; Murphy, M.P.; Lemasters, J.J.; Rockey, D.C.; et al. The mitochondria-targeted antioxidant MitoQ attenuates liver fibrosis in mice. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 14–27. [Google Scholar] [PubMed]
- Marthandan, S.; Murphy, M.P.; Billett, E.; Barnett, Y. An investigation of the effects of MitoQ on human peripheral mononuclear cells. Free Radic. Res. 2011, 45, 351–358. [Google Scholar] [CrossRef]
- Gruber, J.; Fong, S.; Chen, C.B.; Yoong, S.; Pastorin, G.; Schaffer, S.; Cheah, I.; Halliwell, B. Mitochondria-targeted antioxidants and metabolic modulators as pharmacological interventions to slow ageing. Biotechnol. Adv. 2013, 31, 563–592. [Google Scholar] [CrossRef]
- Zuelke, K.A.; Jeffay, S.C.; Zucker, R.M.; Perreault, S.D. Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre-implantation stage embryos. Mol. Reprod. Dev. 2003, 64, 106–112. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kang, J.T.; Park, S.J.; Kim, S.J.; Moon, J.H.; Saadeldin, I.M.; Jang, G.; Lee, B.C. Effect of 7,8-dihydroxyflavone as an antioxidant on in vitro maturation of oocytes and development of parthenogenetic embryos in pigs. J. Reprod. Dev. 2013, 59, 450–456. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; de Simone, A.; Kiddle, G.; Foyer, C.H. Glutathione–linking cell proliferation to oxidative stress. Free Radic. Biol. Med. 2015, 89, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.Y.; Gao, Y.; Jiao, G.Z.; Sun, M.J.; Wu, X.F.; Wang, T.Y.; Li, H.; Tan, J.H. Antioxidant supplementation overcomes the deleterious effects of maternal restraint stress-induced oxidative stress on mouse oocytes. Reproduction 2013, 146, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Cho, H.S.; Yu, I.J. Effect of bovine follicular fluid on reactive oxygen species and glutathione in oocytes, apoptosis and apoptosis-related gene expression of in vitro-produced blastocysts. Reprod. Domest. Anim. = Zuchthyg. 2014, 49, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Smitz, J.E.J.; Sukhikh, G.T.; Mazunin, I. The Role of Mitochondria in Oocyte Maturation. Cells 2021, 10, 2484. [Google Scholar] [CrossRef]
- Yu, X.; Meng, F.; Huang, J.; Li, W.; Zhang, J.; Yin, S.; Zhang, L.; Wang, S. 1-Nitropyrene exposure induces mitochondria dysfunction and impairs oocyte maturation in mice. Ecotoxicol. Environ. Saf. 2022, 242, 113921. [Google Scholar] [CrossRef] [PubMed]
- Sakamuru, S.; Attene-Ramos, M.S.; Xia, M. Mitochondrial Membrane Potential Assay. Methods Mol. Biol. 2016, 1473, 17–22. [Google Scholar] [CrossRef]
- Stojkovic, M.; Machado, S.A.; Stojkovic, P.; Zakhartchenko, V.; Hutzler, P.; Gonçalves, P.B.; Wolf, E. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: Correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol. Reprod. 2001, 64, 904–909. [Google Scholar] [CrossRef]
- Tarazona, A.M.; Rodríguez, J.I.; Restrepo, L.F.; Olivera-Angel, M. Mitochondrial activity, distribution and segregation in bovine oocytes and in embryos produced in vitro. Reprod. Domest. Anim. = Zuchthyg. 2006, 41, 5–11. [Google Scholar] [CrossRef]
- Dalton, C.M.; Szabadkai, G.; Carroll, J. Measurement of ATP in single oocytes: Impact of maturation and cumulus cells on levels and consumption. J. Cell. Physiol. 2014, 229, 353–361. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.Q.; Lu, S.; Guo, Y.L.; Ma, X. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res. 2006, 16, 841–850. [Google Scholar] [CrossRef]
- Zeng, H.T.; Ren, Z.; Yeung, W.S.; Shu, Y.M.; Xu, Y.W.; Zhuang, G.L.; Liang, X.Y. Low mitochondrial DNA and ATP contents contribute to the absence of birefringent spindle imaged with PolScope in in vitro matured human oocytes. Hum. Reprod. 2007, 22, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Reynier, P.; May-Panloup, P.; Chrétien, M.F.; Morgan, C.J.; Jean, M.; Savagner, F.; Barrière, P.; Malthièry, Y. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol. Hum. Reprod. 2001, 7, 425–429. [Google Scholar] [CrossRef]
- Wai, T.; Ao, A.; Zhang, X.; Cyr, D.; Dufort, D.; Shoubridge, E.A. The role of mitochondrial DNA copy number in mammalian fertility. Biol. Reprod. 2010, 83, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Lowes, D.A.; Thottakam, B.M.; Webster, N.R.; Murphy, M.P.; Galley, H.F. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic. Biol. Med. 2008, 45, 1559–1565. [Google Scholar] [CrossRef]
- Fang, L.; Bai, C.; Chen, Y.; Dai, J.; Xiang, Y.; Ji, X.; Huang, C.; Dong, Q. Inhibition of ROS production through mitochondria-targeted antioxidant and mitochondrial uncoupling increases post-thaw sperm viability in yellow catfish. Cryobiology 2014, 69, 386–393. [Google Scholar] [CrossRef]
- Al-Zubaidi, U.; Adhikari, D.; Cinar, O.; Zhang, Q.H.; Yuen, W.S.; Murphy, M.P.; Rombauts, L.; Robker, R.L.; Carroll, J. Mitochondria-targeted therapeutics, MitoQ and BGP-15, reverse aging-associated meiotic spindle defects in mouse and human oocytes. Hum. Reprod. 2021, 36, 771–784. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Z.; Ju, J.; Xing, C.; Li, X.; Shan, M.; Sun, S. DRP1 deficiency induces mitochondrial dysfunction and oxidative stress-mediated apoptosis during porcine oocyte maturation. J. Anim. Sci. Biotechnol. 2020, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, G.S.; Al-Harbi, S.; Almasan, A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol. Biol. 2015, 414, 13–21. [Google Scholar] [CrossRef]
- Chen, X.; Xuan, B.; Xu, D.; Wang, Q.; Cheng, M.; Jin, Y. Crocin supplementation during oocyte maturation enhances antioxidant defence and subsequent cleavage rate. Reprod. Domest. Anim. = Zuchthyg. 2019, 54, 300–308. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Jiao, A.; Sun, J.; Sun, Z.; Zhao, Y.; Han, T.; Zhang, H.; Gao, Q. Effects of limonin on oxidative stress and early apoptosis in oocytes during in vitro maturation. Theriogenology 2024, 218, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Pang, Y.; Hao, H.; Du, W.; Zhao, X.; Zhu, H. Effects of epigallocatechin-3-gallate on bovine oocytes matured in vitro. Asian-Australas. J. Anim. Sci. 2018, 31, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Otsuga, D.; Keegan, B.R.; Brisch, E.; Thatcher, J.W.; Hermann, G.J.; Bleazard, W.; Shaw, J.M. The dynamin-related GTPase, Dnm1p, controls mitochondrial morphology in yeast. J. Cell Biol. 1998, 143, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Jia, B.; Dong, F.; Liu, L.; Rasul, F.; He, J.; Fu, C. Glucose starvation induces mitochondrial fragmentation depending on the dynamin GTPase Dnm1/Drp1 in fission yeast. J. Biol. Chem. 2019, 294, 17725–17734. [Google Scholar] [CrossRef]
- Ren, L.; Fu, B.; Ma, H.; Liu, D. Effects of mechanical delipation in porcine oocytes on mitochondrial distribution, ROS activity and viability after vitrification. CryoLetters 2015, 36, 30–36. [Google Scholar]
- Pin, F.; Huot, J.R.; Bonetto, A. The Mitochondria-Targeting Agent MitoQ Improves Muscle Atrophy, Weakness and Oxidative Metabolism in C26 Tumor-Bearing Mice. Front. Cell Dev. Biol. 2022, 10, 861622. [Google Scholar] [CrossRef]

| Gene | Primer Sequence (5′-3′) | GenBank Accession No. | Product Size (bp) |
|---|---|---|---|
| BAX | F: TGAGCAGATCATGAAGACAGGG | NM_173894.1 | 241 |
| R: CGCCACTCGGAAAAAGACCT | |||
| BCL2 | F: AGGCAGGCGATGAGTTTGAA | NM_001077486.2 | 159 |
| R: AGAAAGAGGGCCAAATGCGA | |||
| CAT | F: GAGGAAACGCCTGTGTGAGA | NM_001035386.2 | 116 |
| R: GGATGCGGGAGCCATATTCA | |||
| DNM1 | F: CTTCACACCTGACCTCGCTT | NM_001076820.1 | 117 |
| R: TTTCTGATGGTGGCCGTGAG | |||
| MFN2 | F: GGGCTCAGAGGAGAAGAGGA | NM_001190269.1 | 72 |
| R: AGCTGTTCGTCCTGGTGAAG | |||
| SOD | F: AAAACACGGTGGGCCAAAAG | NM_174615.2 | 186 |
| R: TTTCCACCTCTGCCCAAGTC | |||
| GAPDH | F: CGCCATCAATGACCCCTTCA | NM_001034034.2 | 71 |
| R: TGCCGTGGGTGGAATCATAC | |||
| mt-ND1 | F: CAGTGAGAATGCCCTCTAGG | 225 | |
| R: TTTACGCCGTACTCCTGT | |||
| β-actin | F: AAGATCAAGATCATCGCGCC | NM_173979.3 | 174 |
| R: GTAACGCAGCTAACAGTCCG |
| Conc. of MitoQ (μM) | NO. of COCs | NO. of Oocytes Matured (PBE) | PBE Rate (% ± SEM) |
|---|---|---|---|
| 0 | 294 | 201 | 68.62 ± 1.17 a |
| 1 | 268 | 194 | 72.16 ± 0.64 b |
| 5 | 311 | 226 | 72.54 ± 0.90 b |
| 10 | 247 | 169 | 68.40 ± 0.57 a |
| Conc. of MitoQ (μM) | NO. of PA Embryos | Cleavage (% ± SEM) | Blastocyst Day7 (% ± SEM) |
|---|---|---|---|
| 0 | 104 | 77 (74.12 ± 0.83) a | 27 (25.88 ± 0.83) a |
| 1 | 113 | 87 (76.95 ± 1.09) b | 35 (30.99 ± 0.44) b |
| 5 | 107 | 83 (77.78 ± 0.93) b | 33 (30.79 ± 0.51) b |
| 10 | 99 | 72 (72.50 ± 0.77) a | 25 (25.03 ± 0.75) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Z.; Shi, J.; Ren, J.; Luo, L.; Liu, D.; Guo, Y.; Sun, B.; Liu, G.; Deng, M.; Li, Y. Mitochondria-Targeted Antioxidant MitoQ Improves In Vitro Maturation and Subsequent Embryonic Development from Culled Cows. Animals 2024, 14, 2929. https://doi.org/10.3390/ani14202929
Feng Z, Shi J, Ren J, Luo L, Liu D, Guo Y, Sun B, Liu G, Deng M, Li Y. Mitochondria-Targeted Antioxidant MitoQ Improves In Vitro Maturation and Subsequent Embryonic Development from Culled Cows. Animals. 2024; 14(20):2929. https://doi.org/10.3390/ani14202929
Chicago/Turabian StyleFeng, Zhihao, Junsong Shi, Jiajie Ren, Lvhua Luo, Dewu Liu, Yongqing Guo, Baoli Sun, Guangbin Liu, Ming Deng, and Yaokun Li. 2024. "Mitochondria-Targeted Antioxidant MitoQ Improves In Vitro Maturation and Subsequent Embryonic Development from Culled Cows" Animals 14, no. 20: 2929. https://doi.org/10.3390/ani14202929
APA StyleFeng, Z., Shi, J., Ren, J., Luo, L., Liu, D., Guo, Y., Sun, B., Liu, G., Deng, M., & Li, Y. (2024). Mitochondria-Targeted Antioxidant MitoQ Improves In Vitro Maturation and Subsequent Embryonic Development from Culled Cows. Animals, 14(20), 2929. https://doi.org/10.3390/ani14202929





