The Application of Duck Embryonic Fibroblasts CCL-141 as a Cell Model for Adipogenesis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Adipogenesis
2.2. Oil Red O Staining and Lipid Droplet Analysis
2.3. Gene Expression Analysis
2.4. Gene Knockout
2.5. Plasmid Transfection and Editing Efficiency Identification
2.6. Pooled Cells with Knocked-Out PPARγ Identification
2.7. Western Blot Analysis
2.8. Cell Counting Kit-8
2.9. Statistical Analysis
3. Results
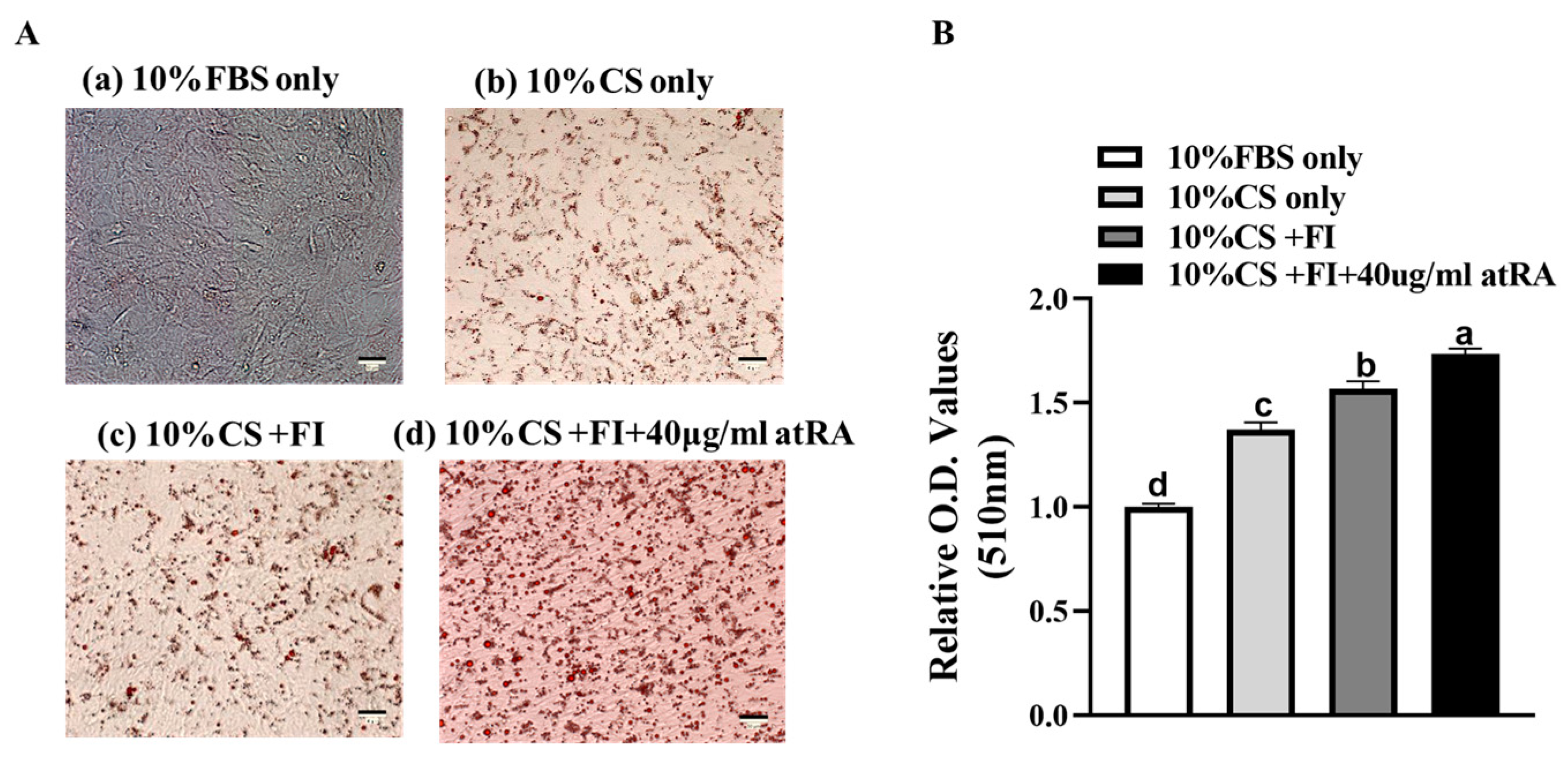
3.1. Induction of Adipogenesis in CCL-141 Cells by Different Culture Components
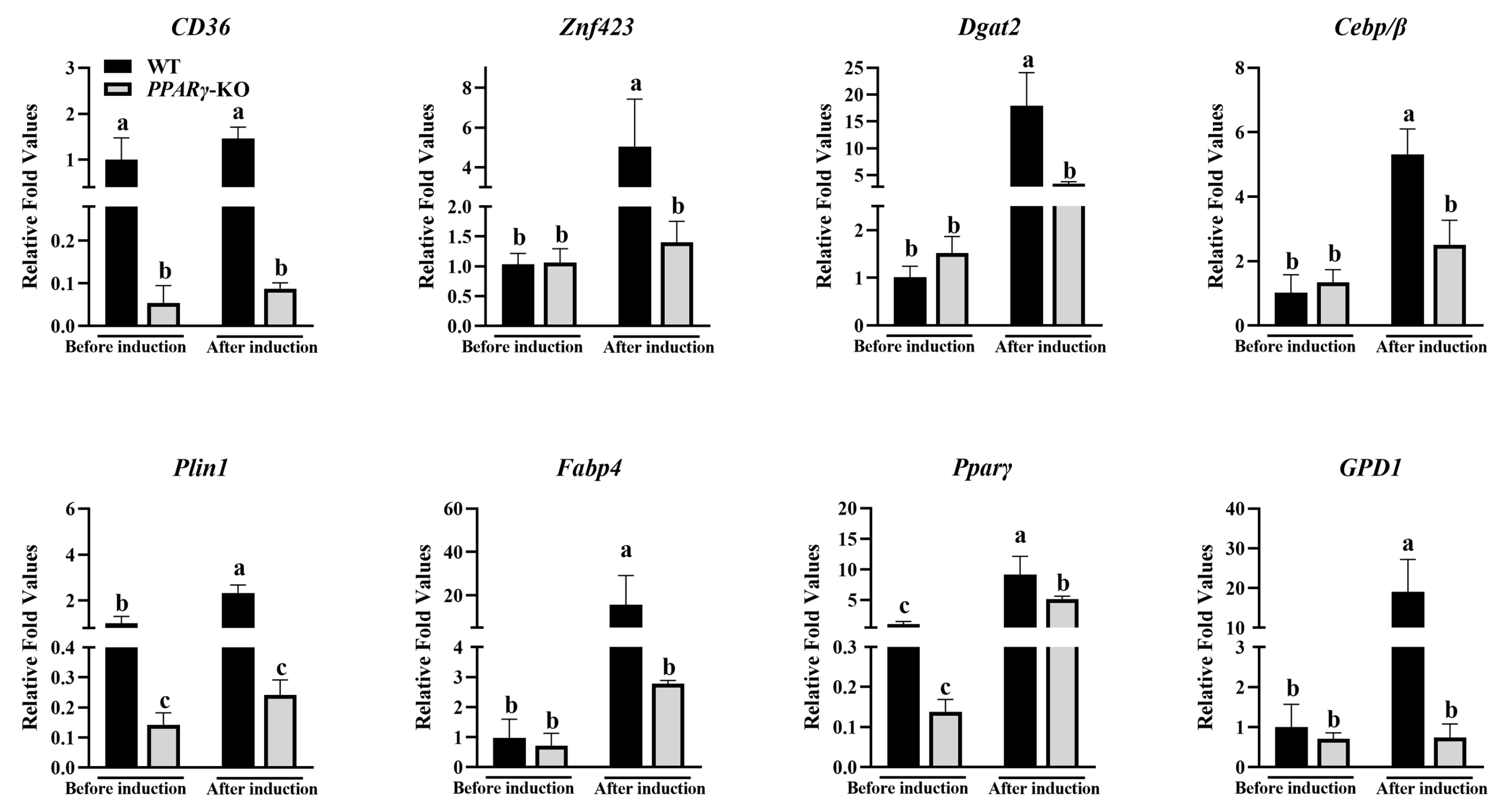
3.2. Expression Levels of Adipogenic Differentiation Marker Genes in CCL-141 Cells under Different Culture Components
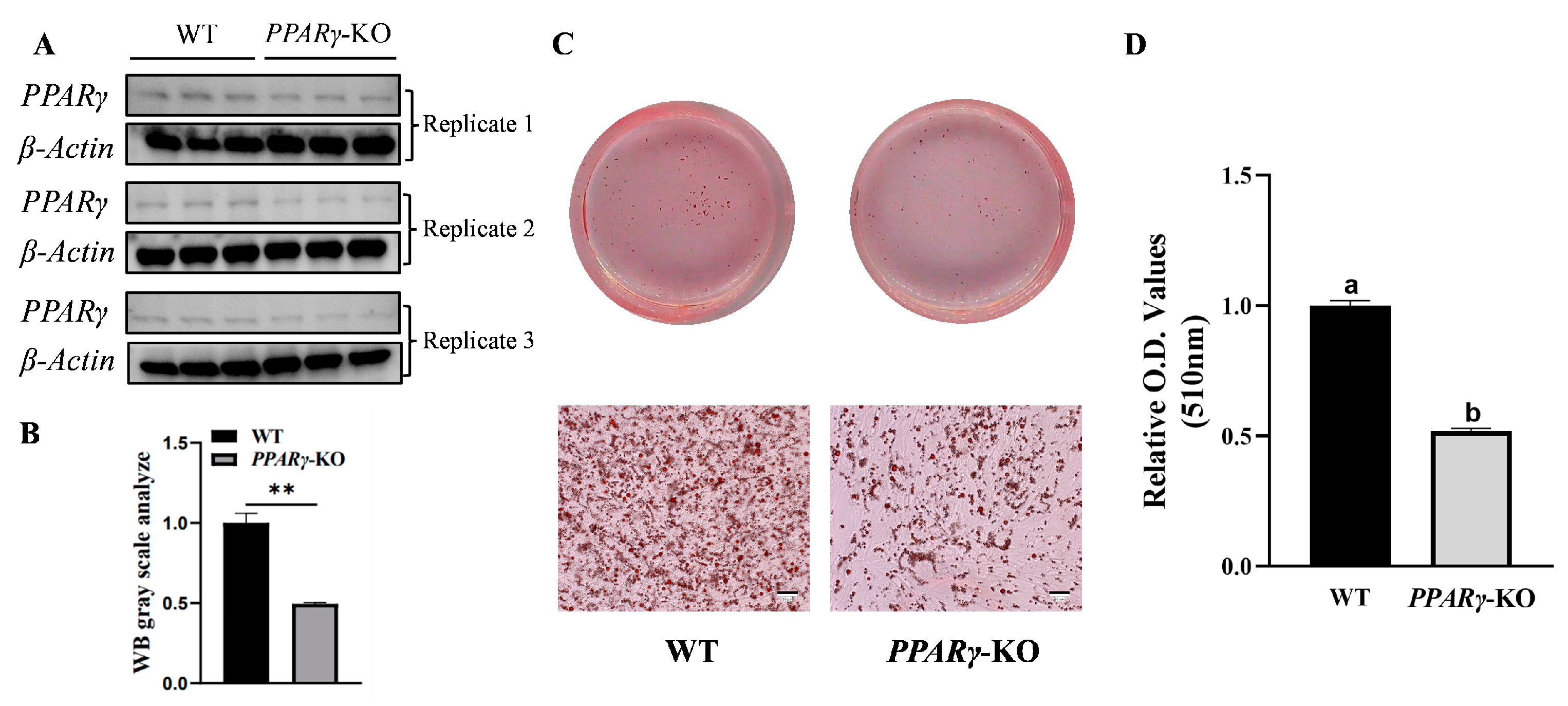
3.3. PPARγ Gene Knockout and Efficiency Identification in CCL-141 Cells
3.4. Inhibition of Expression of Adipogenic Marker Genes in CCL-141 Cells after PPARγ Knockout
3.5. Inhibition of Adipogenesis in CCL-141 Cells after PPARγ Knockout
3.6. Increased Proliferation of CCL-141 Cells after PPARγ Knockout
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hogenkamp, A.; Ehlers, A.; Garssen, J.; Willemsen, L. Allergy modulation by n-3 long chain polyunsaturated fatty acids and fat soluble nutrients of the mediterranean diet. Front. Pharmacol. 2020, 11, 1244. [Google Scholar] [CrossRef] [PubMed]
- Thoene, M.; Van Ormer, M.; Yuil-Valdes, A.; Bruett, T.; Natarajan, S.K.; Mukherjee, M.; Thompson, M.; Nordgren, T.M.; Van Lippevelde, W.; Overby, N.C.; et al. Fat-soluble nutrients and omega-3 fatty acids as modifiable factors influencing preterm birth risk. Placenta 2020, 98, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, T.; Liang, Y.; Jiang, L.; Sui, X. Dietary bioactive lipids: A review on absorption, metabolism, and health properties. J. Agric. Food Chem. 2021, 69, 8929–8943. [Google Scholar] [CrossRef]
- Gonzalez-Gomez, P.L.; Villavicencio, C.P.; Quispe, R.; Schwabl, P.; Cornelius, J.M.; Ramenofsky, M.; Krause, J.S.; Wingfield, J.C. Perspectives on environmental heterogeneity and seasonal modulation of stress response in neotropical birds. Horm. Behav. 2023, 152, 105359. [Google Scholar] [CrossRef] [PubMed]
- Nematbakhsh, S.; Pei, P.C.; Selamat, J.; Nordin, N.; Idris, L.H.; Abdull, R.A. Molecular regulation of lipogenesis, adipogenesis and fat deposition in chicken. Genes 2021, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.F.; Roesler, A.; Kazak, L. Regulation of adipocyte thermogenesis: Mechanisms controlling obesity. Febs J. 2020, 287, 3370–3385. [Google Scholar] [CrossRef]
- Moskaleva, E.Y.; Semochkina, Y.P.; Shuvatova, V.G.; Rodina, A.V.; Krasheninnikova, A.A. Mesenchymal stem cells from mouse adipose tissue stimulate tumor growth. Bull. Exp. Biol. Med. 2019, 167, 145–149. [Google Scholar] [CrossRef]
- Giuliani, G.; Rosina, M.; Reggio, A. Signaling pathways regulating the fate of fibro/adipogenic progenitors (faps) in skeletal muscle regeneration and disease. Febs J. 2022, 289, 6484–6517. [Google Scholar] [CrossRef]
- Huang, C.J.; Choo, K.B. Circular RNA- and microRNA-mediated post-transcriptional regulation of preadipocyte differentiation in adipogenesis: From expression profiling to signaling pathway. Int. J. Mol. Sci. 2023, 24, 4549. [Google Scholar] [CrossRef]
- Huang, C.J.; Choo, K.B.; Chen, C.F. The microRNA-signaling-peroxisome proliferator-activated receptor gamma connection in the modulation of adipogenesis: Bioinformatics projection on chicken. Poult. Sci. 2022, 101, 101950. [Google Scholar] [CrossRef]
- Ding, S.; Li, G.; Chen, S.; Zhu, F.; Hao, J.; Yang, F.; Hou, Z. Comparison of carcass and meat quality traits between lean and fat pekin ducks. Anim. Biosci. 2021, 34, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, X.; Yin, Z.; Hou, Z. The full-length transcriptome provides new insights into the transcript complexity of abdominal adipose and subcutaneous adipose in pekin ducks. Front. Physiol. 2021, 12, 767739. [Google Scholar] [CrossRef]
- Ding, F.; Qiu, J.; Li, Q.; Hu, J.; Song, C.; Han, C.; He, H.; Wang, J. Effects of rosiglitazone on proliferation and differentiation of duck preadipocytes. In Vitro Cell. Dev. Biol.-Anim. 2016, 52, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Yuan, X.; Pan, R.; Yao, W.; Zhong, L.; Song, Q.; Zheng, S.; Wang, Z.; Xu, Q.; et al. Identification of differentially expressed microRNAs during preadipocyte differentiation in Chinese crested duck. Gene 2018, 661, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Xu, Q.; Yuan, X.; Dai, W.; Shen, X.; Wang, Z.; Chang, G.; Wang, Z.; Chen, G. The differentiation of preadipocytes and gene expression related to adipogenesis in ducks (Anas platyrhynchos). PLoS ONE 2018, 13, e196371. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.; Suh, Y.; Cressman, M.; Lee, K. Research note: Adipogenic differentiation of embryonic fibroblasts of chicken, turkey, duck, and quail in vitro by medium containing chicken serum alone. Poult. Sci. 2021, 100, 101277. [Google Scholar] [CrossRef]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef]
- Pham, P.H.; Leacy, A.; Deng, L.; Nagy, E.; Susta, L. Isolation of ontario aquatic bird bornavirus 1 and characterization of its replication in immortalized avian cell lines. Virol. J. 2020, 17, 16. [Google Scholar] [CrossRef]
- Kulprasertsri, S.; Kobayashi, S.; Aoshima, K.; Kobayashi, A.; Kimura, T. Duck tembusu virus induces stronger cellular responses than japanese encephalitis virus in primary duck neurons and fibroblasts. Microbiol. Immunol. 2021, 65, 481–491. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.H.; Suh, Y.; Lee, K. Research note: Potential usage of df-1 cell line as a new cell model for avian adipogenesis. Poult. Sci. 2021, 100, 101057. [Google Scholar] [CrossRef]
- Shui-Sheng, H.; Ling-Zhi, L. Report on the development of waterfowl industry and technology in 2023. Chin. J. Anim. Sci. 2024, 60, 318–321. [Google Scholar]
- Lee, J.; Kim, D.; Karolak, M.C.; Shin, S.; Lee, K. Generation of genome-edited chicken and duck lines by adenovirus-mediated in vivo genome editing. Proc. Natl. Acad. Sci. USA 2022, 119, e2214344119. [Google Scholar] [CrossRef]
- Konoval, O.; Korol, P.; Kostenko, S.; Tabaka, P.; Lizhi, L.; Chepiha, A.; Doroshenko, M.; Sydorenko, O.; Dzhus, P.; Svyrydenko, N.; et al. Efficacy of blastodermal cells and CRISPR/CAS9 method in the creation of transgenic duck (Anas platyrhynchos). Biopolym. Cell 2021, 37, 289–302. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, T.; Zhang, L.; Wang, X.; Liu, N.; Jiang, W.; Fan, X.; Lu, X.; Tian, M.; Wei, L.; et al. The role of duck lgp2 in innate immune response of host anti-tembusu virus. Vet. Microbiol. 2023, 287, 109907. [Google Scholar] [CrossRef]
- Zheng, Q.; Bai, L.; Zheng, S.; Liu, M.; Zhang, J.; Wang, T.; Xu, Z.; Chen, Y.; Li, J.; Duan, Z. Efficient inhibition of duck hepatitis b virus dna by the CRISPR/CAS9 system. Mol. Med. Rep. 2017, 16, 7199–7204. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; Sun, Y.; Sun, D.; Yang, F.; Liu, Y.; Hou, Z. A novel candidate gene cln8 regulates fat deposition in avian. J. Anim. Sci. Biotechnol. 2023, 14, 70. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, Z.; Zhang, F.; Li, X.; Chen, S.; Yang, N.; Porter, T.E.; Hou, Z. Dynamics of transcriptome changes during subcutaneous preadipocyte differentiation in ducks. BMC Genom. 2019, 20, 688. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/CAS systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, T.; Chen, C.H.; Li, W.; Meyer, C.A.; Wu, Q.; Wu, D.; Cong, L.; Zhang, F.; Liu, J.S.; et al. Sequence determinants of improved CRISPR sgRNA design. Genome Res. 2015, 25, 1147–1157. [Google Scholar] [CrossRef]
- Brinkman, E.K.; Chen, T.; Amendola, M.; van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014, 42, e168. [Google Scholar] [CrossRef] [PubMed]
- Concordet, J.P.; Haeussler, M. Crispor: Intuitive guide selection for CRISPR/CAS9 genome editing experiments and screens. Nucleic Acids Res. 2018, 46, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- Stemmer, M.; Thumberger, T.; Del, S.K.M.; Wittbrodt, J.; Mateo, J.L. Cctop: An intuitive, flexible and reliable CRISPR/CAS9 target prediction tool. PLoS ONE 2015, 10, e124633. [Google Scholar] [CrossRef] [PubMed]
- Mallanna, S.K.; Rasool, T.J.; Sahay, B.; Aleyas, A.G.; Ram, H.; Mondal, B.; Nautiyal, B.; Premraj, A.; Sreekumar, E.; Yadav, M.P. Inhibition of anatid herpes virus-1 replication by small interfering RNAs in cell culture system. Virus Res. 2006, 115, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Guo, H.; Deng, Y.; Jiang, J.; Liu, S.; He, W.; Jian, H. Rrm2 regulates osteogenesis of mouse embryo fibroblasts via the wnt/beta-catenin signaling pathway. Exp. Ther. Med. 2022, 24, 605. [Google Scholar] [CrossRef]
- Hwang, M.; Lee, E.J.; Chung, M.J.; Park, S.Y.; Jeong, K.S. Five transcriptional factors reprogram fibroblast into myogenic lineage cells via paraxial mesoderm stage. Cell Cycle 2020, 19, 1804–1816. [Google Scholar] [CrossRef] [PubMed]
- Khuong, T.T.T.; Jeong, D.K. Adipogenic differentiation of chicken epithelial oviduct cells using only chicken serum. In Vitro Cell. Dev. Biol. Anim. 2011, 47, 609–614. [Google Scholar] [CrossRef]
- Gamwell, J.M.; Paphiti, K.; Hodson, L.; Karpe, F.; Pinnick, K.E.; Todorčević, M. An optimised protocol for the investigation of insulin signalling in a human cell culture model of adipogenesis. Adipocyte 2023, 12, 2179339. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Xue, C.; Wang, J.; Wang, Y.; Xu, J.; Li, Z. Exogenous natural epa-enriched phosphatidylcholine and phosphatidylethanolamine ameliorate lipid accumulation and insulin resistance via activation of pparα/γ in mice. Food Funct. 2020, 11, 8248–8258. [Google Scholar] [CrossRef]
- Liu, L.; Chen, C.; Dong, Y.; Cheng, Y.; You, C.; Wang, S.; Ma, H.; Li, Y. Insulin activates lc-pufa biosynthesis of hepatocytes by regulating the pi3k/akt/mtor/srebp1 pathway in teleost siganus canaliculatus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2022, 260, 110734. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.; Suh, Y.; Ko, J.; Lee, K. Transdifferentiation of myoblasts into adipocytes by all-trans-retinoic acid in avian. Front. Cell. Dev. Biol. 2022, 10, 856881. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Zhang, C.; Bai, J.; Song, J.; Hao, B.; Zhang, L.; Xia, G. Effects of vitamin a on yanbian yellow cattle and their preadipocytes by activating akt/mtor signaling pathway and intestinal microflora. Animals 2022, 12, 1477. [Google Scholar] [CrossRef] [PubMed]
- Safonova, I.; Darimont, C.; Amri, E.Z.; Grimaldi, P.; Ailhaud, G.; Reichert, U.; Shroot, B. Retinoids are positive effectors of adipose cell differentiation. Mol. Cell. Endocrinol. 1994, 104, 201. [Google Scholar] [CrossRef] [PubMed]
- Bonet, M.L.; Ribot, J.; Palou, A. Lipid metabolism in mammalian tissues and its control by retinoic acid. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, F.; Paquin, J. Differential effects of retinoids and inhibitors of erk and p38 signaling on adipogenic and myogenic differentiation of p19 stem cells. Stem Cells Dev. 2013, 22, 2003–2016. [Google Scholar] [CrossRef]
- Bonet, M.L.; Ribot, J.; Felipe, F.; Palou, A. Vitamin A and the regulation of fat reserves. Cell. Mol. Life Sci. 2003, 60, 1311–1321. [Google Scholar] [CrossRef]
- He, B.; Wang, X.; Jin, X.; Xue, Z.; Zhu, J.; Wang, C.; Jin, Y.; Fu, Z. Beta-cypermethrin promotes the adipogenesis of 3t3-l1 cells via inducing autophagy and shaping an adipogenesis-friendly microenvironment. Acta Biochim. Biophys. Sin. 2020, 52, 821–831. [Google Scholar] [CrossRef]
- Hodson, L.; Gunn, P.J. The regulation of hepatic fatty acid synthesis and partitioning: The effect of nutritional state. Nat. Rev. Endocrinol. 2019, 15, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Heeren, J.; Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef]
- Faghfouri, A.H.; Khajebishak, Y.; Payahoo, L.; Faghfuri, E.; Alivand, M. Ppar-gamma agonists: Potential modulators of autophagy in obesity. Eur. J. Pharmacol. 2021, 912, 174562. [Google Scholar] [CrossRef]
- Dacic, M.; Shibu, G.; Rogatsky, I. Physiological convergence and antagonism between gr and ppargamma in inflammation and metabolism. Adv. Exp. Med. Biol. 2022, 1390, 123–141. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, C.; Sanchez-Quesada, C.; Martinez-Ramirez, M.J.; Gaforio, J.J. Ppargamma gene as a possible link between acquired and congenital lipodystrophy and its modulation by dietary fatty acids. Nutrients 2022, 14, 4742. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Iglesias, S.; Crocker, M.; OCallaghan, M.; Darling, A.; García-Cazorla, A.; Domingo-Jiménez, R.; Castro, A.; Fernández-Pombo, A.; Ruibal, Á.; Aguiar, P.; et al. Celia’s encephalopathy and c.974dupg in bscl2 gene: A hidden change in a known variant. Neurogenetics 2019, 20, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Bahrami-Nejad, Z.; Zhang, Z.B.; Tholen, S.; Sharma, S.; Rabiee, A.; Zhao, M.L.; Kraemer, F.B.; Teruel, M.N. Early enforcement of cell identity by a functional component of the terminally differentiated state. PLoS Biol. 2022, 20, e3001900. [Google Scholar] [CrossRef]
- Regueira, M.; Gorga, A.; Rindone, G.M.; Pellizzari, E.H.; Cigorraga, S.B.; Galardo, M.N.; Riera, M.F.; Meroni, S.B. Apoptotic germ cells regulate sertoli cell lipid storage and fatty acid oxidation. Reproduction 2018, 156, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Chou, C.A.; Chen, W.Y.; Yang, J.L.; Lee, W.C.; Chen, J.B.; Lee, C.T.; Li, L.C. Empagliflozin ameliorates free fatty acid induced-lipotoxicity in renal proximal tubular cells via the ppargamma/cd36 pathway in obese mice. Int. J. Mol. Sci. 2021, 22, 12408. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Ouyang, Y.; Miao, Y. Peroxisome proliferator-activated receptor gamma regulates genes involved in milk fat synthesis in mammary epithelial cells of water buffalo. Anim. Sci. J. 2021, 92, e13537. [Google Scholar] [CrossRef]
- Lebeck, J.; Cheema, M.U.; Skowronski, M.T.; Nielsen, S.; Praetorius, J. Hepatic aqp9 expression in male rats is reduced in response to pparalpha agonist treatment. Am. J. Physiol. Gastroint. Liver Physiol. 2015, 308, G198–G205. [Google Scholar] [CrossRef]
- Gupta, R.K.; Arany, Z.; Seale, P.; Mepani, R.J.; Ye, L.; Conroe, H.M.; Roby, Y.A.; Kulaga, H.; Reed, R.R.; Spiegelman, B.M. Transcriptional control of preadipocyte determination by zfp423. Nature 2010, 464, 619–623. [Google Scholar] [CrossRef]
- Clarke, S.L.; Robinson, C.E.; Gimble, J.M. Caat/enhancer binding proteins directly modulate transcription from the peroxisome proliferator-activated receptor gamma 2 promoter. Biochem. Biophys. Res. Commun. 1997, 240, 99–103. [Google Scholar] [CrossRef]
- Deng, K.; Ren, C.; Fan, Y.; Liu, Z.; Zhang, G.; Zhang, Y.; You, P.; Wang, F. Mir-27a is an important adipogenesis regulator associated with differential lipid accumulation between intramuscular and subcutaneous adipose tissues of sheep. Domest. Anim. Endocrinol. 2020, 71, 106393. [Google Scholar] [CrossRef]
- Zhao, J.; Xing, Q.; Lu, Y.; Wang, Z. Fatty acid composition in different animal products. Wei Sheng Yan Jiu 2018, 47, 254–259. [Google Scholar] [PubMed]
- Shin, D.M.; Kim, D.H.; Yune, J.H.; Kwon, H.C.; Kim, H.J.; Seo, H.G.; Han, S.G. Oxidative stability and quality characteristics of duck, chicken, swine and bovine skin fats extracted by pressurized hot water extraction. Food Sci. Anim. Resour. 2019, 39, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Kim, I.H. Effects of dietary lipid sources on growth performance and carcass traits in pekin ducks. Poult. Sci. 2020, 99, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Yang, T.; Jia, W.; Bai, M.; Bai, H.; Wang, Z.; Chen, G.; Jiang, Y.; Chang, G. Effects of fatty-type and lean-type on growth performance and lipid droplet metabolism in pekin ducks. Animals 2022, 12, 2268. [Google Scholar] [CrossRef]
- Bai, S.; Wang, G.; Zhang, W.; Zhang, S.; Rice, B.B.; Cline, M.A.; Gilbert, E.R. Broiler chicken adipose tissue dynamics during the first two weeks post-hatch. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2015, 189, 115–123. [Google Scholar] [CrossRef]
- Niu, J.L.; Wei, L.Q.; Luo, Y.Q.; Yang, W.T.; Lu, Q.C.; Zheng, X.X.; Niu, Y.J.; Sheng, W.; Cheng, H.; Zhang, W.J.; et al. Fermented cottonseed meal improves production performance and reduces fat deposition in broiler chickens. Anim. Biosci. 2021, 34, 680–691. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.-D.; Li, X.-Q.; Liu, Y.-T.; Ge, M.-Q.; Hou, Z.-C. The Application of Duck Embryonic Fibroblasts CCL-141 as a Cell Model for Adipogenesis. Animals 2024, 14, 2973. https://doi.org/10.3390/ani14202973
Sun D-D, Li X-Q, Liu Y-T, Ge M-Q, Hou Z-C. The Application of Duck Embryonic Fibroblasts CCL-141 as a Cell Model for Adipogenesis. Animals. 2024; 14(20):2973. https://doi.org/10.3390/ani14202973
Chicago/Turabian StyleSun, Dan-Dan, Xiao-Qin Li, Yong-Tong Liu, Meng-Qi Ge, and Zhuo-Cheng Hou. 2024. "The Application of Duck Embryonic Fibroblasts CCL-141 as a Cell Model for Adipogenesis" Animals 14, no. 20: 2973. https://doi.org/10.3390/ani14202973
APA StyleSun, D.-D., Li, X.-Q., Liu, Y.-T., Ge, M.-Q., & Hou, Z.-C. (2024). The Application of Duck Embryonic Fibroblasts CCL-141 as a Cell Model for Adipogenesis. Animals, 14(20), 2973. https://doi.org/10.3390/ani14202973





