Pre-Embryonic Period Observation Shows a Unique Reproductive Strategy of the Critically Endangered Anji Salamander (Hynobius amjiensis)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Laboratory Observation
3. Results
3.1. Cleavage of the Ovum Stage (Stages 1–7; Figure 1)

3.2. Blastocyst Stage (Stages 8–10; Figure 2)

3.3. Proto-Intestinal Embryo Stage (Stages 11–13; Figure 3)

3.4. Neural Embryonic Stage (Stages 14–18; Figure 4)

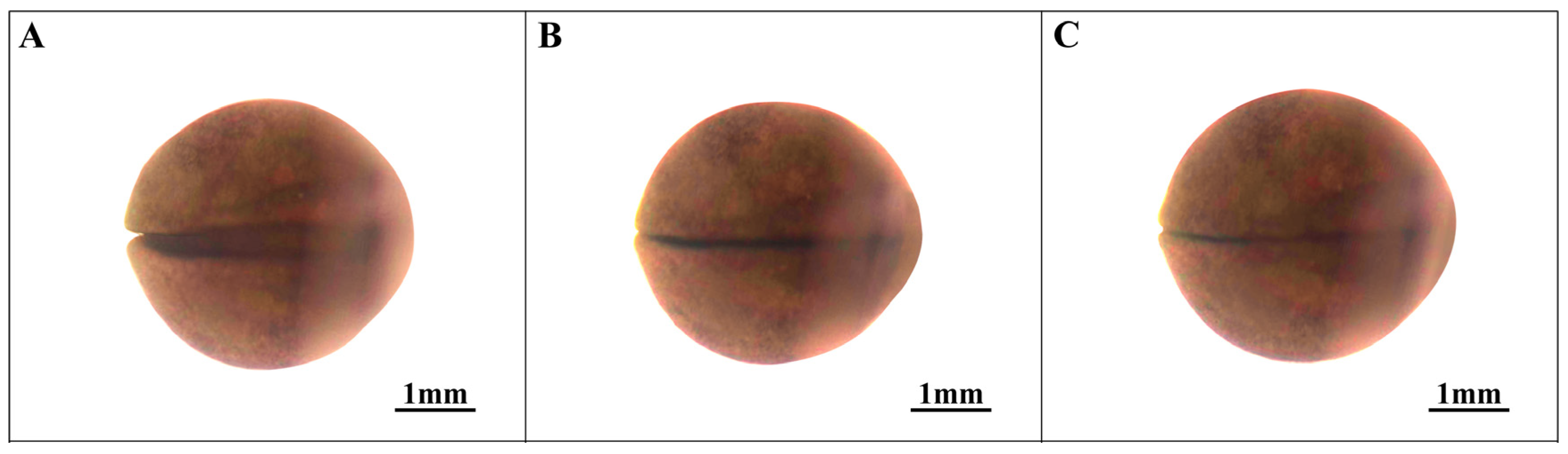
3.5. Organ Formation Stage (Stages 19–21; Figure 6)

3.6. Pre-Incubation Stage (Stages 22–23; Figure 7 and Figure 8)
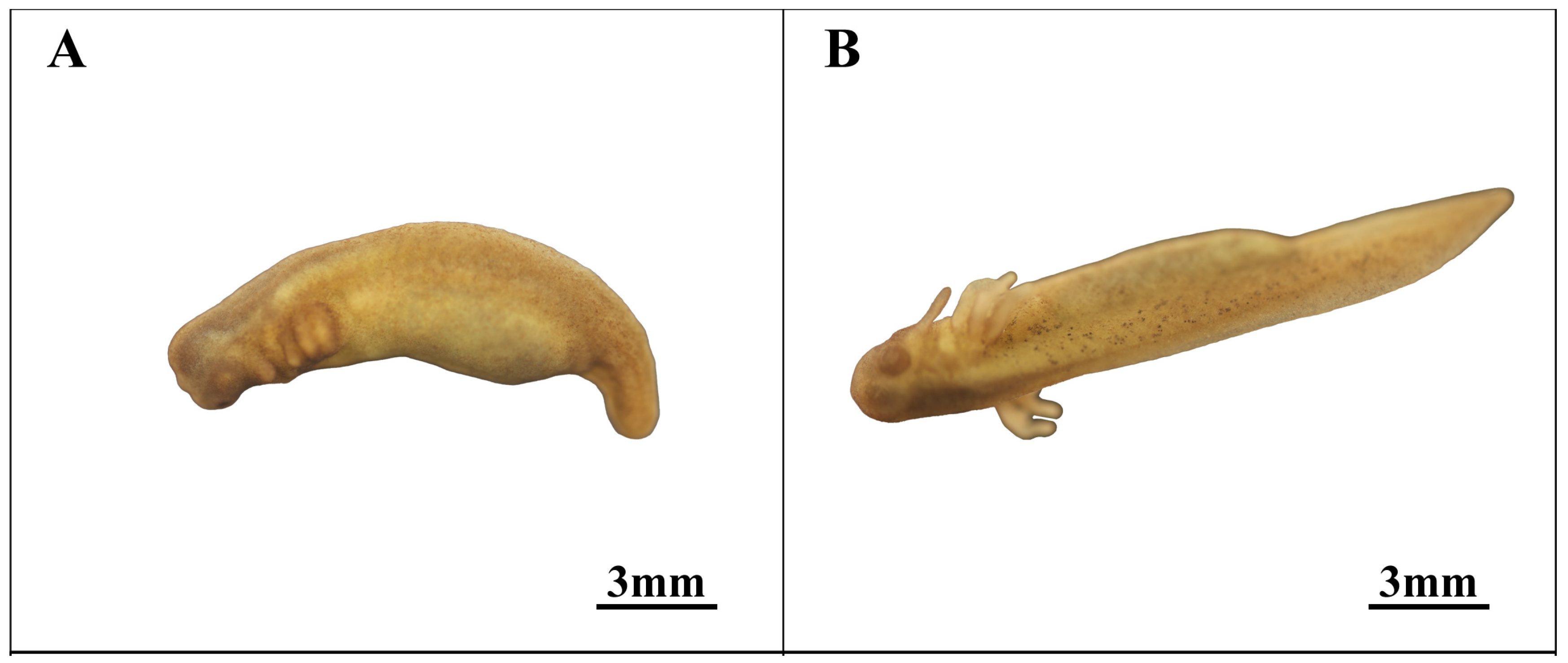

3.7. Incubation Stage (Stage 25; Figure 8)
4. Discussion
4.1. Yolk and Proto-Intestinal Embryo Development
| Species | Body Length (mm) | Egg Diameter (mm) | Number of Eggs/Session | Habitat Altitude (m) |
|---|---|---|---|---|
| Hynobius amjiensis | 150.7–180.5 | 1.6 | 50–150 | 1300–1600 |
| Hynobius formosanus [60] | 58.0–98.0 | 4.3–5.2 | 26–32 | 1800–3650 |
| Hynobius sonani [61] | 90.0–129.0 | 5.0 | 32 | 2600–3100 |
| Hynobius leechii [23] | 85.0–142.0 | 3.0–4.0 | 56–106 | 200–850 |
| Hynobius chinensis [62] | 165.0–205.0 | 2.4–2.8 | 66–189 | 1400–1500 |
| Hynobius guabangshanensis [24] | 125.0–151.0 | 2.6–2.8 | 130–165 | 720 |
| Hynobius yiwuensis | 83.9–136.5 | 2.5–3.1 | 85–96 | 100–200 |
4.2. Reproduction Strategy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, D.; Xu, T.J.; Sun, Y.N. Hynobiidae origin in middle Cretaceous corroborated by the new mitochondrial genome of Hynobius chinensis. Mar. Genom. 2015, 22, 37–44. [Google Scholar] [CrossRef]
- Fei, L.; Hu, S.Q.; Ye, C.Y. Amphibia: Gymnophiona, Urodela. In Fauna Sinica; Science Press: Beijing, China, 2006; Volume 1, pp. 1–471. [Google Scholar]
- Chen, C.S.; Yang, J.; Wu, Y.K.; Fan, Z.Y.; Lu, W.W.; Chen, S.H.; Yu, L.P. The breeding ecology of a critically endangered salamander, Hynobius amjiensis (Caudata: Hynobiidae), endemic to eastern China. Asian Herpetol. Res. 2016, 7, 3–58. [Google Scholar]
- Gu, H.Q.; Ma, X.M.; Wang, J.; Du, Z.H.; Lou, X.Q. Population size and dynamics of Hynobius amjiensis. Sichuan J. Zool. 1999, 18, 9–11. [Google Scholar]
- Duellman, W.E.; Trueb, L. Biology of Amphibians; McGraw Hill Inc.: New York, NY, USA, 1986; pp. 1–670. [Google Scholar]
- Wake, D.B.; Roth, G. The linkage between ontogeny and phylogeny in the evolution of complex systems. In Complex Organismal Functions: Integration and Evolution in Vertebrates; Wiley: Hoboken, NJ, USA, 1989. [Google Scholar]
- Hanken, J. Larvae in Amphibian Development and Evolution. In The Origin and Evolution of Larval Forms; Academic Press: Cambridge, MA, USA, 1999; pp. 61–108. [Google Scholar]
- Wells, K.D. The Ecology and Behavior of Amphibians; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Lack, D.L. Data from: Darwin’s Finches: An Essay on the General Biological Theory of Evolution; Harper: New York, NY, USA, 1947. [Google Scholar]
- Gomez-Mestre, I.; Pyron, R.A.; Wiens, J.J. Phylogenetic analyses reveal unexpected patterns in the evolution of reproductive modes. Evolution 2012, 66, 3687–3700. [Google Scholar] [CrossRef]
- Rollinson, N.; Hutchings, J.A. Why does egg size increase with maternal size? Effects of egg size and egg density on offspring phenotypes in Atlantic salmon (Salmo salar). Evol. Ecol. Res. 2010, 12, 949–960. [Google Scholar]
- Liu, Y.J.; Zhou, S.J.; Hu, J.; Ma, Z.H. Research progress on environmental stress in aquatic animals. J. Tianjin Agric. Univ. 2018, 25, 70–76. [Google Scholar]
- Xu, F.; Yang, W.K.; Li, Y.M. Enlarged egg size increases offspring fitness of a frog species on the Zhoushan Archipelago of China. Sci. Rep. 2019, 9, 11653. [Google Scholar] [CrossRef]
- Furness, A.I.; Venditti, C.; Capellini, I. Terrestrial reproduction and parental care drive rapid evolution in the trade-off between offspring size and number across amphibians. PLoS Biol. 2022, 20, e3001495. [Google Scholar] [CrossRef]
- Yang, J.; Chen, S.H.; Chen, S.H.; Ding, P.; Fan, Z.Y.; Lu, Y.W.; Yu, L.P.; Lin, H.D. Population genetic structure of critically endangered salamander (Hynobius amjiensis) in China: Recommendations for conservation. Genet. Mol. Res. 2016, 15, gmr-15027733. [Google Scholar] [CrossRef]
- Fu, J.Z.; Mark, H.H.; Liu, Z.Z.; Zeng, X.M. Genetic divergence of the southeastern Chinese salamanders of the genus Hynobius. Acta Zool. Sin. 2003, 49, 585–591. [Google Scholar]
- Weisrock, D.W.; Papenfuss, T.J.; Macey, J.R.; Litvinchuk, S.N.; Polymeni, R.; Ugurtas, I.H.; Larson, A. A molecular assessment of phylogenetic relationships and lineage accumulation rates within the family Salamandridae (Amphibia, Caudata). Mol. Phylogenet. Evol. 2006, 41, 368–383. [Google Scholar] [CrossRef]
- Crossland, M.R.; Shine, R. Cues for cannibalism: Cane toad tadpoles use chemical signals to locate and consume conspecific eggs. Oikos 2010, 120, 327–332. [Google Scholar] [CrossRef]
- Crossland, M.R.; Shine, R.; Haramura, T. A biological invasion reduces rates of cannibalism by Japanese toad tadpoles. Sci. Rep. 2023, 13, 9587. [Google Scholar] [CrossRef]
- Dopazo, H.; Alberch, P. Preliminary results on optional viviparity and intrauterine siblicide in Salamandra salamandra populations from northern Spain. Mertensiella 1994, 4, 125–138. [Google Scholar]
- Greven, H. Survey of the oviduct of salamandrids with special reference to the viviparous species. J. Exp. Zool. 1998, 282, 507–525. [Google Scholar] [CrossRef]
- Buckley, D.; Alcobendas, M.; Garcia-Paris, M.; Wake, M.H. Heterochrony, cannibalism, and the evolution of viviparity in Salamandra salamandra. Evol. Dev. 2007, 9, 105–115. [Google Scholar] [CrossRef]
- Park, Y.U.; Yoon, C.S.; Kim, J.H.; Park, J.H.; Cheong, S.W. Numerical variations and spontaneous malformations in the early embryos of the Korean salamander, Hynobius leechii, in the farmlands of Korea. Environ. Toxicol. 2010, 25, 533–544. [Google Scholar] [CrossRef]
- Mi, X.Q.; Deng, X.J.; Guo, K.J.; Niu, Y.D.; Zhou, Y. Preliminary observations on early embryonic development of Hynobius guabangshanensis. Sichuan J. Zool. 2007, 26, 377–378+239. [Google Scholar]
- Hurney, C.A.; Babcock, S.K.; Shook, D.R.; Pelletier, T.M. Normal table of embryonic development in the four-toed salamander, Hemidactylium scutatum. Mech. Dev. 2015, 136, 99–110. [Google Scholar] [CrossRef]
- Luo, J.; Xiao, Y.; Luo, K.; Huang, X. Embryonic development and organogenesis of Chinese giant salamander, Andrias davidianus. Prog. Nat. Sci. 2007, 17, 1303–1311. [Google Scholar]
- Nieuwkoop, P.D.; Faber, J. Normal Table of Xenopus Laevis (Daudin); Garland Publishing: New York, NY, USA, 1994; p. 243. [Google Scholar]
- Zahn, N.; James, Z.C.; Ponferrada, V.G.; Adams, D.S. Normal table of Xenopus development: A new graphical resource. Development 2022, 149, dev200356. [Google Scholar] [CrossRef]
- Bordzilovskaya, N.P.; Dettlaff, T.A.; Duhon, S.T.; Malacinski, G.M. Developmental-stage series of axolotl embryos. In Developmental Biology of the Axolotl; Armstrong, J.B., Malacinski, G.M., Eds.; Oxford University Press: New York, NY, USA, 1989; pp. 201–219. [Google Scholar]
- Collazo, A.; Keller, R. Early development of Ensatina eschscholtzii: An amphibian with a large, yolky egg. EvoDevo 2010, 2, 13. [Google Scholar] [CrossRef]
- Xiong, R.C.; Jiang, J.P.; Fei, L.; Wang, B. Embryonic development of the concave-eared torrent frog with its significance on taxonomy. Zool. Res. 2010, 31, 490–498. [Google Scholar]
- Hervas, F.; Torres, K.; Montenegro, P.; Pino, E.M.D. Development and gastrulation in Hyloxalus vertebralis and Dendrobates auratus (Anura: Dendrobatidae). Amphib. Reptile Conse. 2015, 8, 121–135. [Google Scholar]
- Salazar, N.M.; Del, P.E. Early development of the glass frogs Hyalinobatrachium fleischmanni and Espadarana callistomma (Anura: Centrolenidae) from cleavage to tadpole hatching. Amphib. Reptile Conse. 2015, 8, 89–106. [Google Scholar]
- Jiang, P.; Nelson, J.D.; Leng, N.; Collins, M. Analysis of embryonic development in the unsequenced axolotl: Waves of transcriptomic upheaval and stability. Dev. Biol. 2016, 426, 143–154. [Google Scholar] [CrossRef]
- Zou, P.Z.; Wen, C.Y.; Xu, J.; Chen, J.R. Preliminary study on early embryonic development of Fejervarya limnocharis. Chin. J. Zool. 2001, 36, 5. [Google Scholar]
- Xie, F.; Fei, L.; Li, C.; Ye, C.Y. Study on early individual development of Tylototriton zhenhaiensis. Chin. J. Zool. 2001, 36, 21–25. [Google Scholar]
- Xiang, S.J.; Deng, X.J.; Xu, J.; Xiao, Z.L. Early embryonic development of Tylototriton wenxianensis. Chin. J. Zool. 2010, 45, 127–132. [Google Scholar]
- Yang, G.H.; Yang, Z.Z.; Li, M.R.; Wang, Z.Q.; Song, Y. Preliminary observations on the embryonic development of Cynops orientalis. Bull. Biol. 2011, 46, 51–52+63. [Google Scholar]
- Bernabò, I.; Brunelli, E. Comparative morphological analysis during larval development of three syntopic newt species (Urodela: Salamandridae). Eur. Zool. J. 2019, 86, 38–53. [Google Scholar] [CrossRef]
- Fagotto, F.; Maxfield, F.R. Changes in yolk platelet pH during Xenopus laevis development correlate with yolk utilization. J. Cell Sci. 1995, 107, 3325–3337. [Google Scholar] [CrossRef]
- Ward, R.T. The origin of protein and fatty yolk in Rana pipiens IV. Secondary vesicular yolk formation in frog oocytes. Tissue Cell 1978, 10, 525–534. [Google Scholar] [CrossRef]
- Mallya, S.K.; Partin, J.S.; Valdizan, M.C.; Lennarz, W.J. Proteolysis of the major yolk glycoproteins is regulated by acidification of the yolk platelets in sea urchin embryos. J. Cell Biol. 1992, 117, 1211–1221. [Google Scholar] [CrossRef]
- Ramos, I.; Machado, E.; Masuda, H.; Gomes, F. Open questions on the functional biology of the yolk granules during embryo development. Mol. Reprod. Dev. 2022, 89, 86–94. [Google Scholar] [CrossRef]
- Fagotto, F. Regulation of yolk degradation, or how to make sleepy lysosomes. J. Cell Sci. 1996, 108, 3645–3647. [Google Scholar] [CrossRef]
- Fagotto, F. Yolk degradation in tick eggs: II. Evidence that cathepsin L-like proteinase is stored as a latent, acid-activable proenzyme. Arch. Insect Biochem. Physiol. 1990, 14, 237–252. [Google Scholar] [CrossRef]
- Fausto, A.M.; Gambellini, G.; Mazzini, M.; Cecchettini, A.; Masetti, M.; Giorgi, F. Yolk granules are differentially acidified during embryo development in the stick insect Carausius morosus. Cell Tissue Res. 2001, 305, 433–443. [Google Scholar] [CrossRef]
- Motta, L.S.; da Silva, W.S.; Oliveira, D.M.; de Souza, W.; Machado, E.A. A new model for proton pumping in animal cells: The role of pyrophosphate. Insect Biochem. Mol. Biol. 2004, 34, 19–27. [Google Scholar] [CrossRef]
- Almeida, E.D.; Dittz, U.; Pereira, J.; Walter-Nuno, A.B.; Paiva-Silva, G.O.; Lacerda-Abreu, M.A.; Meyer-Fernandes, J.R.; Ramos, I. Functional characterization of maternally accumulated hydrolases in the mature oocytes of the vector Rhodnius prolixus reveals a new protein phosphatase essential for the activation of the yolk mobilization and embryo development. Front. Physiol. 2023, 14, 1142433. [Google Scholar] [CrossRef]
- Schuel, H.; Wilson, W.L.; Wilson, J.R.; Bressler, R.S. Heterogeneous distribution of ‘lysosomal’ hydrolases in yolk platelets isolated from unfertilized sea urchin eggs by zonal centrifugation. Dev. Biol. 1975, 46, 404–412. [Google Scholar] [CrossRef]
- Nussenzveig, R.H.; Oliveira, P.L.; Masuda, H. Identification of yolk platelet-associated hydrolases in the oocytes of Rhodnius prolixus. Arch. Insect Biochem. Physiol. 1992, 21, 253–262. [Google Scholar] [CrossRef]
- Busson-Mabillot, S. Endosomes transfer yolk proteins to lysosomes in the vitellogenetic oocyte of the trout. Biol. Cell 1984, 51, 53–66. [Google Scholar] [CrossRef]
- Fagotto, F. Yolk degradation in tick eggs: III. Developmentally regulated acidification of the yolk spheres. Dev. Growth Differ. 1991, 33, 57–66. [Google Scholar] [CrossRef]
- Fagotto, F.; Maxfield, F.R. Yolk platelets in Xenopus oocytes maintain an acidic internal pH which may be essential for sodium accumulation. Biol. Cell 1994, 125, 1047–1056. [Google Scholar] [CrossRef]
- Jorgensen, P.; Steen, J.A.J.; Steen, H.; Kirschner, M.W. The mechanism and pattern of yolk consumption provide insight into embryonic nutrition in Xenopus. Development 2009, 136, 1539–1548. [Google Scholar] [CrossRef]
- Komazaki, S.; Hiruma, T. Degradation of yolk platelets in the early amphibian embryo is regulated by fusion with late endosomes. Dev. Growth Differ. 1999, 41, 173–181. [Google Scholar] [CrossRef]
- Perona, R.J.C.B.; Vallejo, C.G. Degradation of yolk in the brine shrimp Artemia. Biochemical and morphological studies on the involvement of the lysosomal system. Biol. Cell 1988, 63, 361–368. [Google Scholar] [CrossRef]
- Selman, G.G.; Pawsey, G.J. The utilization of yolk platelets by tissues of Xenopus embryos studied by a safranin staining method. J. Embryol. Exp. Morphol. 1965, 14, 191–212. [Google Scholar]
- Elinson, R.P. Nutritional endoderm: A way to breach the holoblastic-meroblastic barrier in tetrapods. J. Exp. Zool. B Mol. Dev. Evol. 2009, 312, 526–532. [Google Scholar] [CrossRef]
- Leblanc, J.; Yoder, M.; Brick, I. Morphologic interactions between cells of the anteriad migrating fold during Rana pipiens gastrulation. Ann. N. Y. Acad. Sci. 1988, 529, 148–151. [Google Scholar] [CrossRef]
- Chang, Y.H.; Poyarkov, N.; Vassilieva, A.; Lai, J.S. Reproduction biology, behaviour, and ontogeny in Formosan salamander, Hynobius formosanus (Caudata: Hynobiidae), from Taiwan. In Proceedings of the 15th European Congress of Her pe tology and SEH Ordinary General Meeting, Kusadasi-Aydin, Turkey, 28 September–2 October 2009; p. 141. [Google Scholar]
- Iizuka, K.; Kezer, J.; Seto, T. Karyotypes of two rare species of hynobiid salamanders from Taiwan, Hynobius sonani (Maki) and Hynobius formosanus Maki (Urodela). Genetica 1988, 78, 105–110. [Google Scholar] [CrossRef]
- Piersol, W.H. The habits and larval state of Plethodon cinereus erythronotus. Trans. Can. Inst. 1910, 8, 469–493. [Google Scholar]
- Qu, Y.F.; Zhao, S.Z.; Jiang, X.F.; Lin, L.H.; Ji, X. Can snakes use yolk reserves to maximize body size at hatching? Curr. Zool. 2019, 65, 627–631. [Google Scholar] [CrossRef]
- Allen, J.D.; Zakas, C.; Podolsky, R.D. Effects of egg size reduction and larval feeding on juvenile quality for a species with facultative feeding development. J. Exp. Mar. Biol. Ecol. 2006, 331, 186–197. [Google Scholar] [CrossRef]
- Landberg, T. Embryonic yolk removal affects a suite of larval salamander life history traits. J. Exp. Zool. 2014, 322B, 45–53. [Google Scholar] [CrossRef]
- Gould, S.J. A developmental constraint in Cerion, with comments on the definition and interpretation of constraint in evolution. Evolution 1989, 43, 516–539. [Google Scholar] [CrossRef]
- Sargent, R.G.; Taylor, P.T.; Gross, M.R. Parental care and the evolution of egg size in fishes. Am. Nat. 1987, 129, 32–46. [Google Scholar] [CrossRef]
- Gould, J.; Beranek, C.; Valdez, J.; Mahony, M. Quantity versus quality: A balance between egg and clutch size among Australian amphibians in relation to other life-history variables. Austral Ecol. 2022, 47, 685–697. [Google Scholar] [CrossRef]
- Gatto, C.R.; Robinson, N.J.; Spotila, J.R.; Paladino, F.V.; Tomillo, P.S. Body size constrains maternal investment in a small sea turtle species. Mar. Biol. 2020, 167, 182. [Google Scholar] [CrossRef]
- Summers, K.; McKeon, C.S.; Heying, H. The evolution of parental care and egg size: A comparative analysis in frogs. Proc. R. Soc. B 2006, 273, 687–692. [Google Scholar] [CrossRef]
- Dani, K.G.S.; Kodandaramaiah, U. Plant and animal reproductive strategies: Lessons from offspring size and number trade-offs. Front. Ecol. Evol. 2017, 5, 38. [Google Scholar] [CrossRef]
- Lannoo, M.J.; Townsend, D.S.; Wassersug, R.J. Larval life in the leaves: Arboreal tadpole types, with special attention to the morphology, ecology, and behavior of the oophagous Osteopilus brunneus (Hylidae) larvae. Fieldiana Zool. 1987, 38, 1–31. [Google Scholar]
- Malkmus, R.; Dehling, J.M. Anuran amphibians of Borneo as phytotelm-breeders—A synopsis. Herpetozoa 2008, 20, 165–172. [Google Scholar]
- Vassilieva, A.; Nguyen, T. Restricting living space: Development and larval morphology in sticky frogs (Microhylidae: Kalophrynus) with different reproductive modes. Vertebr. Zool. 2023, 73, 367–382. [Google Scholar] [CrossRef]
- Vera Candioti, M.F.; Nuñez, J.J.; Úbeda, C. Development of the nidicolous tadpoles of Eupsophus emiliopugini (Anura: Cycloramphidae) until metamorphosis, with comments on systematic relationships of the species and its endotrophic developmental mode. Acta Zool. 2011, 92, 27–45. [Google Scholar] [CrossRef]
- Formas, J.R. External morphology, chondrocranium, hyobranchial skeleton, and external and internal oral features of Rhinoderma rufum (Anura, Rhinodermatidae). Zootaxa 2013, 3641, 395–400. [Google Scholar] [CrossRef]
- Cao, Z.H.; Guo, R.Y.; Fang, Z.Y.; Wang, Z.W.; Liu, Y.; Lin, L.H.; Ji, X. Normal table of embryonic development in the Anji salamander Hynobius amjiensis (Hynobiidae). Dev. Biol. 2024, 511, 84–91. [Google Scholar] [CrossRef]
- Kerney, R. Embryonic staging table for a direct-developing salamander, Plethodon cinereus (Plethodontidae). Anat. Rec. 2011, 294, 1796–1808. [Google Scholar] [CrossRef]
- Miller, L. Notes on the eggs and larvae Aneides lugubris. Copeia 1944, 1944, 224–230. [Google Scholar] [CrossRef]
- Jiang, J. Preliminary observations on the embryonic development of Hynobius leechii. J. Dalian Med. Univ 1985, 7, 1–7. [Google Scholar]
- Cai, M.Z.; Zhang, J.; Lin, D.J. Preliminary observations on the embryonic development of Chinese Hynobiidae. J. Herpetol. 1985, 4, 177–180. [Google Scholar]

| Number | Developmental Time | Time of Emergence (Since Incubation)/h | Distinguishing Characteristics |
|---|---|---|---|
| 1 | Fertilized egg (Figure 1A) | 0.0 | The fertilized eggs are spherical, with a grayish-black color; the animal pole matches the plant pole. |
| 2 | 2-cell stage (Figure 1B) | 8.5 | Unequal oogenesis results in the formation of two distinct ovoid spheres. |
| 3 | 4-cell stage (Figure 1C) | 20.0 ± 0.5 | Fertilized eggs are 4 cells. |
| 4 | 8-cell stage (Figure 1D) | 34.7 | Fertilized eggs are 8 cells. |
| 5 | 16-cell stage (Figure 1E) | 68.4 | Fertilized eggs are 16 cells. |
| 6 | 32-cell stage (Figure 1F) | 106.0 ± 0.5 | Fertilized eggs are 32 cells. |
| 7 | Multi-cell stage (Figure 1G) | 146.0 | The germ ball undergoes division, resulting in numerous unequal cells. |
| 8 | Early blastula stage (Figure 2A) | 172.6 | Surface cells, although dense and small, remain distinguishable. |
| 9 | Middle blastula stage (Figure 2B) | 204.3 | The fertilized eggs exhibit irregular cracks and patches with unclear cell boundaries. |
| 10 | Late blastula stage (Figure 2C) | 240.5 | The surface gradually smooths from bottom to top, and cell boundaries become indistinguishable. |
| 11 | Early gastrula stage (Figure 3A) | 252.0 | Short, shallow furrows emerge near the equator, and the dorsal sublabial cells initiate invagination. |
| 12 | Middle gastrula stage (Figure 3B) | 263.4 | The dorsal lip extends nearly halfway, and three gradually increasing depressions appear on the opposite side, eventually joining to form a short concave groove. |
| 13 | Late gastrula stage (Figure 3C) | 266.8 | Yolk deposits are present on both sides where the concave groove converges. |
| 14 | Neural plate stage (Figure 4A) | 274.4 | The yolk plug narrows, and the embryo exhibits slight folding with projections on both sides. |
| 15 | Neural fold stage (Figure 4B) | 300.5 | The lateral nerve folds are prominently elevated and converge toward the center. |
| 16 | Early neural tube stage (Figure 4C,D) | 312.2 | The neural folds do not completely fuse, resulting in a spindle-shaped embryo. |
| 17 | Middle neural tube stage (Figure 4E) | 318.5 | The neural crest is nearly closed, and the embryonic shape is well-defined. |
| 18 | Late neural tube stage (Figure 4F) | 326.8 | The rudimentary head, distinct dorsal ridge, and visible segmental marks are apparent. |
| 19 | Early tail bud stage (Figure 6A) | 345.5 | The head is well-formed, with visible eye vesicles and emerging segmented cheek plates. |
| 20 | Middle tail bud stage (Figure 6B) | 386.0 | Both the head and tail elongate, gill plates segment, ventricles develop, and the tail exhibits distinct features. |
| 21 | Late tail bud stage (Figure 6C) | 428.4 | The body length increases, the tail bud diminishes along with the yolk, and the ventricle develops further, resulting in an overall tadpole form. |
| 22 | Early external gill stage (Figure 6D) | 487.7 | The body coloration is distinct, the head, neck, and ventral regions are pronounced, the outer cheek ridge bears visible bud-like structures, no eye sockets are present, and tail characteristics are evident. |
| 23 | Middle external gill stage (Figure 7A,B) | 551.0 | The well-formed head features developed eye sockets and eye structures, there is a visible ventricular heartbeat, and an independent tail separates from the abdomen. |
| 24 | Late external gill stage (Figure 8A) | 688.7 | The eyes take shape, outer gills develop into whisker-like buds, the tail broadens, and the notochord becomes visible. |
| 25 | Incubation stage (Figure 8B) | 823.4 | Development is essentially complete, marked by the emergence of the eye and increased activity within the yolk membrane. |
| 26 | Incubation | 1054.6 | The film is broken during incubation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Chen, K.; Mei, Y.; Yang, J.; Chen, C. Pre-Embryonic Period Observation Shows a Unique Reproductive Strategy of the Critically Endangered Anji Salamander (Hynobius amjiensis). Animals 2024, 14, 3007. https://doi.org/10.3390/ani14203007
Qiu Y, Chen K, Mei Y, Yang J, Chen C. Pre-Embryonic Period Observation Shows a Unique Reproductive Strategy of the Critically Endangered Anji Salamander (Hynobius amjiensis). Animals. 2024; 14(20):3007. https://doi.org/10.3390/ani14203007
Chicago/Turabian StyleQiu, Yu, Kaiyang Chen, Yiyun Mei, Jia Yang, and Cangsong Chen. 2024. "Pre-Embryonic Period Observation Shows a Unique Reproductive Strategy of the Critically Endangered Anji Salamander (Hynobius amjiensis)" Animals 14, no. 20: 3007. https://doi.org/10.3390/ani14203007
APA StyleQiu, Y., Chen, K., Mei, Y., Yang, J., & Chen, C. (2024). Pre-Embryonic Period Observation Shows a Unique Reproductive Strategy of the Critically Endangered Anji Salamander (Hynobius amjiensis). Animals, 14(20), 3007. https://doi.org/10.3390/ani14203007





