Temporal Range Dynamics of the Lataste’s Viper (Vipera latastei Boscá, 1878) in Doñana (Spain): Insights into Anthropogenically Driven Factors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
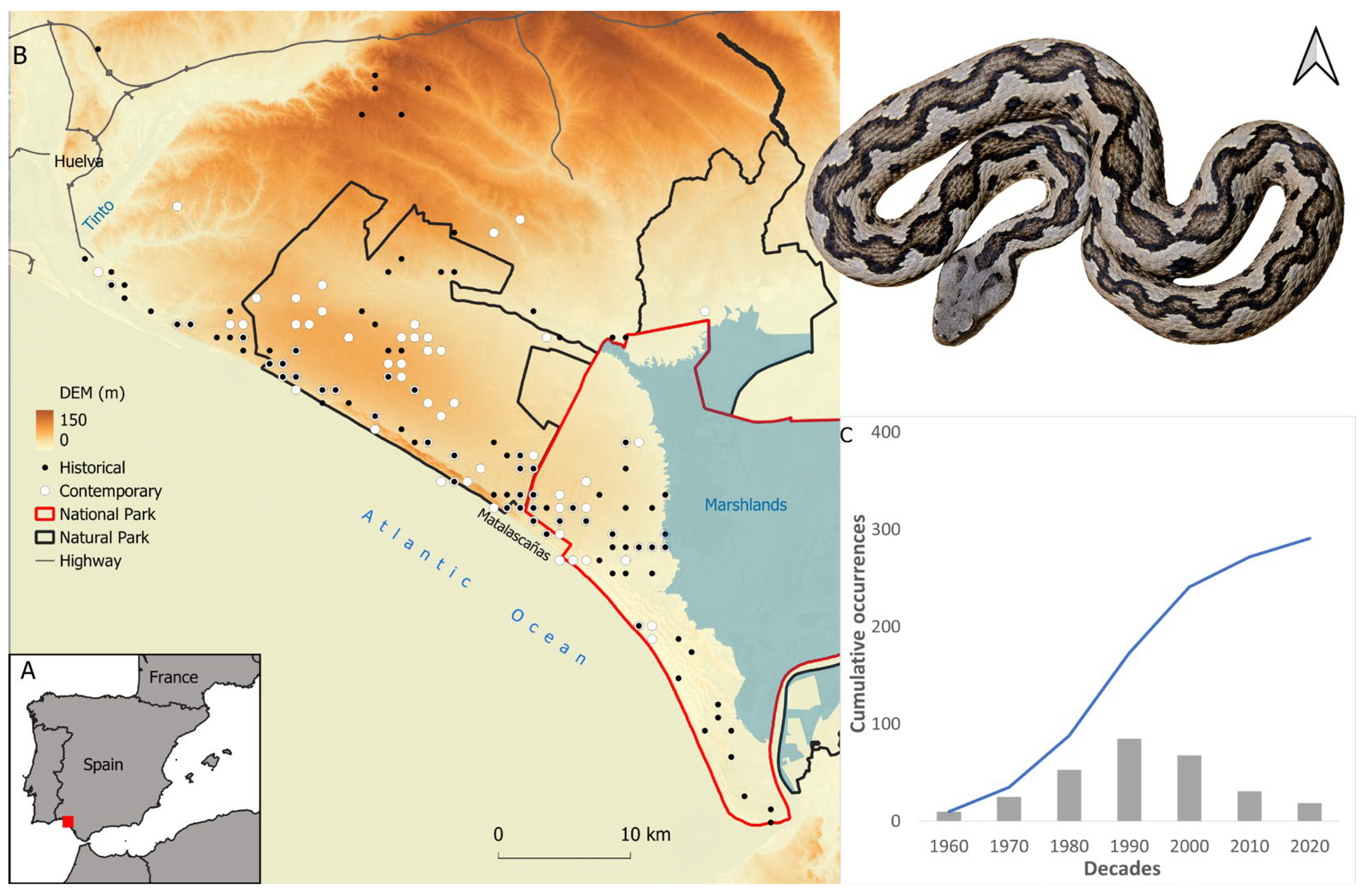
2.1. Study Area
2.2. Species Records
2.3. Climatic Factors
2.4. Normalized Difference Vegetation Index (NDVI) Factors
2.5. Ecological Niche-Based Models
3. Results
3.1. Models’ Performance
3.2. Environmental Factors Related to Species Occurrence
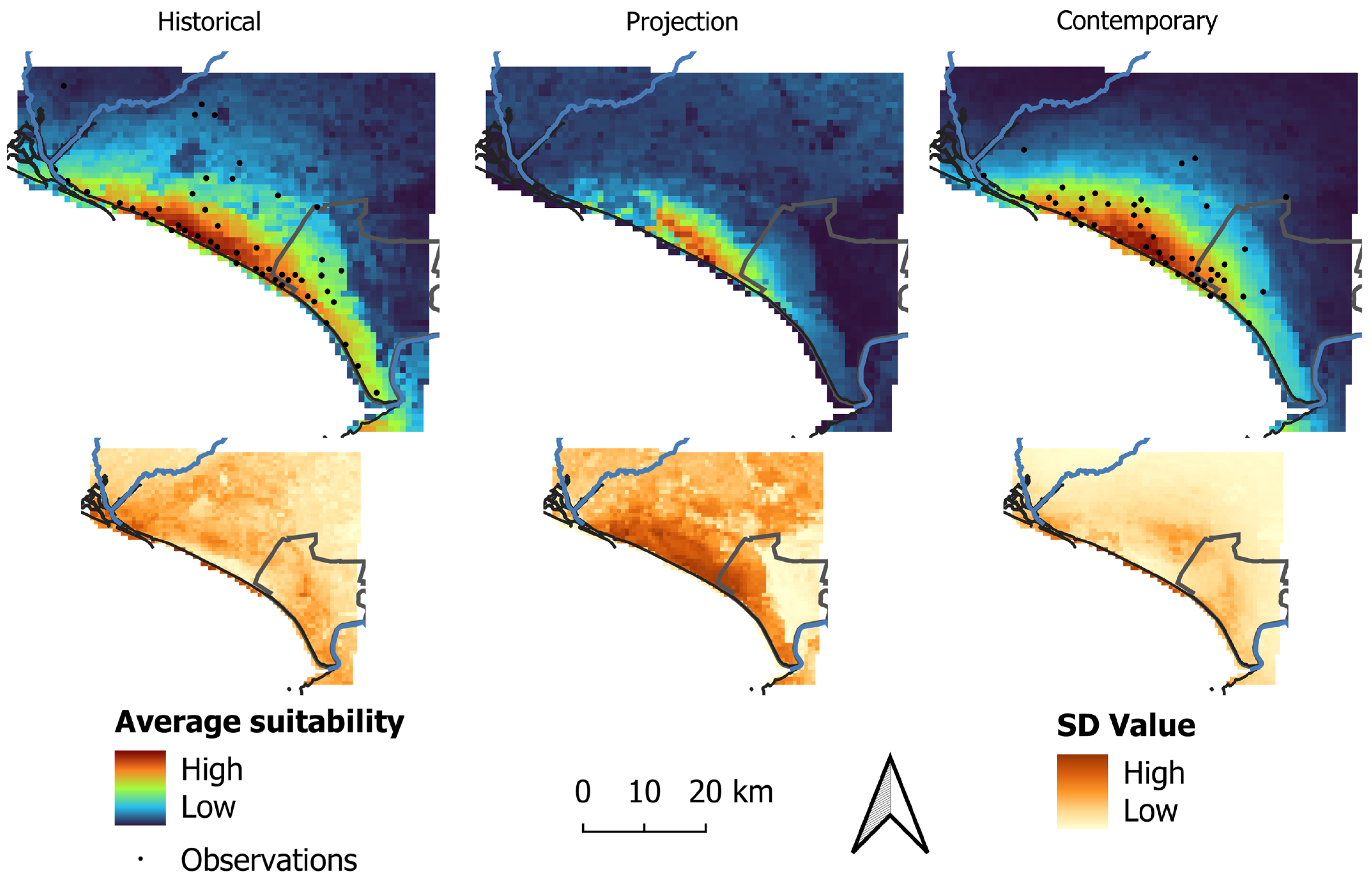
3.3. Predicted Occurrence
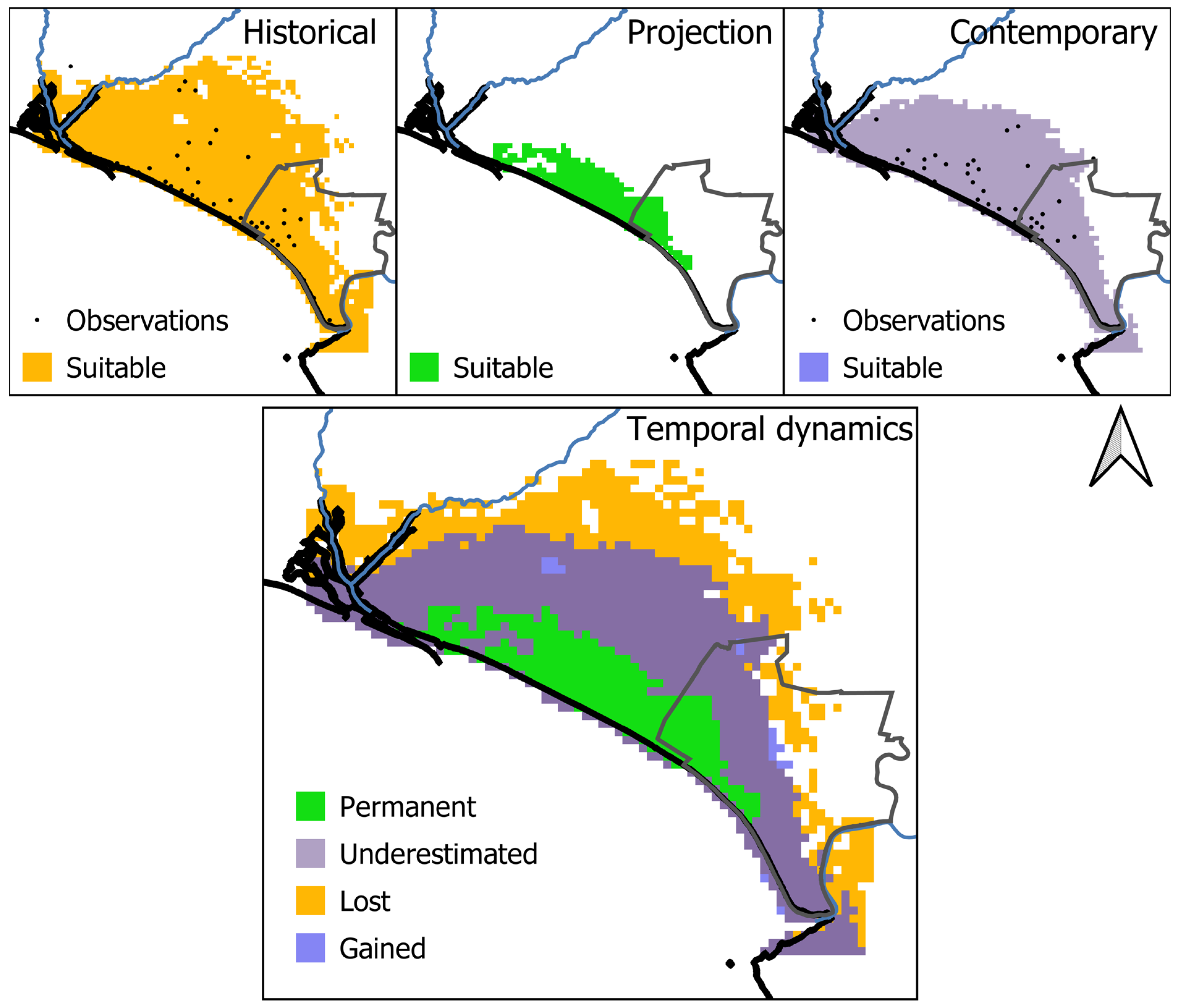
3.4. Range Dynamics
4. Discussion
4.1. Temporal Rarefication of the Lataste’s Viper in Doñana
4.2. A Distribution Driven by Cool Temperatures
4.3. A Reduction in the Distribution Range Driven by Climate Change
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Travis, J.M.J. Climate Change and Habitat Destruction: A Deadly Anthropogenic Cocktail. Proceedings of the Royal Society of London. Ser. B Biol. Sci. 2003, 270, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Butchart, S.H.M.; Walpole, M.; Collen, B.; Van Strien, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global Biodiversity: Indicators of Recent Declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, M.; Santini, L. Human Pressures Predict Species’ Geographic Range Size Better than Biological Traits. Global Change Biology 2015, 21, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.C.; Klein Goldewijk, K.; Siebert, S.; Lightman, D.; Ramankutty, N. Anthropogenic transformation of the biomes, 1700 to 2000. Glob. Ecol. Biogeogr. 2010, 19, 589–606. [Google Scholar] [CrossRef]
- IPBES. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Díaz, S., Settele, J., Brondízio, E.S., Ngo, H.T., Guèze, M., Agard, J., Arneth, A., Balvanera, P., Brauman, K.A., Butchart, S.H.M., et al., Eds.; IPBES Secretariat: Bonn, Germany, 2019; 56p. [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Habibullah, M.S.; Din, B.H.; Tan, S.H.; Zahid, H. Impact of climate change on biodiversity loss: Global evidence. Environ. Sci. Pollut. Res. 2022, 29, 1073–1086. [Google Scholar] [CrossRef]
- Teplitsky, C.; Mills, J.A.; Alho, J.S.; Yarrall, J.W.; Merilä, J. Bergmann’s Rule and Climate Change Revisited: Disentangling Environmental and Genetic Responses in a Wild Bird Population. Proc. Natl. Acad. Sci. USA 2008, 105, 13492–13496. [Google Scholar] [CrossRef] [PubMed]
- Myers, P.; Lundrigan, B.L.; Hoffman, S.M.G.; Haraminac, A.P.; Seto, S.H. Climate-induced changes in the small mammal communities of the Northern Great Lakes Region. Glob. Chang. Biol. 2009, 15, 1434–1454. [Google Scholar] [CrossRef]
- Woodroffe, R.; Groom, R.; McNutt, J.W. Hot dogs: High ambient temperatures impact reproductive success in a tropical carnivore. J. Anim. Ecol. 2017, 86, 1329–1338. [Google Scholar] [CrossRef]
- Riddell, E.A.; Iknayan, K.J.; Hargrove, L.; Tremor, S.; Patton, J.L.; Ramirez, R.; Wolf, B.O.; Beissinger, S.R. Exposure to climate change drives stability or collapse of desert mammal and bird communities. Science 2021, 371, 633–636. [Google Scholar] [CrossRef]
- Franklin, J. Mapping Species Distributions: Spatial Inference and Prediction, 1st ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar] [CrossRef]
- Sillero, N.; Arenas-Castro, S.; Enriquez-Urzelai, U.; Gomes Vale, C.; Sousa-Guedes, D.; Martínez-Freiría, F.; Real, R.; Barbosa, A.M. Want to model a species niche? A step-by-step guideline on correlative ecological niche modelling. Ecol. Modell. 2021, 456, 109671. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Araújo, M.B.; Guilhaumon, F.; Rodrigues Neto, D.; Pozo Ortego, I.; Gómez Calmaestra, R. Impactos, Vulnerabilidad y Adaptación de la Biodiversidad Española Frente al Cambio Climático; 2. Fauna de vertebrados; Dirección General de Medio Natural y Política Forestal. Ministerio de Medio Ambiente, y Medio Rural y Marino: Madrid, Spain, 2011.
- Woodin, S.A.; Hilbish, T.J.; Helmuth, B.; Jones, S.J.; Wethey, D.S. Climate change, species distribution models, and physiological performance metrics: Predicting when biogeographic models are likely to fail. Ecol. Evol. 2013, 3, 3334–3346. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Freiría, F.; Tarroso, P.; Rebelo, H.; Brito, J.C. Contemporary niche contraction affects climate change predictions for elephants and giraffes. Divers. Distrib. 2016, 22, 432–444. [Google Scholar] [CrossRef]
- Khalatbari, L.; Yusefi, G.H.; Martínez-Freiría, F.; Jowkar, H.; Brito, J.C. Availability of prey and natural habitats are related with temporal dynamics in range and habitat suitability for Asiatic cheetah. Hystrix 2018, 29, 145–151. [Google Scholar] [CrossRef]
- Urbina-Cardona, J.N.; Flores-Villela, O. Ecological-niche modeling and prioritization of conservation-area networks for Mexican herpetofauna. Conserv. Biol. 2010, 24, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- [IPCC] Intergovernmental Panel on Climate Change. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014.
- MITECO. Doñana, Technical Information. National Parks Autonomous Agency, 2024. Available online: https://www.miteco.gob.es/es/parques-nacionales-oapn/red-parques-nacionales/parques-nacionales/donana/ficha-tecnica.html (accessed on 15 May 2024).
- Zorrilla-Miras, P.; Palomo, I.; Gómez-Baggethun, E.; Martín-López, B.; Lomas, P.L.; Montes, C. Effects of land-use change on wetland ecosystem services: A case study in the Doñana marshes (SW Spain). Landsc. Urban Plan. 2014, 122, 160–174. [Google Scholar] [CrossRef]
- Palomo, I.; Martín-López, B.; Zorrilla-Miras, P.; García Del Amo, D.; Montes, C. Deliberative mapping of ecosystem services within and around Doñana National Park (SW Spain) in relation to land use change. Reg. Environ. Change 2014, 14, 237–251. [Google Scholar] [CrossRef]
- De Felipe, M.; Aragonés, D.; Díaz-Paniagua, C. Thirty-four years of Landsat monitoring reveal long-term effects of groundwater abstractions on a World Heritage Site wetland. Sci. Total Environ. 2023, 880, 163329. [Google Scholar] [CrossRef]
- Sousa, A.; García-Murillo, P.; Morales, J.; García-Barrón, L. Anthropogenic and natural effects on the coastal lagoons in the southwest of Spain (Doñana National Park). ICES J. Mar. Sci. 2009, 66, 1508–1514. [Google Scholar] [CrossRef]
- Naranjo-Fernández, N.; Guardiola-Albert, C.; Aguilera, H.; Serrano-Hidalgo, C.; Rodríguez-Rodríguez, M.; Fernández-Ayuso, A.; Ruiz-Bermudo, F.; Montero-González, E. Relevance of Spatio-Temporal Rainfall Variability Regarding Groundwater Management Challenges under Global Change: Case Study in Doñana (SW Spain). Stoch. Environ. Res. Risk Assess. 2020, 34, 1289–1311. [Google Scholar] [CrossRef]
- Cox, N.; Young, B.E.; Bowles, P.; Fernandez, M.; Marin, J.; Rapacciuolo, G.; Böhm, M.; Brooks, T.M.; Hedges, S.B.; Hilton-Taylor, C.; et al. A Global Reptile Assessment Highlights Shared Conservation Needs of Tetrapods. Nature 2022, 605, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Maritz, B.; Penner, J.; Martins, M.; Crnobrnja-Isailović, J.; Spear, S.; Alencar, L.R.V.; Sigala-Rodriguez, J.; Messenger, K.; Clark, R.W.; Soorae, P.; et al. Identifying Global Priorities for the Conservation of Vipers. Biol. Conserv. 2016, 204, 94–102. [Google Scholar] [CrossRef]
- Santos, X.; Brito, J.C.; Pleguezuelos, J.M.; Llorente, G.A. Comparing Filippi and Luiselli’s (2000) Method with a Cartographic Approach to Assess the Conservation Status of Secretive Species: The Case of the Iberian Snake-Fauna. Amphibia-Reptilia 2007, 28, 17–23. [Google Scholar] [CrossRef]
- Martínez-Freiría, F.; Santos, X.; Pleguezuelos, J.M. Vipera latastei. The IUCN Red List of Threatened Species 2024, e.T221202039A137859691. Available online: https://www.iucnredlist.org/en (accessed on 2 September 2024).
- Dufresnes, C.; Ghielmi, S.; Halpern, B.; Martínez-Freiría, F.; Mebert, K.; Jelić, D.; Crnobrnja-Isailović, J.; Gippner, S.; Jablonski, D.; Joger, U.; et al. Phylogenomic insights into the diversity and evolution of Palearctic vipers. Mol. Phylogenet. Evol. 2024, 197, 108095. [Google Scholar] [CrossRef] [PubMed]
- Freitas, I.; Ursenbacher, S.; Mebert, K.; Zinenko, O.; Schweiger, S.; Wüster, W.; Brito, J.C.; Crnobrnja-Isailović, J.; Halpern, B.; Fahd, S.; et al. Evaluating taxonomic inflation: Towards evidence-based species delimitation in Eurasian vipers (Serpentes: Viperinae). Amphibia-Reptilia 2020, 41, 285–311. [Google Scholar] [CrossRef]
- Martínez-Freiría, F.; Freitas, I.; Zuffi, M.A.L.; Golay, P.; Ursenbacher, S.; Velo-Antón, G. Climatic refugiaboosted allopatric diversification in Western Mediterraneanvipers. J. Biogeogr. 2020, 47, 1698–1713. [Google Scholar] [CrossRef]
- Martínez-Freiría, F.; Freitas, I.; Velo-Antón, G.; Lucchini, N.; Fahd, S.; Larbes, S.; Pleguezuelos, J.M.; Santos, X.; Brito, J.C. Integrative Taxonomy Reveals Two Species and Intraspecific Differentiation in the Vipera Latastei–Monticola Complex. J. Zool. Syst. Evol. Res. 2021, 59, 2278–2306. [Google Scholar] [CrossRef]
- Pleguezuelos, J.M.; Santos, X. Vipera latasti. In Atlas y Libro Rojo de los Anfibios y Reptiles de España; Pleguezuelos, J.M., Márquez, R., Lizana, M., Eds.; Dirección General de Conservación de la Naturaleza y Asociación Herpetológica Española: Madrid, Spain, 2002; pp. 298–300. Available online: https://www.researchgate.net/publication/237066172_Atlas_Y_Libro_Rojo_de_Los_Anfibios_Y_Reptiles_de_Espana (accessed on 13 September 2023).
- Brito, J.C. Vipera latastei Boscá, 1878. In Atlas dos Anfíbios e Répteis de Portugal; Loureiro, A., Ferrand de Almeida, N., Carretero, M.A., Paulo, O.S., Eds.; Instituto da Conservação da Natureza e da Biodiversidade: Lisboa, Portugal, 2008. [Google Scholar]
- Brito, J.C. Víbora hocicuda—Vipera latastei. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Marco, A., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2017; Available online: http://www.vertebradosibericos.org/ (accessed on 13 September 2023).
- Santos, X.; Brito, J.C.; Sillero, N.; Pleguezuelos, J.M.; Llorente, G.A.; Fahd, S.; Parellada, X. Inferring Habitat-Suitability Areas with Ecological Modelling Techniques and GIS: A Contribution to Assess the Conservation Status of Vipera latastei. Biol. Conserv. 2006, 130, 416–425. [Google Scholar] [CrossRef]
- Saint Girons, H. Systématique de Vipera latastei latastei Bosca, 1878 et description de Vipera latastei gaditana, subsp.n. (Reptilia, Viperidae). Rev. Suisse De Zool. 1977, 84, 599–607. [Google Scholar] [CrossRef]
- Valverde, J.A. Estructura de una Comunidad Mediterránea de Vertebrados Terrestres. 1967. Available online: https://digital.csic.es/handle/10261/114370 (accessed on 13 September 2023).
- Andreu, A.C. Seguimiento de anfibios y reptiles en Doñana. Boletín De La Asoc. Herpetológica Española 2014, 25, 73–93. [Google Scholar]
- Díaz-Delgado, R.; Aragonés, D.; Afán, I.; Bustamante, J. Long-Term Monitoring of the Flooding Regime and Hydroperiod of Doñana Marshes with Landsat Time Series (1974–2014). Remote Sens. 2016, 8, 775. [Google Scholar] [CrossRef]
- Duarte, C.; Montes, C.; Agustí, S.; Martino, P.; Bernués, M.; Kalff, J. Biomasa de macrófitos acuáticos en la marisma del Parque Nacional de Doñana (SW España): Importancia y factores ambientales que controlan su distribución. Limnetica 1990, 6, 1–11. [Google Scholar] [CrossRef]
- Gimena, E.C.; Arellano, M.M.; del Olmo, C.M.; Laso, C.M. Las Aguas Subterráneas en Doñana. Aspectos Ecológicos y Sociales; Agencia Andaluza del Agua. Consejería de Medio Ambiente de Andalucía: Sevilla, Spain, 2010.
- Rivas-Martínez, S.; Costa, M.; Castroviejo, S.; Valdés, E. La vegetación de Doñana (Huelva, España). Lazaroa 1980, 2, 5–190. [Google Scholar]
- Granados, M.; Martin, A.; Garcia-Novo, F. Long-Term Vegetation Changes on the Stabilized Dunes of Doñana National Park (SW Spain). Vegetatio 1988, 75, 73–80. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The Next Generation Python-Based GIS Toolkit for Landscape Genetic, Biogeographic and Species Distribution Model Analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Freiría, F.; Sillero, N.; Lizana, M.; Brito, J.C. GIS-Based Niche Models Identify Environmental Correlates Sustaining a Contact Zone between Three Species of European Vipers. Divers. Distrib. 2008, 14, 452–461. [Google Scholar] [CrossRef]
- Mizsei, E.; Uveges, B.; Vagi, B.; Szabolcs, M.; Lengyel, S.; Pfliegler, W.P.; Nagy, Z.T.; Toth, J.P. Species Distribution Modelling Leads to the Discovery of New Populations of One of the Least Known European Snakes, Vipera Ursinii Graeca, in Albania. Amphibia-Reptilia 2016, 37, 55–68. [Google Scholar] [CrossRef]
- Booth, T.H. Checking Bioclimatic Variables That Combine Temperature and Precipitation Data before Their Use in Species Distribution Models. Austral Ecol. 2022, 47, 1506–1514. [Google Scholar] [CrossRef]
- Running, S.W. Estimating Terrestrial Primary Productivity by Combining Remote Sensing and Ecosystem Simulation. In Remote Sensing of Biosphere Functioning; Hobbs, R.J., Mooney, H.A., Eds.; Ecological Studies; Springer: New York, NY, USA, 1990; pp. 65–86. [Google Scholar] [CrossRef]
- Myneni, R.B.; Hall, F.G.; Sellers, P.J.; Marshak, A.L. The Interpretation of Spectral Vegetation Indexes. IEEE Trans. Geosci. Remote Sens. 1995, 33, 481–486. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent Software for Modelling Species and Distributions. 2006. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 9 September 2023).
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The Effect of Sample Size and Species Characteristics on Performance of Different Species Distribution Modeling Methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Brito, J.C.; Fahd, S.; Martínez-Freiría, F.; Tarroso, P.; Larbes, S. Climate change and peripheral populations: Predictions for a relict Mediterranean viper. Acta Herpetol. 2011, 6, 105–118. [Google Scholar] [CrossRef]
- Martínez-Freiría, F.; Velo-Antón, G.; Brito, J.C. Trapped by Climate: Interglacial Refuge and Recent Population Expansion in the Endemic Iberian Adder Vipera Seoanei. Divers. Distrib. 2015, 21, 331–344. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Brown, J.A.; Jiménez-Valverde, A.; Real, R. modEvA. modTools (blog). 4 March 2014. Available online: https://modtools.wordpress.com/packages/modeva/ (accessed on 9 September 2023).
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for Customizable and Reproducible Modeling of Species’ Niches and Distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A Review of Methods for the Assessment of Prediction Errors in Conservation Presence/Absence Models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Freitas, I.; Fahd, S.; Velo-Antón, G.; Martínez-Freiría, F. Chasing the Phantom: Biogeography and Conservation of Vipera latastei-monticola in the Maghreb (North Africa). Amphibia-Reptilia 2018, 39, 145–161. [Google Scholar] [CrossRef]
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M.; et al. The Importance of Correcting for Sampling Bias in MaxEnt Species Distribution Models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting Thresholds for the Prediction of Species Occurrence with Presence-Only Data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Greene, H.W. Snakes: The Evolution of Mystery in Nature; Univ of California Press: Berkeley, CA, USA, 1997. [Google Scholar]
- Martínez-Freiría, F.; Brito, J.C.; Freitas, I.; Pleguezuelos, J.M.; Santos, X.; Velo-Antón, G. Luces y sombras en la biología de la víbora hocicuda. Quercus 2023, 452, 20–27. [Google Scholar]
- Freitas, I.; Tarroso, P.; Zuazo, Ó.; Zaldívar, R.; Álvarez, J.; Meijide-Fuentes, M.; Martínez-Freiría, F. Local Niches Explain Coexistence in Environmentally-Distinct Contact Zones between Western Mediterranean Vipers. Sci. Rep. 2023, 13, 21113. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Hofer, U. Predicting Reptile Distributions at the Mesoscale: Relation to Climate and Topography. J. Biogeogr. 2003, 30, 1233–1243. [Google Scholar] [CrossRef]
- Sow, A.S.; Martínez-Freiría, F.; Dieng, H.; Fahd, S.; Brito, J.C. Biogeographical Analysis of the Atlantic Sahara Reptiles: Environmental Correlates of Species Distribution and Vulnerability to Climate Change. J. Arid. Environ. 2014, 109, 65–73. [Google Scholar] [CrossRef]
- Chamorro, D.; Martínez-Freiría, F.; Real, R.; Muñoz, A.-R. Understanding Parapatry: How Do Environment and Competitive Interactions Shape Iberian Vipers’ Distributions? Edited by D. Chapple. J. Biogeogr. 2021, 48, 1322–1335. [Google Scholar] [CrossRef]
- Scaramuzzi, A.; Freitas, I.; Sillero, N.; Martínez-Freiría, F. Meso-Habitat Distribution Patterns and Ecological Requirements of Two Mediterranean Vipers Depict Weak Competition in a Contact Zone. J. Zool. 2023, 320, 308–321. [Google Scholar] [CrossRef]
- Scheele, B.C.; Foster, C.N.; Banks, S.C.; Lindenmayer, D.B. Niche Contractions in Declining Species: Mechanisms and Consequences. Trends Ecol. Evol. 2017, 32, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Montes-Vega, M.J.; Guardiola-Albert, C.; Rodríguez-Rodríguez, M. Calculation of the SPI, SPEI, and GRDI Indices for Historical Climatic Data from Doñana National Park: Forecasting Climatic Series (2030–2059) Using Two Climatic Scenarios RCP 4.5 and RCP 8.5 by IPCC. Water 2023, 15, 2369. [Google Scholar] [CrossRef]
- Valladares, F.; Matesanz, S.; Guilhaumon, F.; Araújo, M.B.; Balaguer, L.; Benito-Garzón, M.; Cornwell, W.; Gianoli, E.; van Kleunen, M.; Naya, D.E.; et al. The Effects of Phenotypic Plasticity and Local Adaptation on Forecasts of Species Range Shifts under Climate Change. Ecol. Lett. 2014, 17, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Refsnider, J.M.; Janzen, F.J. Behavioural Plasticity May Compensate for Climate Change in a Long-Lived Reptile with Temperature-Dependent Sex Determination. Biol. Conserv. 2012, 152, 90–95. [Google Scholar] [CrossRef]
- Beever, E.A.; Hall, L.E.; Varner, J.; Loosen, A.E.; Dunham, J.B.; Gahl, M.K.; Smith, F.A.; Lawler, J.J. Behavioral flexibility as a mechanism for coping with climate change. Front. Ecol. Environ. 2017, 15, 299–308. [Google Scholar] [CrossRef]
- Briscoe, N.J.; Morris, S.D.; Mathewson, P.D.; Buckley, L.B.; Jusup, M.; Levy, O.; Maclean, I.M.D.; Pincebourde, S.; Riddell, E.A.; Roberts, J.A.; et al. Mechanistic forecasts of species responses to climate change: The promise of biophysical ecology. Glob. Change Biol. 2023, 29, 1451–1470. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Freiría, F.; Lizana, M.; do Amaral, J.P.; Brito, J.C. Spatial and temporal segregation allows coexistence in a hybrid zone among two Mediterranean vipers (Vipera aspis and V. latastei). Amphibia-Reptilia 2010, 31, 195–212. [Google Scholar] [CrossRef]
- Arenas-Castro, S.; Sillero, N. Cross-Scale Monitoring of Habitat Suitability Changes Using Satellite Time Series and Ecological Niche Models. Sci. Total Environ. 2021, 784, 147172. [Google Scholar] [CrossRef]
- Zuazo, Ó.; Freitas, I.; Zaldívar, R.; Martínez-Freiría, F. Coexistence and Intermediate Morphological Forms between Vipera aspis and V. latastei in the Intensive Agriculture Fields of North-Western Iberian System. Boletín De La Asoc. Herpetológica Española 2019, 30, 35–41. [Google Scholar]
- Green, A.J.; Guardiola-Albert, C.; Bravo-Utrera, M.Á.; Bustamante, J.; Camacho, A.; Camacho, C.; Contreras-Arribas, E.; Espinar, J.L.; Gil-Gil, T.; Gomez-Mestre, I.; et al. Groundwater Abstraction has Caused Extensive Ecological Damage to the Doñana World Heritage Site, Spain. Wetlands 2024, 44, 20. [Google Scholar] [CrossRef]
- Matas-Granados, L.; Pizarro, M.; Cayuela, L.; Domingo, D.; Gómez, D.; García, M.B. Long-Term Monitoring of NDVI Changes by Remote Sensing to Assess the Vulnerability of Threatened Plants. Biol. Conserv. 2022, 265, 109428. [Google Scholar] [CrossRef]
- Brito, J.C. Seasonal and Daily Activity Patterns of Vipera latastei in Northern Portugal. Amphibia-Reptilia 2003, 24, 497–508. [Google Scholar] [CrossRef]
- Dullinger, S.; Gattringer, A.; Thuiller, W.; Moser, D.; Zimmermann, N.E.; Guisan, A.; Willner, W.; Plutzar, C.; Leitner, M.; Mang, T.; et al. Extinction debt of high-mountain plants under twenty-first-century climate change. Nat. Clim. Chang. 2012, 2, 619–622. [Google Scholar] [CrossRef]
- Elsen, P.R.; Tingley, M.W. Global Mountain Topography and the Fate of Montane Species under Climate Change. Nat. Clim. Chang. 2015, 5, 772–776. [Google Scholar] [CrossRef]
- Madsen, T.; Loman, J.; Bauwens, D.; Stille, B.; Anderberg, H.; Anderberg, L.; Ujvari, B. The Impact of an Extreme Climatic Event on Adder (Vipera berus) Demography in Southern Sweden. Biol. J. Linn. Soc. 2023, 138, 282–288. [Google Scholar] [CrossRef]
- Antunes, B.; Lourenço, A.; Caeiro-Dias, G.; Dinis, M.; Gonçalves, H.; Martínez-Solano, I.; Tarroso, P.; Velo-Antón, G. Combining Phylogeography and Landscape Genetics to Infer the Evolutionary History of a Short-Range Mediterranean Relict, Salamandra salamandra longirostris. Conserv. Genet. 2018, 19, 1411–1424. [Google Scholar] [CrossRef]
- Vörös, J.; Ursenbacher, S.; Jelić, D.; Tomović, L.; Crnobrnja-Isailović, J.; Ajtić, R.; Sterijovski, B.; Zinenko, O.; Ghira, I.; Strugariu, A.; et al. Well-known species, unexpected results: High genetic diversity in declining Vipera ursinii in central, eastern and southeastern Europe. Amphibia-Reptilia 2022, 43, 407–423. [Google Scholar] [CrossRef]
- Madsen, T.; Ujvari, B.; Bauwens, D.; Gruber, B.; Georges, A.; Klaassen, M. Polyandry and Non-Random Fertilisation Maintain Long-Term Genetic Diversity in an Isolated Island Population of Adders (Vipera berus). Heredity 2023, 130, 64–72. [Google Scholar] [CrossRef]

| Code | Meaning (and Units) | Historical | Contemporary |
|---|---|---|---|
| AnnTemp | Annual mean temperature (°C) | 29.079–29.192 | 29.118–29.226 |
| TempWet | Mean temperature of wettest quarter (°C) | 28.515–28.705 | 28.803–28.967 |
| TempDri | Mean temperature of driest quarter (°C) | 29.628–29.896 | 29.71–30 |
| PrecDri | Precipitation of driest month (mm) | 3.751–1.274 | 0.881–0.498 |
| PrecSeas | Precipitation seasonality (coefficient of variation) | 72.966–77.987 | 66.263–70.08 |
| Autumn SD | Autumn standard deviation | 0.03–0.238 | 0.026–0.271 |
| Spring M | Spring mean | −0.499–0.568 | −0.503–0.637 |
| Spring SD | Spring standard deviation | 0.02–0.304 | 0.028–0.279 |
| Summer SD | Summer standard deviation | 0.023–0.199 | 0.012–0.226 |
| Variables | Historical | Contemporary |
|---|---|---|
| AnnTemp | 0/0 | 0.004/0.049 |
| TempWet | 8.262/24.348 | 0.755/2.266 |
| TempDri | 73.079/48.555 | 91.69/79.650 |
| PrecDri | 0.153/0.136 | 1.377/6.594 |
| PrecSeas | 4.815/3.92 | 0.061/0.402 |
| Autumn SD | 3.421/4.641 | 0.174/0.163 |
| Spring M | 0.663/0.805 | 1.214/3.367 |
| Spring SD | 8.378/15.229 | 4.328/7.04 |
| Summer SD | 1.23/2.364 | 0.398/0.470 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona-González, R.; Carro, F.; González de la Vega, J.P.; Martínez-Freiría, F. Temporal Range Dynamics of the Lataste’s Viper (Vipera latastei Boscá, 1878) in Doñana (Spain): Insights into Anthropogenically Driven Factors. Animals 2024, 14, 3025. https://doi.org/10.3390/ani14203025
Carmona-González R, Carro F, González de la Vega JP, Martínez-Freiría F. Temporal Range Dynamics of the Lataste’s Viper (Vipera latastei Boscá, 1878) in Doñana (Spain): Insights into Anthropogenically Driven Factors. Animals. 2024; 14(20):3025. https://doi.org/10.3390/ani14203025
Chicago/Turabian StyleCarmona-González, Rafael, Francisco Carro, Juan Pablo González de la Vega, and Fernando Martínez-Freiría. 2024. "Temporal Range Dynamics of the Lataste’s Viper (Vipera latastei Boscá, 1878) in Doñana (Spain): Insights into Anthropogenically Driven Factors" Animals 14, no. 20: 3025. https://doi.org/10.3390/ani14203025
APA StyleCarmona-González, R., Carro, F., González de la Vega, J. P., & Martínez-Freiría, F. (2024). Temporal Range Dynamics of the Lataste’s Viper (Vipera latastei Boscá, 1878) in Doñana (Spain): Insights into Anthropogenically Driven Factors. Animals, 14(20), 3025. https://doi.org/10.3390/ani14203025







