Prediction of Potential Suitable Distribution Areas for Northeastern China Salamander (Hynobius leechii) in Northeastern China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Distribution Data Collection and Processing
2.3. Environmental Variables
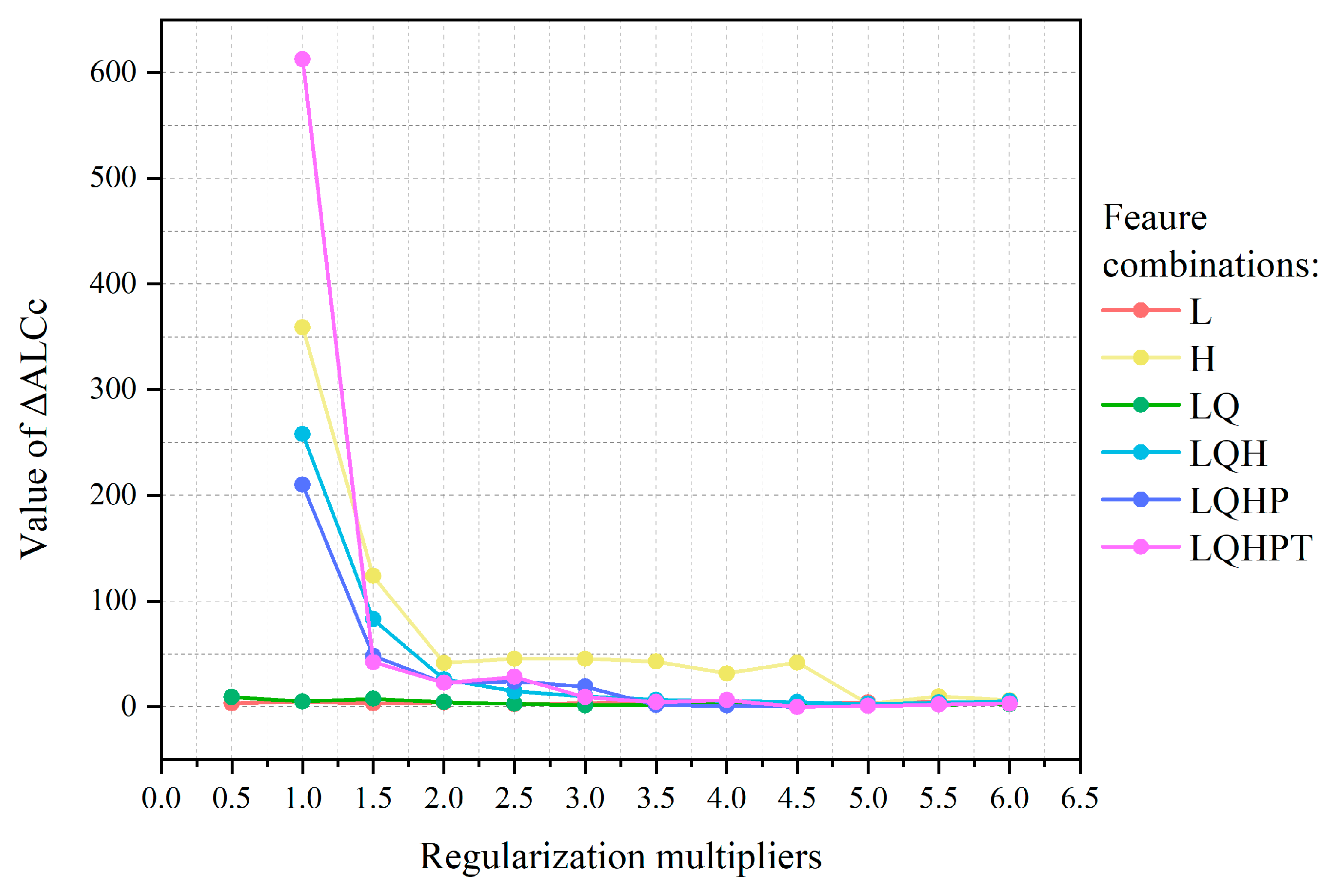
2.4. Parameter Optimization and Model Construction
2.5. Analysis of the Change in Potential Suitable Growth Area
3. Results
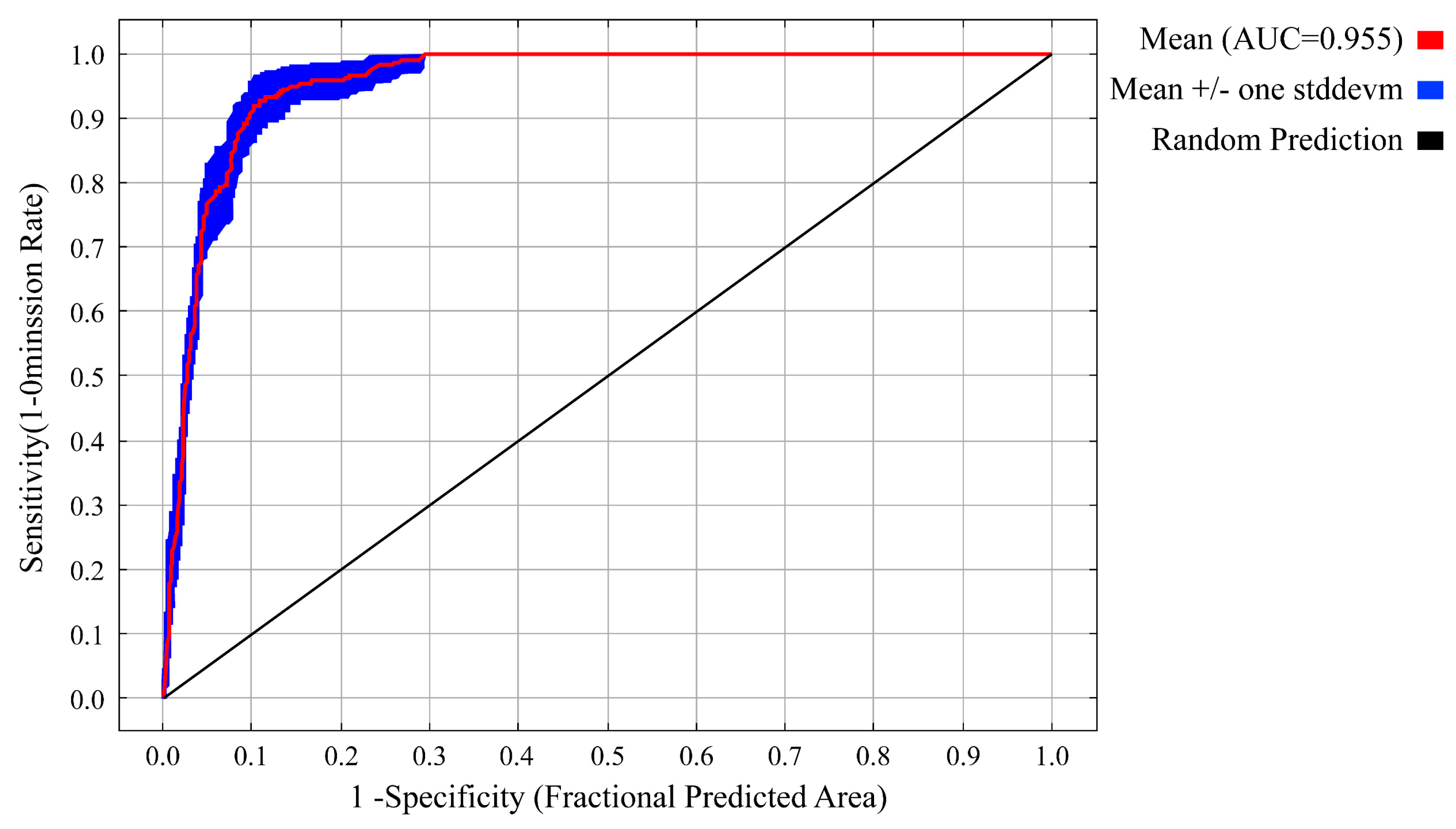
3.1. Model Optimization and Accuracy Evaluation
3.2. The Importance of Environmental Variables
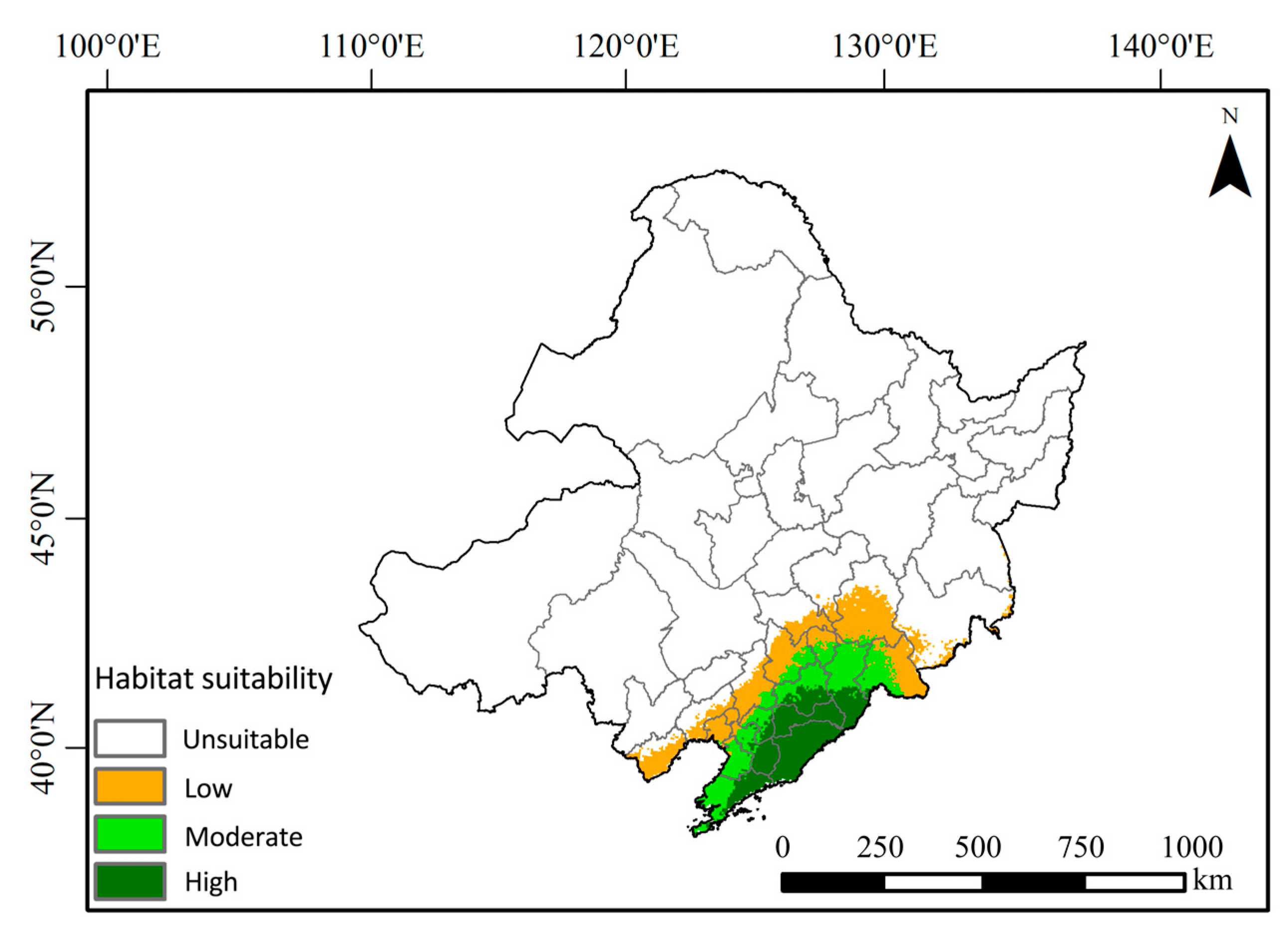
3.3. Distribution and Area of Suitable Habitat for Northeastern China Salamander in Northeastern China Under Modern Climate Conditions
3.4. Potential Distribution and Changes in Northeastern China Salamander in Northeastern China Under Future Climate Scenarios
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panetta, A.M.; Stanton, M.L.; Harte, J. Climate warming drives local extinction: Evidence from observation and experimentation. Sci. Adv. 2018, 4, eaaq1819. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lv, J.; Ai, L. The impacts of climate change on the biodiversity: Vulnerability and adaptation. Ecol. Environ. Sci. 2009, 18, 693–703, (In Chinese with English Abstract). [Google Scholar]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, F.B.N.; De Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef]
- Han, X.; Sun, B.; Zhang, Q.; Teng, L.; Zhang, F.; Liu, Z. Metabolic regulation reduces the oxidative damage of arid lizards in response to moderate heat events. Integr. Zool. 2023. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, C.; Pan, X.; Storey, K.B.; Zhang, W. Distinct metabolic responses to thermal stress between invasive freshwater turtle Trachemys scripta elegans and native freshwater turtles in China. Integr. Zool. 2024. [Google Scholar] [CrossRef]
- National Forestry and Grassland Administration Ministry of Agriculture and Rural Affairs. List of National Key Protected Wild Animals. Chin. J. Wildl. 2021, 42, 605–640. (In Chinese) [Google Scholar]
- Vidal, M.A.; Henríquez, N.; Torres-Díaz, C.; Collado, G.; Acuña-Rodríguez, I.S. Identifying strategies for effective biodiversity preservation and species status of Chilean amphibians. Biology 2024, 13, 169. [Google Scholar] [CrossRef]
- Warren, R.; VanDerWal, J.; Price, J.; Welbergen, J.A.; Atkinson, I.; Ramirez-Villegas, J.; Osborn, T.J.; Jarvis, A.; Shoo, L.P.; Williams, S.E.; et al. Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nat. Clim. Chang. 2013, 3, 678–682. [Google Scholar] [CrossRef]
- Duan, R.; Kong, X.; Huang, M.; Varela, S.; Ji, X. The potential effects of climate change on amphibian distribution, range fragmentation and turnover in China. PeerJ 2016, 4, e2185. [Google Scholar] [CrossRef]
- Shi, N.; Guo, N.; Liu, G.; Wang, Q.; Han, Y.; Xiao, N. Spatial distribution pattern and influencing factors of amphibians and reptiles in Beijing. Acta. Ecol. Sin. 2022, 42, 3806–3821, (In Chinese with English Abstract). [Google Scholar]
- Ali, W.; Javid, A.; Hussain, A.; Bukhari, S.M. Diversity and habitat preferences of amphibians and reptiles in Pakistan: A review. J. Asia-Pac. Biodivers. 2018, 11, 173–187. [Google Scholar] [CrossRef]
- Wang, B. Geographical Distribution Pattern and Climate Change Impact on Amphibians in the Karst Aeras of Southwest China. Ph.D. Thesis, Central South University of Forestry and Technology, Changsha, China, 2020. (In Chinese). [Google Scholar]
- Yan, S. Spatial Distribution Pattern for Salamander (Paramesotriton) Species in the Mountains of Southern China. Master’s Thesis, Guizhou Normal University, Guiyang, China, 2023. (In Chinese). [Google Scholar]
- Nottingham, S.; Pelletier, T.A. The impact of climate change on western Plethodon salamanders’ distribution. Ecol. Evol. 2021, 11, 9370–9384. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Peng, H.; Peng, S. The development and evaluation of species distribution models. Acta. Ecol. Sin. 2015, 35, 557–567, (In Chinese with English Abstract). [Google Scholar]
- Thapa, A.; Wu, R.; Hu, Y.; Nie, Y.; Singh, P.B.; Khatiwada, J.R.; Yan, L.; Gu, X.; Wei, F. Predicting the potential distribution of the endangered red panda across its entire range using MaxEnt modeling. Ecol. Evol. 2018, 8, 10542–10554. [Google Scholar] [CrossRef]
- Alfaya, P.; Casanovas, J.G.; Lobón-Rovira, J.; Matallanas, B.; Cruz, A.; Arana, P.; Alonso, G. Using MaxEnt algorithm to assess habitat suitability of a potential Iberian lynx population in central Iberian Peninsula. Community. Ecol. 2019, 20, 266–276. [Google Scholar] [CrossRef]
- Costa, G.C.; Nogueira, C.; Machado, R.B.; Colli, G.R. Sampling bias and the use of ecological niche modeling in conservation planning: A field evaluation in a biodiversity hotspot. Biodivers. Conserv. 2010, 19, 883–899. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H. Exploring the advantages of the maximum entropy model in calibrating cellular automata for urban growth simulation: A comparative study of four methods. GIScience Remote Sens. 2022, 59, 71–95. [Google Scholar] [CrossRef]
- Lissovsky, A.A.; Dudov, S.V. Species-Distribution Modeling: Advantages and Limitations of Its Application. 2. MaxEnt. Biol. Bull. Rev. 2021, 11, 265–275. [Google Scholar] [CrossRef]
- Qian, T.; Qin, S.; Wu, Z.; Xi, C.; Wang, J. Impacts of human interference on the potential distribution of Yunnan snub-nosed monkeys by MaxEnt model. Acta Theriol. Sin. 2022, 42, 349–361, (In Chinese with English Abstract). [Google Scholar]
- Tang, Y.; Pi, J.; Liu, X.; Xiang, J.; Zeng, C.; Li, D. Predicting potential distribution of Corbicula fluminea under climate change scenarios using MaxEnt model. Acta Ecol. Sin. 2023, 43, 4250–4259, (In Chinese with English Abstract). [Google Scholar]
- Luo, M.; Wang, H.; Lyu, Z. Evaluating the performance of species distribution models Biomod2 and MaxEnt using the giant panda distribution data. Chin. J. Appl. Ecol. 2017, 28, 4001–4006, (In Chinese with English Abstract). [Google Scholar]
- Ying, B.; Tian, K.; Guo, H.; Yang, X.; Li, W.; Li, Q.; Luo, Y.; Zhang, X. Predicting potential suitable habitats of Kandelia obovata in China under future climatic scenarios based on MaxEnt model. Acta Ecol. Sin. 2024, 44, 224–234, (In Chinese with English Abstract). [Google Scholar]
- Groff, L.A.; Marks, S.B.; Hayes, M.P. Using ecological niche models to direct rare amphibian surveys: A case study using the Oregon Spotted Frog (Rana pretiosa). Herpetol. Conserv. Biol. 2014, 9, 354–368. [Google Scholar]
- Pourhallaji, M.; Dargahi, M.D.; Kami, H.G.; Pouyani, E.R.; Abed, M.H. Species distribution modeling and environmental suitability of the Southern crested newt, Triturus karelinii (Strauch, 1870) (Amphibia: Caudata) in Iran. J. Wildl. Biodivers 2021, 5, 44–52. [Google Scholar]
- Kim, H.W.; Adhikari, P.; Chang, M.H.; Seo, C. Potential distribution of amphibians with different habitat characteristics in response to climate change in South Korea. Animals 2021, 11, 2185. [Google Scholar] [CrossRef]
- Borzée, A.; Litvinchuk, S.N.; Ri, K.; Andersen, D.; Nam, T.Y.; Jon, G.H.; Man, H.S.; Choe, J.S.; Kwon, S.; Othman, S.N.; et al. Update on Distribution and Conservation Status of Amphibians in the Democratic People’s Republic of Korea: Conclusions Based on Field Surveys, Environmental Modelling, Molecular Analyses and Call Properties. Animals 2021, 11, 2057. [Google Scholar] [CrossRef]
- Zhang, P.; Grenouillet, G.; Dong, X.; Zheng, Y.; Lek, S.; Chang, J. Capturing response differences of species distribution to climate and human pressures by incorporating local adaptation: Implications for the conservation of a critically endangered species. J. Environ. Manag. 2021, 284, 111998. [Google Scholar] [CrossRef]
- Ma, Q.; Wan, L.; Shi, S.; Wang, Z. Impact of climate change on the distribution of three rare salamanders (Liua shihi, Pseudohynobius jinfo, and Tylototriton wenxianensis) in Chongqing, China, and their conservation implications. Animals 2024, 14, 672. [Google Scholar] [CrossRef]
- Tao, J.; Hu, Y.; Jiang, J.; Yang, W.; Zhao, T.; Su, S. Prediction of potential suitable distribution areas for an endangered salamander in China. Animals 2024, 14, 1390. [Google Scholar] [CrossRef]
- Mu, C.; Qi, X.; Xie, L.; Qian, T.; Zhou, Z.; Lu, Y.; Mo, Y.; Li, P. MaxEnt-Based prediction on the geographical distribution of Hainan steam treefrog (Buergeria oxycephala) in Hainan island. Chin. J. Wildl. 2021, 42, 809–816, (In Chinese with English Abstract). [Google Scholar]
- Xia, X.; Li, Y.; Yang, D.D.; Pi, Y.Y. Potential geographical distribution of Rana hanluica in China under climate change. Chin. J. Appl. Ecol. 2021, 32, 4307–4314, (In Chinese with English Abstract). [Google Scholar]
- Huang, Y.J.; Lu, J.B.; Wang, F.T.; Lin, Y.H.; Liu, L.; Mi, H.X.; Mo, F.Q.; Fang, J.; Li, J.L. Predicting the potential geographic distribution of Hainan Odorous Frog (Odorrana hainanensis) in Hainan province by MaxEnt. Chin. J. Zool. 2017, 52, 30–41, (In Chinese with English Abstract). [Google Scholar]
- Zhao, W. The Amphibia and Reptilia Fauna of Heilongjiang, 1st ed.; Science Press: Beijing, China, 2008; pp. 24–27. (In Chinese) [Google Scholar]
- Fei, L.; Ye, C.; Jiang, J. Colorful Illustrations of Amphibians in China, 1st ed.; Sichuan Publishing Group Sichuan Science and Technology Press: Chengdu, China, 2010; pp. 38–39. (In Chinese) [Google Scholar]
- Jiang, C.; Zhang, X.; Xie, W.; Wang, R.; Feng, C.; Ma, L.; Li, Q.; Yang, Q.; Wang, H. Predicting the potential distribution of the fall armyworm Spodoptera frugiperda (J.E. Smith) under climate change in China. Glob. Ecol. Conserv. 2022, 33, e01994. [Google Scholar] [CrossRef]
- Yuan, C.C.; Qin, N.; Li, Y.N.; Zhang, H.T.; Zhang, Q.P.; Zhang, D.X. The Characteristics of Spatial and Temporal Changes of Black Soil Resources Occupied by Urban Expansion in Northeast China and its Influencing Factors from 1986–2018. Chin. J. Soil Sci. 2024, 55, 610–621. (In Chinese) [Google Scholar]
- Chen, X.X. The Impact of Snowpack on Soil Hydrothermal in Typical Cropland Regions of Northeast China. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2023. [Google Scholar]
- Ye, X.; Zhang, M.; Lai, W.; Yang, M.; Fan, H.; Zhang, G.; Chen, S.; Liu, B. Prediction of potential suitable distribution of Phoebe bournei based on MaxEnt optimization model. Acta. Ecol. Sin. 2021, 41, 8135–8144, (In Chinese with English Abstract). [Google Scholar]
- Sillero, N. What does ecological modelling model? A proposed classification of ecological niche models based on their underlying methods. Ecol. Model. 2011, 222, 1343–1346. [Google Scholar] [CrossRef]
- Mota, J.F.; Pérez-García, F.J.; Jiménez, M.L.; Amate, J.J.; Peñas, J. Phytogeographical relationships among high mountain areas in the Baetic Ranges (South Spain). Global. Ecol. Biogeogr. 2002, 11, 497–504. [Google Scholar] [CrossRef]
- Yang, X.Q.; Kushwaha, S.P.S.; Saran, S.; Xu, J.; Roy, P.S. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda, L. in Lesser Himalayan foothills. Ecol. Eng. 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Li, Y.; Lu, L.; Tian, X.; Duan, Y. Prediction of the suitable habitat distribution of Oriental White Stork in Shandong Province. Acta. Ecol. Sin. 2023, 43, 2194–2201, (In Chinese with English Abstract). [Google Scholar]
- Phillips, J.S.; Anderson, P.R.; Schapire, E.R. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- John, E.; Bunting, P.; Hardy, A.; Roberts, O.; Giliba, R.; Silayo, D.S. Modelling the impact of climate change on Tanzanian forests. Divers. Distrib. 2020, 26, 1663–1686. [Google Scholar] [CrossRef]
- Zhang, K.L.; Yao, L.J.; Meng, J.S.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Stickley, S.F.; Fraterrigo, J.M. Microclimate species distribution models estimate lower levels of climate-related habitat loss for salamanders. Nat. Conserv. 2023, 72, 126333. [Google Scholar] [CrossRef]
- Wan, C.; Fang, K.; Wu, J.; Mu, X.; Dong, F.; Zhang, H. Prediction of potential distribution of Gambusia affinis in China based on MaxEnt model. Freshw. Fish. 2024, 54, 3–10, (In Chinese with English Abstract). [Google Scholar]
- Karuppaiah, V.; Maruthadurai, R.; Das, B.; Soumia, P.S.; Gadge, A.S.; Thangasamy, A.; Ramesh, S.V.; Shirsat, D.V.; Mahajan, V.; Krishna, H.; et al. Predicting the potential geographical distribution of onion thrips, Thrips tabaci in India based on climate change projections using MaxEnt. Sci. Rep. 2023, 13, 7934. [Google Scholar] [CrossRef]
- Wu, J. The hazard and unsureness of reducing habitat ranges in response to climate warming for 91 amphibian species in China. Acta Oecol. 2020, 108, 103640. [Google Scholar] [CrossRef]
- Daniel, E.; Axel, H. Vegetation cover and occurrence of salamanders in the western Mediterranean. Integr. Zool. 2021, 17, 456–467. [Google Scholar]
- Wan, B.; Chen, G.; Poon ES, K.; Fung, H.S.; Lau, A.; Sin, S.Y.W. Environmental factors and host sex influence the skin microbiota structure of Hong Kong newt (Paramesotriton hongkongensis) in a coldspot of chytridiomycosis in subtropical East Asia. Integr. Zool. 2024. [Google Scholar] [CrossRef]
- Qian, H.; Wang, X.; Wang, S.; Li, Y. Environmental determinants of amphibian and reptile species richness in China. Ecography 2007, 30, 471–482. [Google Scholar] [CrossRef]
- Zheng, Z.; Gong, D.; Sun, C. Elevational pattern of species richness and species range size of herpetofauna in Baishuijiang Nature Reserve: A test of Rapoport’s rule. J. Ecol. 2014, 33, 537–546, (In Chinese with English Abstract). [Google Scholar]
- Xu, X.; Zhao, W.; Liu, P. Effect of environmental temperature on body temperature during reproductive period and embryonic development in different geographic populations of Rana dybowskii. Acta. Ecol. Sin. 2018, 38, 2965–2973, (In Chinese with English Abstract). [Google Scholar]
- Siemens, A.L.; Bogart, J.P.; Linton, J.E.; Norris, D.R. Predicting the occurrence of an endangered salamander in a highly urbanized landscape. Endang. Species. Res. 2023, 52, 81–95. [Google Scholar] [CrossRef]
- Bakare, A.G.; Kour, G.; Akter, M.; Iji, P.A. Impact of climate change on sustainable livestock production and existence of wildlife and marine species in the South Pacific island countries: A review. Int. J. Biometeorol. 2020, 64, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, C.; Mi, C.; Han, L.; Li, M.; Xu, W.; Yang, W. Potential impacts of climate change on suitable habitats of Marco Polo sheep in China. Chin. J. Appl. Ecol. 2021, 32, 3127–3135, (In Chinese with English Abstract). [Google Scholar]
- Widmer, B.W.; Gehring, T.M.; Heumann, B.W.; Nicholson, K.E. Climate change and range restriction of common salamanders in eastern Canada and the United States. J. Wildl. Manag. 2022, 86, e22235. [Google Scholar] [CrossRef]
- Zhao, Z. Prediction of Future Changes in the Suitable Distribution Area of Rare and Endangered Amphibian Species in the Southern Hengduan Mountains and Their Optimization Conservation. Master’s Thesis, Lanzhou University, Lanzhou, China, 2022. [Google Scholar]
- Ballesteros-Barrera, C.; Tapia-Pérez, O.; Zárate-Hernández, R.; Leyte-Manrique, A.; Martínez-Bernal, A.; Vargas-Miranda, B.; Martínez-Coronel, M.; Ortiz-Burgos, S. The potential effect of climate change on the distribution of endemic Anurans from Mexico’s tropical dry forest. Diversity 2022, 14, 650. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Yao, M.; Wei, M.; Nie, H. Geographic distribution pattern and dispersal route of Urodela in China. Chin. J. Zool. 2018, 53, 1–16, (In Chinese with English Abstract). [Google Scholar]
- Vasilakos, C.; Kavroudakis, D.; Georganta, A. Machine learning classification ensemble of multitemporal sentinel-2 images: The case of a mixed mediterranean ecosystem. Remote Sens. 2020, 12, 2005. [Google Scholar] [CrossRef]
- Zellmer, A.J.; Slezak, P.; Katz, T.S. Clearing up the crystal ball: Understanding uncertainty in future climate suitability projections for amphibians. Herpetologica 2020, 76, 108–120. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, N.; Liu, G.; Li, J. Prediction of the potential geographical distribution of five species of Scutiger in the south of Hengduan Mountains Biodiversity Conservation Priority Zone. Acta. Ecol. Sin. 2022, 42, 2636–2647, (In Chinese with English Abstract). [Google Scholar]
- Saupe, E.E.; Barve, V.; Myers, C.E.; Soberón, J.; Barve, N.; Hensz, C.M.; Peterson, A.T.; Owens, H.L.; Lira-Noriega, A. Variation in niche and distribution model performance: The need for a priori assessment of key causal factors. Ecol. Model. 2012, 237–238, 11–22. [Google Scholar] [CrossRef]
- Xie, J.; Fei, X.; Qiu, L.; Lou, X.; Huang, J.; Zheng, R. Effects of climate change on habitat suitability of Hynobius yiwuensis in Yiwu city. J. Zhejiang Norm. Univ. Nat. Sci. 2023, 46, 66–73, (In Chinese with English Abstract). [Google Scholar]
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzée, A.; Hamidy, A.; Aowphol, A.; Jean, A.; et al. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 2023, 622, 308–314. [Google Scholar] [CrossRef]
- Zhang, Z.; Mammola, S.; Xian, W.; Zhang, H. Modelling the potential impacts of climate change on the distribution of ichthyoplankton in the Yangtze Estuary, China. Divers. Distrib. 2019, 26, 126–137. [Google Scholar] [CrossRef]
- Rohman, M.; Prasetyo, L.B.; Kusrini, M.D. Predicting spatial distribution of Asian Horned Frog (Megophrys montana Kuhl & Van Hasselt 1882) in Java Island using citizen science’s data. IOP Conf. Ser. Earth. Environ. Sci. 2021, 771, 012027. [Google Scholar]


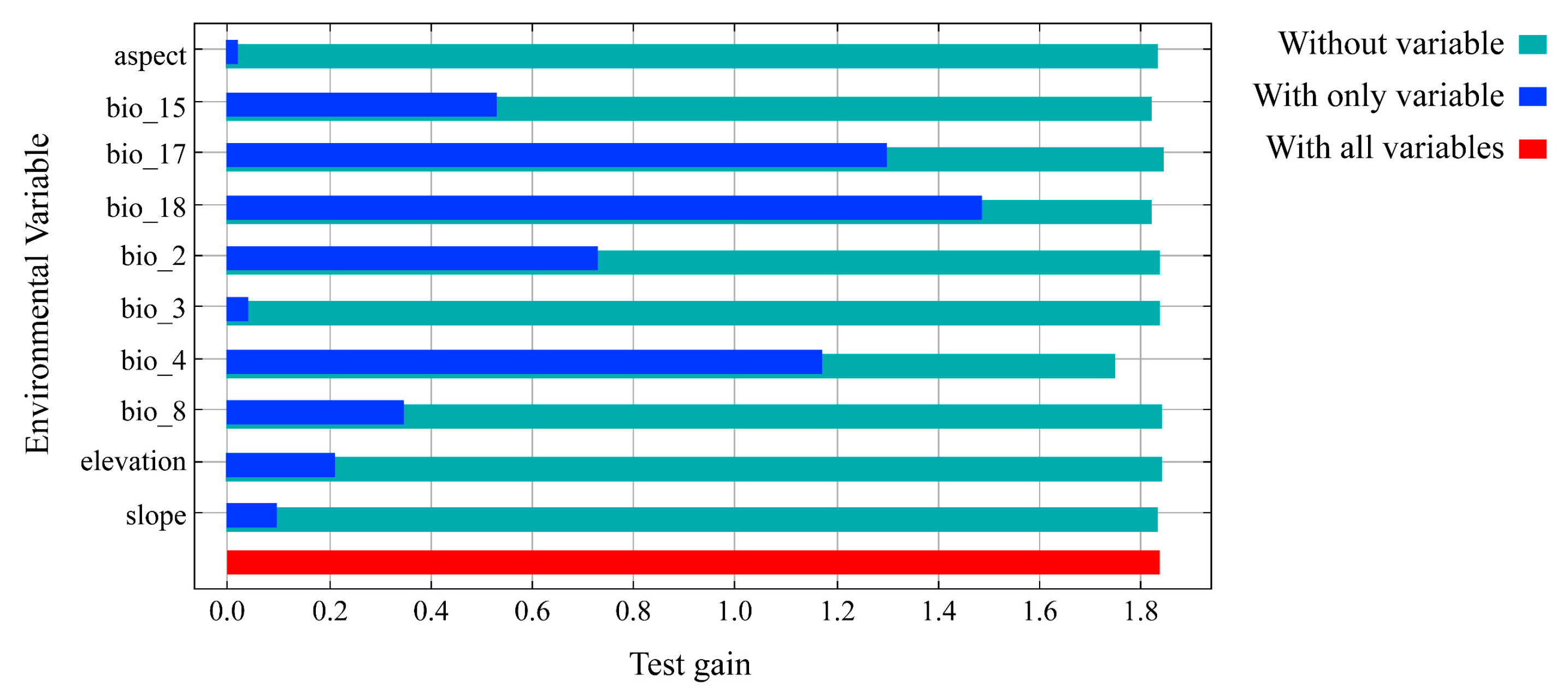


| Code | Environmental Variable | Percentage Contribution (%) | Permutation Importance (%) |
|---|---|---|---|
| bio18 | Precipitation of Warmest Quarter | 52 | 48.5 |
| bio17 | Precipitation of Driest Quarter | 26.6 | 21.4 |
| bio4 | Temperature Seasonality | 9.6 | 14.8 |
| bio8 | Mean Temperature of Wettest Quarter | 7.4 | 14.6 |
| bio15 | Precipitation Seasonality | 2.4 | 0.1 |
| bio3 | Mean Diurnal Range | 1.2 | 0 |
| bio2 | Mean Diurnal Range | 0.5 | 0 |
| Asp | Aspect | 0.1 | 0.4 |
| Ele | Elevation | 0.1 | 0 |
| Slo | Slope | 0 | 0.1 |
| Grade | Current | 2050 | 2070 | ||||
|---|---|---|---|---|---|---|---|
| SSP126 | SSP245 | SSP585 | SSP126 | SSP245 | SSP585 | ||
| Low | 7.325 | 20.011 | 16.813 | 34.124 | 14.083 | 31.829 | 30.468 |
| (12.686) | (9.488) | (26.799) | (6.758) | (24.504) | (23.143) | ||
| Moderate | 4.900 | 23.173 | 22.608 | 25.169 | 25.592 | 19.448 | 18.732 |
| (18.273) | (17.708) | (20.269) | (20.692) | (14.548) | (13.832) | ||
| High | 4.753 | 22.520 | 28.671 | 33.091 | 25.345 | 39.756 | 42.963 |
| (17.767) | (23.918) | (28.338) | (20.592) | (35.003) | (38.210) | ||
| Total | 16.978 | 65.704 | 68.092 | 92.384 | 65.020 | 91.033 | 92.163 |
| (48.726) | (51.114) | (75.406) | (48.042) | (74.055) | (75.185) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Zhou, M.; Zhang, T.; Zhao, W.; Liu, P. Prediction of Potential Suitable Distribution Areas for Northeastern China Salamander (Hynobius leechii) in Northeastern China. Animals 2024, 14, 3046. https://doi.org/10.3390/ani14213046
Han L, Zhou M, Zhang T, Zhao W, Liu P. Prediction of Potential Suitable Distribution Areas for Northeastern China Salamander (Hynobius leechii) in Northeastern China. Animals. 2024; 14(21):3046. https://doi.org/10.3390/ani14213046
Chicago/Turabian StyleHan, Lei, Minghang Zhou, Ting Zhang, Wenge Zhao, and Peng Liu. 2024. "Prediction of Potential Suitable Distribution Areas for Northeastern China Salamander (Hynobius leechii) in Northeastern China" Animals 14, no. 21: 3046. https://doi.org/10.3390/ani14213046
APA StyleHan, L., Zhou, M., Zhang, T., Zhao, W., & Liu, P. (2024). Prediction of Potential Suitable Distribution Areas for Northeastern China Salamander (Hynobius leechii) in Northeastern China. Animals, 14(21), 3046. https://doi.org/10.3390/ani14213046






