Developmental Thermal Reaction Norms of Leatherback Marine Turtles at Nesting Beaches
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bibliography Analysis
2.1.1. Northwest Atlantic RMU—French Guiana and Suriname
2.1.2. East Pacific RMU—Costa Rica
2.1.3. East Pacific RMU—Mexico
2.1.4. West Pacific RMU—Malaysia
2.2. Model of Embryo Growth
2.3. Pattern of TSD
2.4. Comparison of Models
3. Results
3.1. Bibliography Analysis
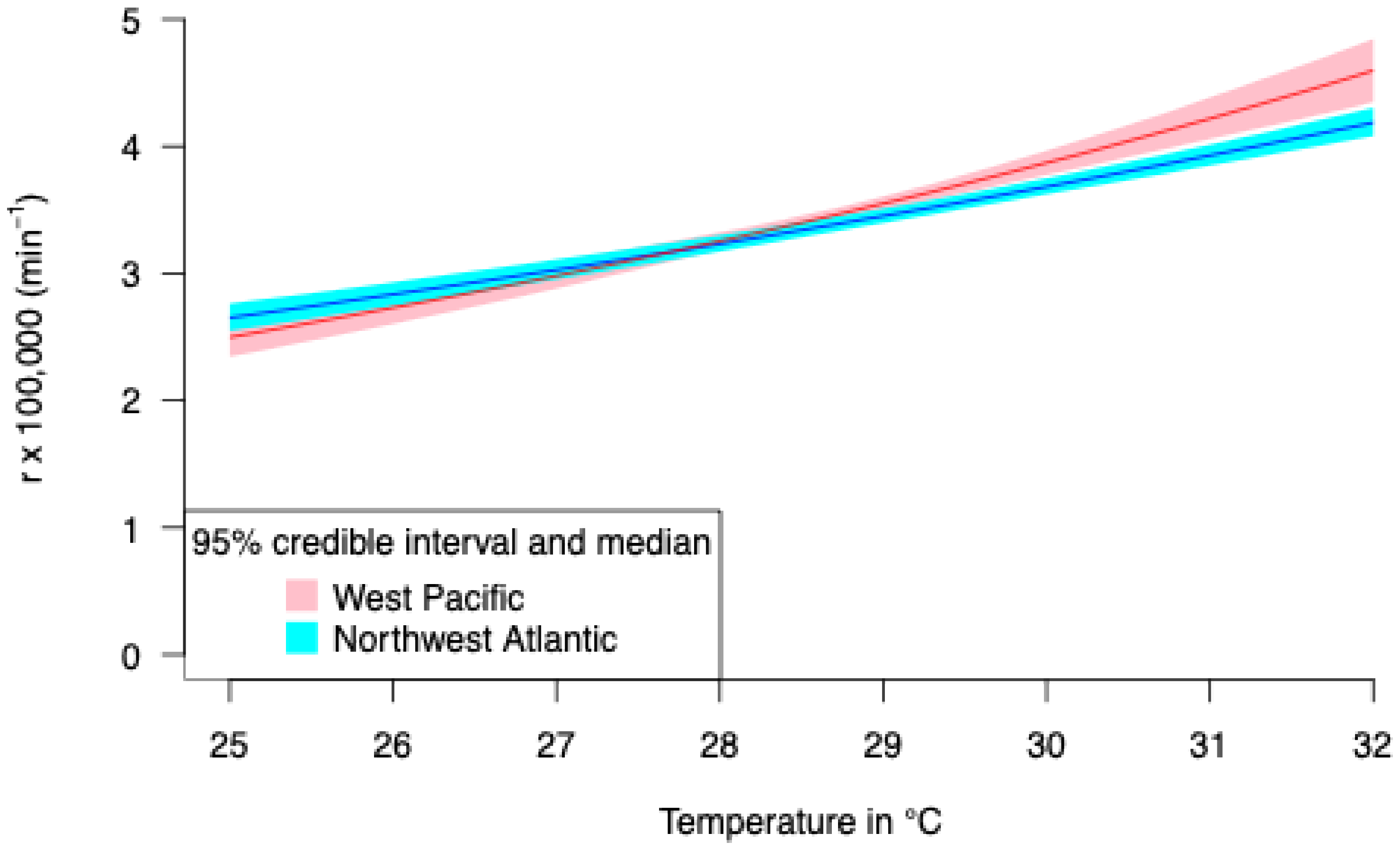
3.2. Thermal Reaction Norm of Embryo Growth
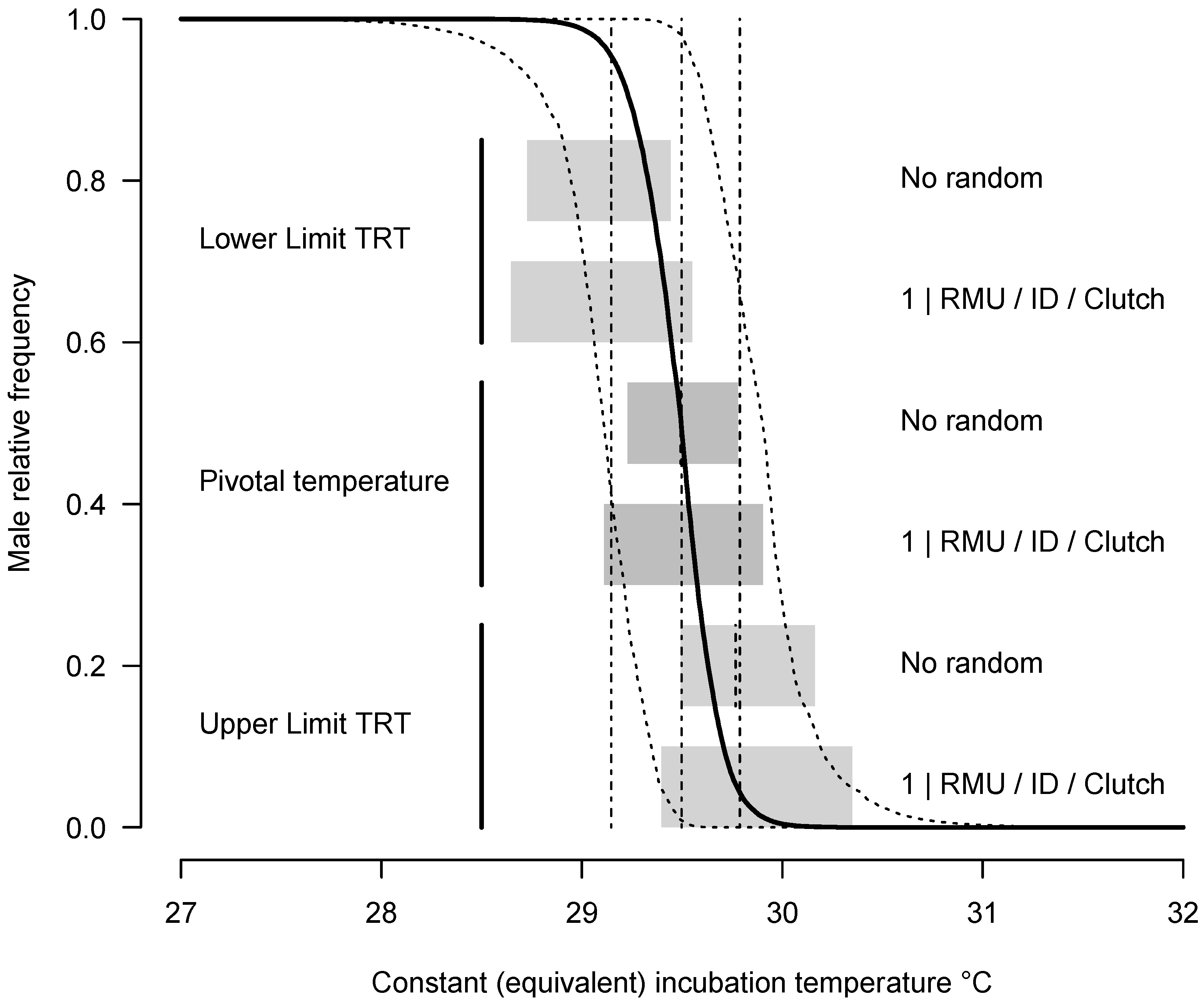
3.3. Pattern of TSD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Temperature | Published Hatching Success | Number of Incubated Eggs (Inferred) | Hatched (Inferred) | Non-Hatched (Inferred) | Inferred Hatching Success |
|---|---|---|---|---|---|
| 28 °C | 73.9% | 23 | 17 | 6 | 73.9% |
| 29 °C | 45.5% | 22 | 10 | 12 | 45.5% |
| 29.5 °C | 63.6% | 22 | 14 | 8 | 63.6% |
| 30 °C | 69.6% | 23 | 16 | 7 | 69.6% |
| 30.5 °C | 45.5% | 22 | 10 | 12 | 45.5% |
| 31 °C | 54.6% | 22 | 12 | 10 | 54.6% |
| 31.5 °C | 36.4% | 22 | 8 | 14 | 36.4% |
| 32 °C | 50.0% | 38 | 19 | 19 | 50% |
| 33 °C | 0.0% | ? | 0 | ? |
| Temperatures | Hatched Embryos | Sex Ratio in [20] Text | Sex Ratio in [20] Graph | %Error | Sex Ratio in [24] Graph | %Error |
|---|---|---|---|---|---|---|
| 29 °C | 10 | 0.0635 | 0.0433 | |||
| 29.5 °C | 14 | 0.6 | 0.6012 | 0.2% | 0.6036 | 0.6% |
| 30 °C | 16 | 0.9385 | 0.9635 |
| DatabaseTSD 9.4 [31] | ROSIE 1.0.3 [32] | Consensus | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperatures | Hatched | M | F | S | M | F | S | M | F | S |
| 28 °C | 17 | 17 | 0 | 17 | 17 | 0 | 17 | 17 | 0 | 17 |
| 29 °C | 10 | 9 | 1 | 10 | 9 | 1 | 10 | 9 | 1 | 10 |
| 29.5 °C | 14 | 4 | 6 | 10 | 4 | 6 | 10 | 4 | 6 | 10 |
| 30 °C | 16 | 1 | 15 | 16 | 1 | 13 | 14 | 1 | 15 | 16 |
| 30.5 °C | 10 | 0 | 16 | 16 | 0 | 16 | 16 | 0 | 10 | 10 |
| 31 °C | 12 | 0 | 10 | 10 | 0 | 10 | 10 | 0 | 12 | 12 |
| 31.5 °C | 8 | 0 | 12 | 12 | 0 | 12 | 12 | 0 | 8 | 8 |
| 32 °C | 19 | 0 | 8 | 8 | 0 | 8 | 8 | 0 | 19 | 19 |
| 33 °C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
References
- Charnier, M. Action de la température sur la sex-ratio chez l’embryon d’Agama agama (Agamidae, Lacertilien). Comptes Rendus des Séances de la Société de Biol. et de ses Fil. 1966, 160, 620–622. [Google Scholar]
- Pieau, C. Sur la proportion sexuelle chez les embryons de deux Chéloniens (Testudo graeca L. et Emys orbicularis L.) issus d’oeufs incubés artificiellement. Comptes Rendus de l’Académie des Sci. Paris Série D 1971, 272, 3071–3074. [Google Scholar]
- Pieau, C. Effets de la température sur le développement des glandes génitales chez les embryons de deux Chéloniens, Emys orbicularis L. et Testudo graeca L. Comptes Rendus de l’Académie des Sci. Paris Série D 1972, 274, 719–722. [Google Scholar]
- Krueger, C.J.; Janzen, F.J. On the origin of patterns of temperature-dependent sex determination. Evolution 2023, 77, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Mrosovsky, N.; Pieau, C. Transitional range of temperature, pivotal temperatures and thermosensitive stages for sex determination in reptiles. Amphib.-Reptil. 1991, 12, 169–179. [Google Scholar] [CrossRef]
- Hulin, V.; Delmas, V.; Girondot, M.; Godfrey, M.H.; Guillon, J.-M. Temperature-dependent sex determination and global change: Are some species at greater risk? Oecologia 2009, 160, 493–506. [Google Scholar] [CrossRef]
- Ewert, M.A.; Lang, J.W.; Nelson, C.E. Geographic variation in the pattern of temperature-dependent sex determination in the American snapping turtle (Chelydra serpentina). J. Zool. 2005, 265, 81–95. [Google Scholar] [CrossRef]
- Carter, A.W.; Bowden, R.M.; Paitz, R.T. Seasonal shifts in sex ratios are mediated by maternal effects and fluctuating incubation temperatures. Funct. Ecol. 2017, 31, 876–884. [Google Scholar] [CrossRef]
- Bull, J.J.; Vogt, R.C.; Bulmer, M.G. Heritability of sex ratio in turtles with environmental sex determination. Evolution 1982, 36, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Janzen, F.J.; Paukstis, G.L. Environmental sex determination in reptiles: Ecology, evolution, and experimental design. Quart. Rev. Biol. 1991, 66, 149–179. [Google Scholar] [CrossRef]
- Abreu-Grobois, F.A.; Morales-Mérida, B.A.; Hart, C.E.; Guillon, J.-M.; Godfrey, M.H.; Navarro, E.; Girondot, M. Recent advances on the estimation of the thermal reaction norm for sex ratios. PeerJ 2020, 8, e8451. [Google Scholar] [CrossRef] [PubMed]
- Angilletta, M.J.; Wilson, R.S.; Navas, C.A.; James, R.S. Tradeoffs and the evolution of thermal reaction norms. Trends Ecol. Evol. 2003, 18, 234–240. [Google Scholar] [CrossRef]
- Bentley, B.P.; Stubbs, J.L.; Whiting, S.D.; Mitchell, N.J.; Husak, J. Variation in thermal traits describing sex determination and development in Western Australian sea turtle populations. Funct. Ecol. 2020, 34, 2302–2314. [Google Scholar] [CrossRef]
- Carter, A.L.; Janzen, F.J. Predicting the effects of climate change on incubation in reptiles: Methodological advances and new directions. J. Exp. Biol. 2021, 224. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, A.J.; Miller, J.H.; Suzuki, D.T.; Lewontin, R.C.; Gelbart, W.M. Norm of reaction and phenotypic distribution. In An Introduction to Genetic Analysis, 7th ed.; Griffiths, A.J., Ed.; W. H. Freeman: New York, NY, USA, 2000. [Google Scholar]
- Stan Development Team. Stan Modeling Language Users Guide and Reference Manual, 2.33; Stan Development Team: Stanford, CA, USA, 2023. [Google Scholar]
- Rimblot, F.; Fretey, J.; Lescure, J.; Pieau, C. Influence de la température sur la differenciation sexuelle des gonades chez la tortue luth (Dermochelys coriacea); étude en incubation artificielle et naturelle. In Proceedings of the Bases Biologiques de l’Aquaculture, Montpellier, France, 12–16 December 1983; pp. 355–362. [Google Scholar]
- Rimblot, F.; Fretey, J.; Mrosovsky, N.; Lescure, J.; Pieau, C. Sexual differentiation as a function of the incubation temperature of eggs in the sea-turtle Dermochelys coriacea (Vandelli, 1761). Amphib.-Reptil. 1985, 85, 83–92. [Google Scholar] [CrossRef]
- Rimblot-Baly, F.; Lescure, J.; Fretey, J.; Pieau, C. Sensibilité à la température de la différenciation sexuelle chez la tortue Luth, Dermochelys coriacea (Vandelli, 1761); application des données de l’incubation artificielle à l’étude de la sex-ratio dans la nature. Ann. Des Sci. Nat. Zool. 1986, 8, 277–290. [Google Scholar]
- Binckley, C.A.; Spotila, J.R.; Wilson, K.S.; Paladino, F.V. Sex determination and sex ratios of Pacific Leatherback Turtles, Dermochelys coriacea. Copeia 1998, 1998, 291–300. [Google Scholar] [CrossRef]
- Chevalier, J.; Godfrey, M.H.; Girondot, M. Significant difference of temperature-dependent sex determination between French Guiana (Atlantic) and Playa Grande (Costa-Rica, Pacific) Leatherbacks (Dermochelys coriacea). Ann. Des Sci. Nat.-Zool. Et Biol. Anim. 1999, 20, 147–152. [Google Scholar] [CrossRef]
- Mrosovsky, N.; Kamel, S.; Rees, A.F.; Margaritoulis, D. Pivotal temperature for loggerhead turtles (Caretta caretta) from Kyparissia Bay, Greece. Can. J. Zool.-Rev. Can. De Zool. 2002, 80, 2118–2124. [Google Scholar] [CrossRef]
- Chan, E.H.; Liew, H.C. Incubation temperatures and sex-ratios in the Malaysian leatherback turtle Dermochelys coriacea. Biol. Conserv. 1995, 74, 169–174. [Google Scholar] [CrossRef]
- Binckley, C.A.; Spotila, J.R. Sex determination and hatching sex ratios of the leatherback sea turtles. In The Leatherback Turtle; Spotila, J., Tomillo, P.S., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2015; pp. 84–93. [Google Scholar]
- Lescure, J.; Rimblot, F.; Fretey, J.; Renous, S.; Pieau, C. Influence of the incubation-temperature of eggs on the hatchling sex-ratio in the leatherback, Dermochelys coriacea. Bull. De La Société Zool. De Fr.-Evol. Et Zool. 1985, 110, 355–359. [Google Scholar]
- Lescure, J.; Rimblot-Baly, F.; Pieau, C.; Fretey, J. Effect of temperature on sex differentiation in Dermochelys coriacea: Sex determination of hatchlings. In Proceedings of the Second Western Atlantic Turtle Symposium, Mayaguez, Puerto Rico, 12–16 October 1987; pp. 330–331. [Google Scholar]
- Desvages, G.; Girondot, M.; Pieau, C. Sensitive stages for the effects of temperature on gonadal aromatase activity in embryos of the marine turtle Dermochelys coriacea. Gen. Comp. Endocrinol. 1993, 92, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Pieau, C.; Fretey, J.; Rimblot, F.; Lescure, J. Influence de la température d’incubation des oeufs sur la différenciation sexuelle des tortues. Son importance dans l’élevage des tortues. Acta Zool. Et Pathol. Antverp. 1984, 78, 277–296. [Google Scholar]
- Lescure, J.; Rimblot-Baly, F.; Fretey, J.; Pieau, C. Critical-temperature for sexual-differentiation and reproductive strategies in marine turtles. J. De Physiol. 1987, 82, A28. [Google Scholar]
- Rimblot-Baly, F. Contribution à la Biologie du Développement et de la reproduction chez la tortue luth (Dermochelys coriacea, vandelli, 1761); Université Paris-Sud: Orsay, France, 1991. [Google Scholar]
- Girondot, M. Embryogrowth: Tools to Analyze the Thermal Reaction Norm of Embryo Growth, 9.5; The Comprehensive R Archive Network: Orsay, France, 2024. [Google Scholar] [CrossRef]
- Krueger, C.J.; Janzen, F.J. ROSIE, a database of reptilian offspring sex ratios and sex-determining mechanisms, beginning with Testudines. Sci. Data 2022, 9, 22. [Google Scholar] [CrossRef]
- Renous, S.; Rimblot-Baly, F.; Fretey, J.; Pieau, C. Caractéristique du développement embryonnaire de la Tortue Luth, Dermochelys coriacea (Vandelli, 1761). Ann. Des Sci. Nat.-Zool. Et Biol. Anim. 1989, 10, 197–229. [Google Scholar]
- Bels, V.; Rimblot-Baly, F.; Lescure, J. Croissance et maintien en captivité de la tortue luth Dermochelys coriacea (Vandelli, 1761). Rev. Fr. Aquariol. 1988, 15, 59–64. [Google Scholar]
- Rohatgi, A. WebPlotDigitizer, 4.6; Automeris-io: San Francisco, CA, USA, 2019. [Google Scholar]
- Hilterman, M.; Goverse, E. Nesting and nest success of the leatherback turtle (Dermochelys coriacea) in Suriname, 1999–2005. Chelonian Conserv. Biol. 2007, 6, 87–100. [Google Scholar] [CrossRef]
- Banerjee, S.M.; Frey, A.; Kurle, C.M.; Perrault, J.R.; Stewart, K.R. Morphological variation in leatherback (Dermochelys coriacea) hatchlings at Sandy Point National Wildlife Refuge, US Virgin Islands. Endanger. Species Res. 2020, 41, 361–372. [Google Scholar] [CrossRef]
- Benabib Nisenbaum, M. Efecto de la Temperatura de Incubación, la Posición del nido y la fecha de Anidadación en la Determinación del sexo de Dermochelys coriacea. Master’s Thesis, Universidad Nacional Autónoma de México, México City, Mexico, 1984. [Google Scholar]
- Mrosovsky, N.; Benabib, M. An assessment of two methods of sexing hatchling sea turtles. Copeia 1990, 1990, 589–591. [Google Scholar] [CrossRef]
- Fuentes, M.M.P.B.; Monsinjon, J.; Lopez, M.; Lara, P.; Santos, A.; dei Marcovaldi, M.A.G.; Girondot, M. Sex ratio estimates for species with temperature-dependent sex determination differ according to the proxy used. Ecol. Model. 2017, 365, 55–67. [Google Scholar] [CrossRef]
- Georges, A.; Limpus, C.J.; Stoutjesdijk, R. Hatchling sex in the marine turtle Caretta caretta is determined by proportion of development at a temperature, not daily duration of exposure. J. Exp. Zool. 1994, 270, 432–444. [Google Scholar] [CrossRef]
- Girondot, M.; Monsinjon, J.; Guillon, J.-M. Delimitation of the embryonic thermosensitive period for sex determination using an embryo growth model reveals a potential bias for sex ratio prediction in turtles. J. Therm. Biol. 2018, 73, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.H.; Liew, H.C. The Leatherback Turtle: A Malaysian Heritage; Tropical Press Sdn. Bhd.: Kuala Kumpur, Malayasia, 1989; p. 49. [Google Scholar]
- Gumbel, E.J. Statistics of Extremes; Columbia University Press: New York, NY, USA, 1958; p. 378. [Google Scholar]
- Morales-Mérida, B.A.; Bustamante, D.M.; Monsinjon, J.; Girondot, M. Reaction norm of embryo growth rate dependent on incubation temperature in the olive ridley sea turtle, Lepidochelys olivacea, from Pacific Central America. J. Embryol. 2018, 1, 12–24. [Google Scholar]
- Schoolfield, R.M.; Sharpe, P.J.; Magnuson, C.E. Non-linear regression of biological temperature-dependent rate models based on absolute reaction-rate theory. J. Theor. Biol. 1981, 88, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.P.; Posnik, Z.A.; Hurley, B.J.; DiMatteo, A.D.; Bandimere, A.; Rodriguez, I.; Maxwell, S.M.; Meyer, L.; Brenner, H.; Jensen, M.P.; et al. Marine turtle regional management units 2.0: An updated framework for conservation and research of wide-ranging megafauna species. Endanger. Species Res. 2023, 52, 209–223. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Monsinjon, J.; Guillon, J.-M.; Wyneken, J.; Girondot, M. Thermal reaction norm for sexualization: The missing link between temperature and sex ratio for temperature-dependent sex determination. Ecol. Model. 2022, 473, 110119. [Google Scholar] [CrossRef]
- YalçIi Özdilek, Ş.; Sönmez, B.; Kaska, Y. Sex ratio estimations of Chelonia mydas hatchlings at Samandağ Beach, Turkey. Turk. J. Zool. 2016, 40, 552–560. [Google Scholar] [CrossRef]
- Calderón-Peña, R.; Betancourt-Avila, R.; Rodríguez-Fajardo, E.; Martínez-González, Y.; Azanza-Ricardo, J. Sex ratio of the green sea turtle Chelonia mydas (Testudines: Cheloniidae) hatchlings in the Guanahacabibes Peninsula, Cuba. Rev. De Biol. Trop. 2020, 68, 777–784. [Google Scholar] [CrossRef]
- Morales Mérida, A.; Ruiz, A.; Navarro, E.; Sifuentes-Romero, I.; Abreu-Grobois, F.A.; Girondot, M. Contrasting incubation data for Lepidochelys olivacea highlight the need for more experimentation and detailed reporting. Mar. Turt. Newsl. 2015, 145, 15–19. [Google Scholar]
- Monsinjon, J.; Jribi, I.; Hamza, A.; Ouerghi, A.; Kaska, Y.; Girondot, M. Embryonic growth rate thermal reaction norm of Mediterranean Caretta caretta embryos from two different thermal habitats, Turkey and Libya. Chelonian Conserv. Biol. 2017, 16, 172–179. [Google Scholar] [CrossRef]
- Spotila, J.; Tomillo, P.S. The Leatherback Turtle; Johns Hopkins University Press: Baltimore, MD, USA, 2015; p. 246. [Google Scholar]
- Spotila, J.R.; Reina, R.D.; Steyermark, A.C.; Plotkin, P.T.; Paladino, F.V. Pacific leatherback turtles faces extinction. Nature 2000, 405, 529–530. [Google Scholar] [CrossRef] [PubMed]
- National Marine Fisheries Service; U.S. Fish and Wildlife Service. Endangered Species act. Status Review of the Leatherback Turtle (Dermochelys coriacea); Report to the National Marine Fisheries Service Office of Protected Resources and U.S. Fish and Wildlife Service; NMFS (National Marine Fisheries Service): Silver Spring, MD, USA, 2020; p. 396.
- Chevalier, J.; Cazelles, B.; Girondot, M. Apports scientifiques à la stratégie de conservation des tortues luths en Guyane française. JATBA Rev. D’éthnobiologie 1998, 40, 485–507. [Google Scholar] [CrossRef]
- Chevallier, D.; Girondot, M.; Péron, C.; Martin, J.; Bonola, M.; Chevalier, J.; de Thoisy, B.; Kelle, L.; Le Maho, Y.; Gardel, A.; et al. Beach erosion aggravates the drastic decline in marine turtle populations in French Guiana. Reg. Environ. Change 2023, 23, 116. [Google Scholar] [CrossRef]
- Molfetti, E.; Vilaca, S.T.; Georges, J.Y.; Plot, V.; Delcroix, E.; Le Scao, R.; Lavergne, A.; Barrioz, S.; dos Santos, F.R.; de Thoisy, B. Recent demographic history and present fine-scale structure in the Northwest Atlantic leatherback (Dermochelys coriacea) turtle population. PLoS ONE 2013, 8, e58061. [Google Scholar] [CrossRef]
- Dutton, P.H.; Bowen, B.W.; Owens, D.W.; Barragan, A.; Davies, S.K. Global phylogeography of the leatherback turtle (Dermochelys coriacea). J. Zool. 1999, 247, 397–409. [Google Scholar] [CrossRef]
- Eckert, K.L.; Eckert, S.A.; Adams, T.W.; Tucker, A.D. Inter-nesting migrations by leatherback sea turtles (Dermochelys coriacea) in the West Indies. Herpetologica 1989, 45, 190–194. [Google Scholar]
- Hulin, V.; Girondot, M.; Godfrey, M.H.; Guillon, J.-M. Mixed and uniform brood sex ratio strategy in turtles: The facts, the theory and their consequences. In Biology of Turtles, Wyneken, J., Bels, V., Godfrey, M.H., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 279–300. [Google Scholar]
- Morales Mérida, A.; Helier, A.; Cortés-Gómez, A.A.; Girondot, M. Hatching success rather than temperature-dependent sex determination as the main driver of olive ridley (Lepidochelys olivacea) nest density in the Pacific Coast of Central America. Animals 2021, 11, 3168. [Google Scholar] [CrossRef]
- Bell, B.A.; Spotila, J.R.; Paladino, F.V.; Reina, R.D. Low reproductive success of leatherback turtles, Dermochelys coriacea, is due to high embryonic mortality. Biol. Conserv. 2003, 115, 131–138. [Google Scholar] [CrossRef]
- Seaman, H.A.; Milton, S.L. Impacts of nest temperatures on leatherback reproductive success, hatchling morphology, and performance in South Florida. Endanger. Species Res. 2023, 51, 305–317. [Google Scholar] [CrossRef]
- Rafferty, A.R.; Santidrián Tomillo, P.; Spotila, J.R.; Paladino, F.V.; Reina, R.D. Embryonic death is linked to maternal identity in the leatherback turtle (Dermochelys coriacea). PLoS ONE 2011, 6, e21038. [Google Scholar] [CrossRef] [PubMed]
- Perrault, J.R.; Miller, D.L.; Garner, J.; Wyneken, J. Mercury and selenium concentrations in leatherback sea turtles (Dermochelys coriacea): Population comparisons, implications for reproductive success, hazard quotients and directions for future research. Sci. Total Environ. 2013, 463–464, 61–71. [Google Scholar] [CrossRef]
- Rafferty, A.R.; Johnstone, C.P.; Garner, J.A.; Reina, R.D. A 20-year investigation of declining leatherback hatching success: Implications of climate variation. R. Soc. Open Sci. 2017, 4, 170196. [Google Scholar] [CrossRef]
- Heppell, S.S. Application of life-history theory and population model analysis to turtle conservation. Copeia 1998, 1998, 367–375. [Google Scholar] [CrossRef]
- Heppell, S.S. On the importance of eggs. Mar. Turt. Newsl. 1997, 76, 6–8. [Google Scholar]
- Spencer, R.-J.; Janzen, F.J. Demographic consequences of adaptive growth and the ramifications for conservation of long-lived organisms. Biol. Conserv. 2010, 143, 1951–1959. [Google Scholar] [CrossRef]

| Incubation Temperatures | Males | Females | Sexed |
|---|---|---|---|
| 28 °C | 17 | 0 | 17 |
| 29 °C | 9 | 1 | 10 |
| 29.5 °C | 4 | 6 | 10 |
| 30 °C | 1 | 15 | 16 |
| 30.5 °C | 0 | 10 | 10 |
| 31 °C | 0 | 12 | 12 |
| 31.5 °C | 0 | 8 | 8 |
| 32 °C | 0 | 19 | 19 |
| 33 °C | 0 | 0 | 0 |
| Nest | Mean ± SE | Median CTE|TSP | 95% CI CTE|TSP | Males | Females |
|---|---|---|---|---|---|
| 1C | 27.04 ± 0.58 | 27.31 °C | 27.30; 27.31 | 11 | 0 |
| 3C | 29.21 ± 1.01 | 28.90 °C | 28.89; 28.91 | 9 | 0 |
| 4C | 28.95 ± 1.34 | 28.63 °C | 28.62; 28.64 | 9 | 0 |
| Oven | 30.42 ± 0.80 | 30.43 °C | 30.43; 30.43 | 0 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girondot, M.; Krueger, C.J.; Cléomène, C.; Tran, Z.; Chevallier, D.; Janzen, F.J. Developmental Thermal Reaction Norms of Leatherback Marine Turtles at Nesting Beaches. Animals 2024, 14, 3050. https://doi.org/10.3390/ani14213050
Girondot M, Krueger CJ, Cléomène C, Tran Z, Chevallier D, Janzen FJ. Developmental Thermal Reaction Norms of Leatherback Marine Turtles at Nesting Beaches. Animals. 2024; 14(21):3050. https://doi.org/10.3390/ani14213050
Chicago/Turabian StyleGirondot, Marc, Caleb J. Krueger, Camille Cléomène, Zeenat Tran, Damien Chevallier, and Fredric J. Janzen. 2024. "Developmental Thermal Reaction Norms of Leatherback Marine Turtles at Nesting Beaches" Animals 14, no. 21: 3050. https://doi.org/10.3390/ani14213050
APA StyleGirondot, M., Krueger, C. J., Cléomène, C., Tran, Z., Chevallier, D., & Janzen, F. J. (2024). Developmental Thermal Reaction Norms of Leatherback Marine Turtles at Nesting Beaches. Animals, 14(21), 3050. https://doi.org/10.3390/ani14213050








