Surviving on a Rock, but for How Long? Deviations in the Thermoregulatory Strategy of the Milos Wall Lizard (Podarcis milensis)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study System
2.2. Thermal Measurements
2.3. Lab Measurements (Preferred and Set-Point Temperatures; Tpref and Tset)
2.4. Effectiveness of Thermoregulation (E)
2.5. Statistical Analysis
3. Results
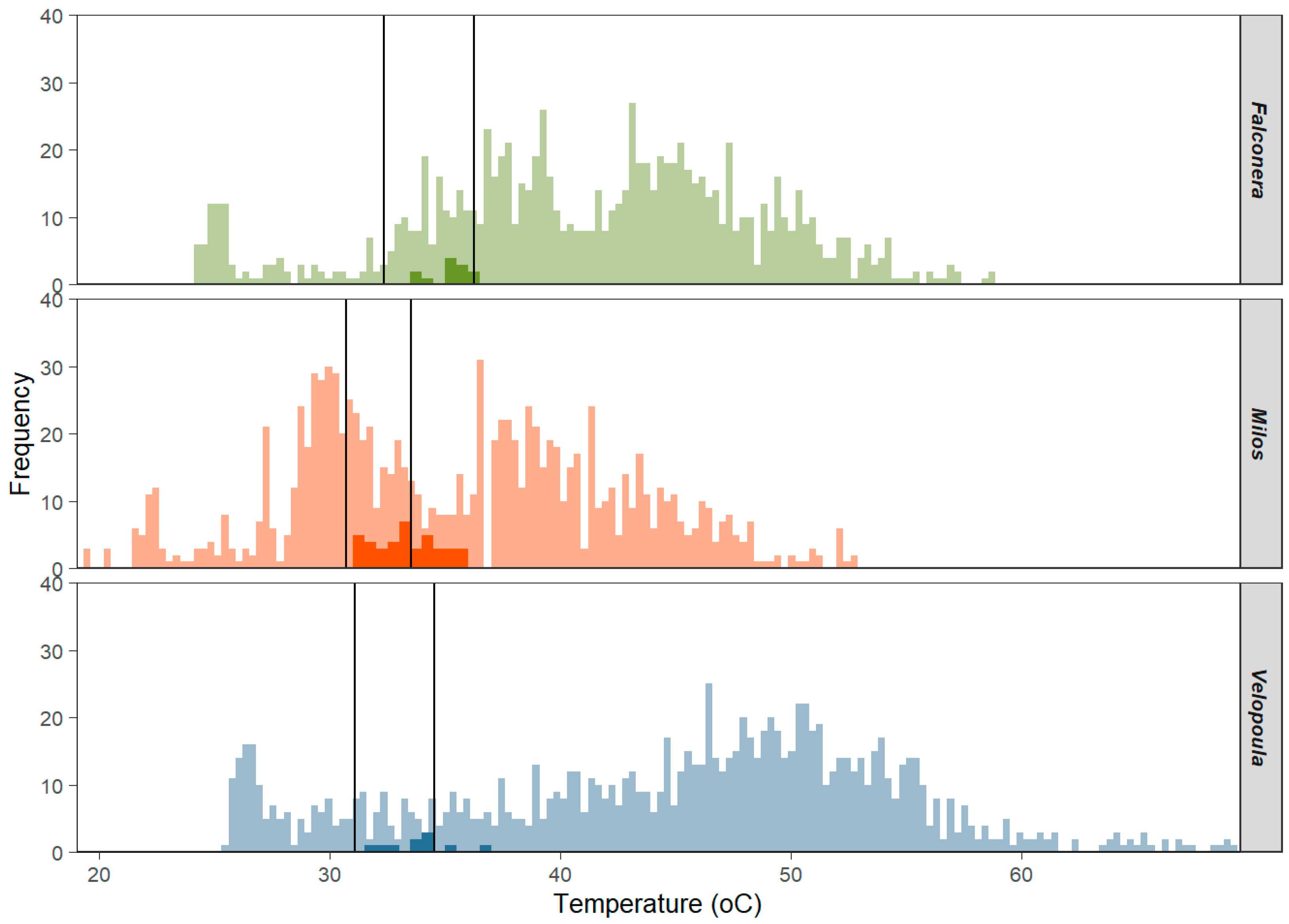
3.1. Body Temperatures (Tb and Tpref)
3.2. Operative Temperatures (Te)
3.3. Effectiveness of Thermoregulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography: Ecology, Evolution and Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Whittaker, R.J.; Fernández-Palacios, J.M.; Matthews, T.J.; Borregaard, M.K.; Triantis, K.A. Island biogeography: Taking the long view of nature’s laboratories. Science 2017, 357, eaam8326. [Google Scholar] [CrossRef] [PubMed]
- Van Valen, L.M. Pattern and the balance of nature. Evol. Theor. 1973, 1, 31–49. [Google Scholar]
- Adler, G.H.; Levins, R. The island syndrome in rodent populations. Q. Rev. Biol. 1994, 69, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Milberg, P.; Tyrberg, T. Naïve birds and noble savages—A review of man-caused prehistoric extinctions of island birds. Ecography 1993, 16, 229–250. [Google Scholar] [CrossRef]
- Calsbeek, R.; Cox, R.M. Experimentally assessing the relative importance of predation and competition as agents of selection. Nature 2010, 465, 613–616. [Google Scholar] [CrossRef]
- Losos, J.B.; Pringle, R.M. Competition, predation and natural selection in island lizards. Nature 2011, 475, E1–E2. [Google Scholar] [CrossRef]
- Sagonas, K.; Pafilis, P.; Valakos, E.D. Effects of insularity on digestive performance: Living in islands induces shifts in physiological and morphological traits in a Mediterranean lizard? Sci. Nat. 2015, 102, 55–62. [Google Scholar] [CrossRef]
- Huey, R.B.; Webster, T.P. Thermal biology of Anolis lizards in a complex fauna: The Christatellus group on Puerto Rico. Ecology 1976, 57, 985–994. [Google Scholar] [CrossRef]
- Grbac, I.; Bauwens, D. Constraints on temperature regulation in two sympatric Podarcis lizards during autumn. Copeia 2001, 2001, 178–186. [Google Scholar] [CrossRef]
- Karameta, E.; Gavriilidi, I.; Sfenthourakis, S.; Pafilis, P. Seasonal Variation in the Thermoregulation Pattern of an Insular Agamid Lizard. Animals 2023, 13, 3195. [Google Scholar] [CrossRef]
- Bartholomew, G.A. Physiological control of body temperatures. In Biology of the Reptilia; Gans, C., Pough, F.H., Eds.; Academic Press: New York, NY, USA, 1982; Volume 12, Physiology C, Physiological Ecology; pp. 167–211. [Google Scholar]
- Angilletta, M.J.J. Thermal Adaptation. A Theoritical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Meiri, S.; Bauer, A.M.; Chirio, L.; Colli, G.R.; Das, I.; Doan, T.M.; Feldman, A.; Herrera, F.-C.; Novosolov, M.; Pafilis, P.; et al. Are lizards feeling the heat? A tale of ecology and evolution under two temperatures. Global Ecol. Biogeogr. 2013, 22, 834–845. [Google Scholar] [CrossRef]
- Garcia-Porta, J.; Irisarri, I.; Kirchner, M.; Rodríguez, A.; Kirchhof, S.; Brown, J.L.; MacLeod, A.; Turner, A.P.; Ahmadzadeh, F.; Albaladejo, G.; et al. Environmental temperatures shape thermal physiology as well as diversification and genome-wide substitution rates in lizards. Nat. Commun. 2019, 10, 4077. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Camacho, F.J.; Reguera, S.; Moreno-Rueda, G.; Pleguezuelos, J.M. Patterns of seasonal activity in a Mediterranean lizard along a 2200 m altitudinal gradient. J. Therm. Biol. 2013, 38, 64–69. [Google Scholar] [CrossRef]
- Sears, M.W.; Angilletta, M.J.J. Costs and benefits of thermoregulation revisited: Both the heterogeneity and spatial structure of temperature drive energetic costs. Am. Nat. 2015, 185, E94–E102. [Google Scholar] [CrossRef]
- Sunday, J.M.; Bates, A.E.; Kearney, M.R.; Colwell, R.K.; Dulvy, N.K.; Longino, J.T.; Huey, R.B. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl. Acad. Sci. USA 2014, 111, 5610–5615. [Google Scholar] [CrossRef]
- Sagonas, K.; Valakos, E.D.; Pafilis, P. The impact of insularity on the thermoregulation of a Mediterranean lizard. J. Therm. Biol. 2013, 38, 480–486. [Google Scholar] [CrossRef]
- Ortega, Z.; Martin-Vallejo, F.J. Main factors affecting lacertid lizard thermal ecology. Integr. Zool. 2019, 14, 293–305. [Google Scholar] [CrossRef]
- Ortega, Z.; Mencía, A.; Pérez-Mellado, V. The peak of thermoregulation effectiveness: Thermal biology of the Pyrenean rock lizard, Iberolacerta bonnali (Squamata, Lacertidae). J. Therm. Biol. 2016, 56, 77–83. [Google Scholar] [CrossRef]
- Piantoni, C.; Navas, C.A.; Ibargüengoytía, N.R. Vulnerability to climate warming of four genera of New World iguanians based on their thermal ecology. Anim. Conserv. 2016, 19, 391–400. [Google Scholar] [CrossRef]
- Schwaner, T.D. A field study of thermoregulation in black tiger snakes (Notechis ater niger: Elapidae) on the Franklin Islands, South Australia. Herpetologica 1989, 45, 393–401. [Google Scholar]
- Albaladejo-Robles, G.; Rodríguez, N.; Rodríguez-Concepción, B.; Nogales, M.; Vences, M. Limited ecophysiological variation in the Canary Island lizard Gallotia galloti (Oudart, 1839) across an elevational range of over 3500 m (Squamata: Lacertidae). Herpetol. Notes 2022, 15, 87–96. [Google Scholar]
- Karameta, E.; Sfenthourakis, S.; Pafilis, P. Are all islands the same? A comparative thermoregulatory approach in four insular populations. Amphibia-Reptilia 2022, 44, 59–69. [Google Scholar] [CrossRef]
- Triantis, K.A.; Vardinoyannis, K.; Tsolaki, E.P.; Botsaris, I.; Lika, K.; Mylonas, M. Re-approaching the small island effect. J. Biogeogr. 2006, 33, 914–923. [Google Scholar] [CrossRef]
- Sfenthourakis, S.; Triantis, K.A. Habitat diversity, ecological requirements of species and the Small Island Effect. Divers. Distrib. 2009, 15, 131–140. [Google Scholar] [CrossRef]
- Ortega, Z.; Pérez-Mellado, V.; Garrido, M.; Guerra, C.; Villa-García, A.; Alonso-Fernández, T. Seasonal changes in thermal biology of Podarcis lilfordi (Squamata, Lacertidae) consistently depend on habitat traits. J. Therm. Biol. 2014, 39, 32–39. [Google Scholar] [CrossRef]
- Pafilis, P.; Lymberakis, P.; Sagonas, K.; Valakos, E. The particularities of a remote islet shape the thermoregulatory profile of an endemic Mediterranean lizard. J. Therm. Biol. 2016, 61, 55–60. [Google Scholar] [CrossRef]
- Ortega, Z.; Mencía, A.; Giroux, A.; Pérez-Mellado, V. Broad seasonal changes in thermoregulation of Podarcis lilfordi (Squamata, Lacertidae) at Binicodrell islet (Menorca, Spain). Herpetozoa 2019, 32, 57–63. [Google Scholar] [CrossRef]
- Reppa, A.; Agori, A.F.; Santikou, P.; Parmakelis, A.; Pafilis, P.; Valakos, E.D.; Sagonas, K. Small Island Effects on the Thermal Biology of the Endemic Mediterranean Lizard Podarcis gaigeae. Animals 2023, 13, 2965. [Google Scholar] [CrossRef]
- Pafilis, P.; Herrel, A.; Kapsalas, G.; Vasilopoulou-Kampitsi, M.; Fabre, A.-C.; Foufopoulos, J.; Donihue, C.M. Habitat shapes the thermoregulation of Mediterranean lizards introduced to replicate experimental islets. J. Therm. Biol. 2019, 84, 368–374. [Google Scholar] [CrossRef]
- Huey, R.B.; Kearney, M.R.; Krockenberger, A.; Holtum, J.A.M.; Jess, M.; Williams, S.E. Predicting organismal vulnerability to climate warming: Roles of behaviour, physiology and adaptation. Philos. Trans. R. Soc. B 2012, 367, 1665–1679. [Google Scholar] [CrossRef]
- Pontes-da-Silva, E.; Magnusson, W.E.; Sinervo, B.; Caetano, G.H.; Miles, D.B.; Colli, G.R.; Diele-Viegas, L.M.; Fenker, J.; Santos, J.C.; Werneck, F.P. Extinction risks forced by climatic change and intraspecific variation in the thermal physiology of a tropical lizard. J. Therm. Biol. 2018, 73, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Sinervo, B.; Méndez-de-la-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Villagrán-Santa Cruz, M.; Lara-Resendiz, R.; Martínez-Méndez, N.; Calderón-Espinosa, M.L.; Meza-Lázaro, R.N.; et al. Erosion of Lizard Diversity by Climate Change and Altered Thermal Niches. Science 2010, 328, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Dubiner, S.; Aguilar, R.; Anderson, R.O.; Arenas Moreno, D.M.; Avila, L.J.; Boada-Viteri, E.; Castillo, M.; Chapple, D.G.; Chukwuka, C.O.; Cree, A.; et al. A global analysis of field body temperatures of active squamates in relation to climate and behaviour. Global Ecol. Biogeogr. 2024, 33, e13808. [Google Scholar] [CrossRef]
- Kubisch, E.L.; Fernández, J.B.; Ibargüengoytía, N.R. Thermophysiological plasticity could buffer the effects of global warming on a Patagonian lizard. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2023, 339, 590–601. [Google Scholar] [CrossRef]
- Ortega, Z.; Mencía, A.; Pérez-Mellado, V. Are mountain habitats becoming more suitable for generalist than cold-adapted lizards thermoregulation? PeerJ 2016, 4, e2085. [Google Scholar] [CrossRef]
- Díaz, J.A.; Izquierdo-Santiago, R.; Llanos-Garrido, A. Lizard thermoregulation revisited after two decades of global warming. Funct. Ecol. 2022, 36, 3022–3035. [Google Scholar] [CrossRef]
- Adamopoulou, C.; Valakos, E.D. Thermal ecology and activity cycle of Podarcis milensis in a sandy coastal area. Isr. J. Zool. 2005, 51, 39–52. [Google Scholar] [CrossRef]
- Hertz, P.E.; Huey, R.B.; Stevenson, R.D. Evaluating temperature regulation by field-active ectotherms: The fallacy of the inappropriate question. Am. Nat. 1993, 142, 796–818. [Google Scholar] [CrossRef]
- Lymberakis, P.; Pafilis, P.; Poulakakis, N.; Sotiropoulos, K.; Valakos, E.D. The Amphibians and Reptiles of the Aegean Sea. In Biogeography and Biodiversity of the Aegean. In honour of Prof. Moysis Mylonas; Sfenthourakis, S., Pafilis, P., Parmakelis, A., Poulakakis, N., Triantis, K.A., Eds.; Broken Hill Publishers Ltd.: Nicosia, Cyprus, 2018; pp. 169–189. [Google Scholar]
- Pafilis, P.; Maragou, P. Atlas of Amphibian and Reptiles of Greece; Broken Hill Publishers Ltd.: Nicosia, Cyprus, 2020; p. 231. [Google Scholar]
- Adamopoulou, C.; Valakos, E. Small clutch size in a Mediterranean endemic lacertid (Podarcis milensis). Copeia 2000, 2000, 610–614. [Google Scholar] [CrossRef]
- Adamopoulou, C.; Valakos, E.D.; Pafilis, P. Summer diet of Podarcis milensis, Podarcis gaigeae and Podarcis erhardii (Sauria: Lacertidae). Bonn Zool. Bull. 1999, 48, 275–282. [Google Scholar]
- Natural Environment and Climate Change Agency. The Greek Red List of Threatened Species. Available online: https://redlist.necca.gov.gr/en/ (accessed on 22 August 2024).
- Blouin-Demers, G.; Weatherhead, P.J. Thermal ecology of black rat snakes (Elaphe obsoleta) in a thermally challenging environment. Ecology 2001, 82, 3025–3043. [Google Scholar] [CrossRef]
- Van Damme, R.; Bauwens, D.; Verheyen, R.F. Selected body temperatures in the lizard Lacerta vivipara: Variation within and between populations. J. Therm. Biol. 1986, 11, 219–222. [Google Scholar] [CrossRef]
- Carretero, M.A.; Roig, J.M.; Llorente, G.A. Variation in preferred body temperature in an oviparous population of Lacerta (Zootoca) vivipara. Herpetol. J. 2005, 15, 51–55. [Google Scholar]
- Carneiro, D.; García-Muñoz, E.; Kaliontzopoulou, A.; Llorent, G.A.; Carretero, M.A. Comparing ecophysiology traits in two Podarcis wall lizards with overlapping ranges. Salamandra 2015, 51, 335–344. [Google Scholar]
- Belasen, A.; Brock, K.; Li, B.; Chremou, D.; Valakos, E.; Pafilis, P.; Sinervo, B.; Foufopoulos, J. Fine with heat, problems with water: Microclimate alters water loss in a thermally adapted insular lizard. Oikos 2017, 126, 447–457. [Google Scholar] [CrossRef]
- Maia-Carneiro, T.; Dorigo, T.A.; Rocha, C.F.D. Influences of Seasonality, Thermal Environment and Wind Intensity on the Thermal Ecology of Brazilian Sand Lizards In A Restinga Remnant. S. Am. J. Herpetol. 2012, 7, 241–251. [Google Scholar] [CrossRef]
- Spears, S.; Pettit, C.; Berkowitz, S.; Collier, S.; Colwell, C.; Livingston, E.H.; McQueen, W.; Vaughn, P.L.; Bodensteiner, B.L.; Leos-Barajas, V.; et al. Lizards in the wind: The impact of wind on the thermoregulation of the common wall lizard. J. Therm. Biol. 2024, 121, 103855. [Google Scholar] [CrossRef]
- Neel, L.K.; McBrayer, L.D. Habitat management alters thermal opportunity. Funct. Ecol. 2018, 32, 2029–2039. [Google Scholar] [CrossRef]
- Basson, C.H.; Levy, O.; Angilletta Jr, M.J.; Clusella-Trullas, S. Lizards paid a greater opportunity cost to thermoregulate in a less heterogeneous environment. Funct. Ecol. 2017, 31, 856–865. [Google Scholar] [CrossRef]
- Kapsalas, G.; Gavriilidi, I.; Adamopoulou, C.; Foufopoulos, J.; Pafilis, P. Effective thermoregulation in a newly established population of Podarcis siculus in Greece: A possible advantage for a successful invader. Acta Herpetol. 2016, 11, 111–118. [Google Scholar]
- Androulidakis, Y.S.; Krestenitis, Y.N. Sea Surface Temperature Variability and Marine Heat Waves over the Aegean, Ionian, and Cretan Seas from 2008–2021. J. Mar. Sci. Eng. 2022, 10, 42. [Google Scholar] [CrossRef]
- Nastos, P.T.; Kostianoy, A.G.; Serykh, I.V. The Aegean Sea Air Temperature Changes. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Monasterio, C.; Salvador, A.; Iraeta, P.; Díaz, J.A. The effects of thermal biology and refuge availability on the restricted distribution of an alpine lizard. J. Biogeogr. 2009, 36, 1673–1684. [Google Scholar] [CrossRef]
- Zhu, Z.; Du, W.; Zhang, C.; Yu, W.; Zhao, X.; Liu, Z.; Zeng, Z. Altitudinal variation in thermal vulnerability of Qinghai-Tibetan Plateau lizards under climate warming. Curr. Zool. 2024, zoae031. [Google Scholar] [CrossRef]
- Mi, C.; Ma, L.; Wang, Y.; Wu, D.; Du, W.; Sun, B. Temperate and tropical lizards are vulnerable to climate warming due to increased water loss and heat stress. Proc. R. Soc. B 2022, 289, 20221074. [Google Scholar] [CrossRef]
- Theodosiou, A.; Anastasiou, I.; Karameta, E.; Sagonas, K.; Kostantinidis, T.; Foufopoulos, J.; Valakos, E.D.; Pafilis, P. Dry stonewalls support biodiversity: A case study from the Aegean Sea. In Proceedings of the 14th International Congress on Dry Stone, El Jadida, Morocco, 23 September 2014. [Google Scholar]
- Rivera-Rea, J.; Macotela, L.; Moreno-Rueda, G.; Suárez-Varón, G.; Bastiaans, E.; Quintana, E.; González-Morales, J.C. Thermoregulatory behavior varies with altitude and season in the sceloporine mesquite lizard. J. Therm. Biol. 2023, 114, 103539. [Google Scholar] [CrossRef]




| Population | Tb (°C) | Tpref (°C) | Te (°C) | db (°C) | de (°C) | EH | EB |
|---|---|---|---|---|---|---|---|
| Falconera islet | 35.23 ± 0.85 | 33.14 ± 1.19 | 41.25 ± 7.27 | 0.22 ± 0.28 | 7.19 ± 5.40 | 0.97 | 6.97 |
| (33.80–36.20) | (31.66–35.17) | (24.40–58.90) | (0.0–0.68) | (0.0–23.38) | |||
| N = 12 | N = 13 | N = 28 | N = 12 | N = 28 | |||
| Velopoula islet | 34.00 ± 1.52 | 32.24 ± 0.46 | 44.84 ± 9.59 | 0.56 ± 0.95 | 12.10 ± 7.78 | 0.95 | 11.54 |
| (31.70–37.00) | (31.59–33.25) | (25.60–69.40) | (0.0–3.04) | (0.0–35.44) | |||
| N = 10 | N = 11 | N = 28 | N = 10 | N = 28 | |||
| Milos Island | 33.38 ± 1.37 | 32.03 ± 0.84 | 35.68 ± 6.67 | 0.49 ± 0.74 | 4.76 ± 4.38 | 0.89 | 4.27 |
| (31.20–35.90) | (30.23–33.80) | (19.40–52.90) | (0.0–2.37) | (0.0–19.37) | |||
| N = 40 | N = 18 | N = 28 | N = 40 | N = 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pafilis, P.; Adamopoulou, C.; Antonopoulos, A.; Deimezis-Tsikoutas, A.; Christopoulos, A.; Sagonas, K. Surviving on a Rock, but for How Long? Deviations in the Thermoregulatory Strategy of the Milos Wall Lizard (Podarcis milensis). Animals 2024, 14, 3087. https://doi.org/10.3390/ani14213087
Pafilis P, Adamopoulou C, Antonopoulos A, Deimezis-Tsikoutas A, Christopoulos A, Sagonas K. Surviving on a Rock, but for How Long? Deviations in the Thermoregulatory Strategy of the Milos Wall Lizard (Podarcis milensis). Animals. 2024; 14(21):3087. https://doi.org/10.3390/ani14213087
Chicago/Turabian StylePafilis, Panayiotis, Chloe Adamopoulou, Antonis Antonopoulos, Aris Deimezis-Tsikoutas, Apostolos Christopoulos, and Kostas Sagonas. 2024. "Surviving on a Rock, but for How Long? Deviations in the Thermoregulatory Strategy of the Milos Wall Lizard (Podarcis milensis)" Animals 14, no. 21: 3087. https://doi.org/10.3390/ani14213087
APA StylePafilis, P., Adamopoulou, C., Antonopoulos, A., Deimezis-Tsikoutas, A., Christopoulos, A., & Sagonas, K. (2024). Surviving on a Rock, but for How Long? Deviations in the Thermoregulatory Strategy of the Milos Wall Lizard (Podarcis milensis). Animals, 14(21), 3087. https://doi.org/10.3390/ani14213087






