Canine Distemper Virus in Sardinia, Italy: Detection and Phylogenetic Analysis in Foxes †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. Pathology and Immunohistochemistry
2.3. Nucleic Acid Extraction, RT-PCR Amplification, and Sequencing
2.4. Sequence and Phylogenetic Analyses
3. Results
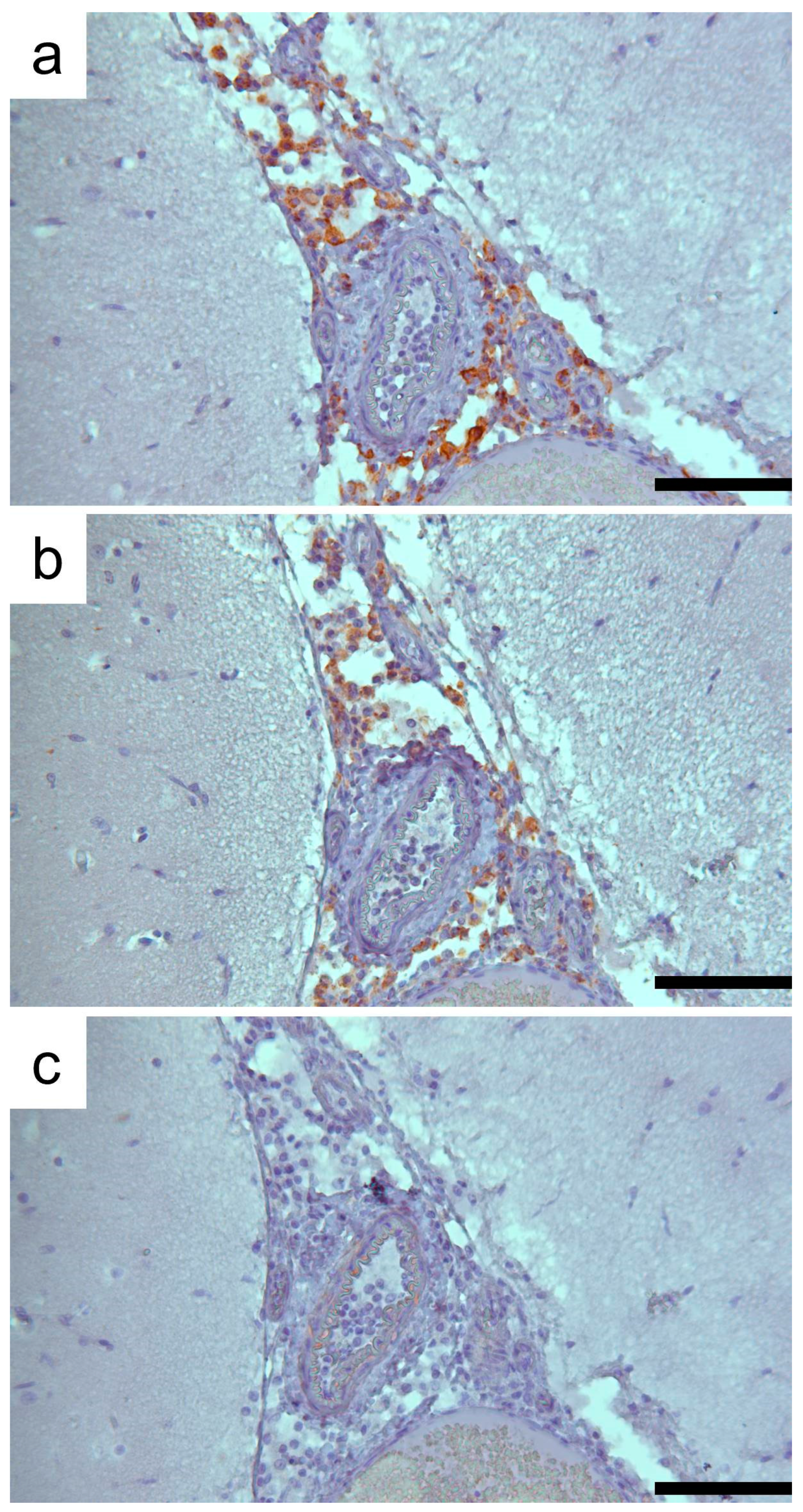
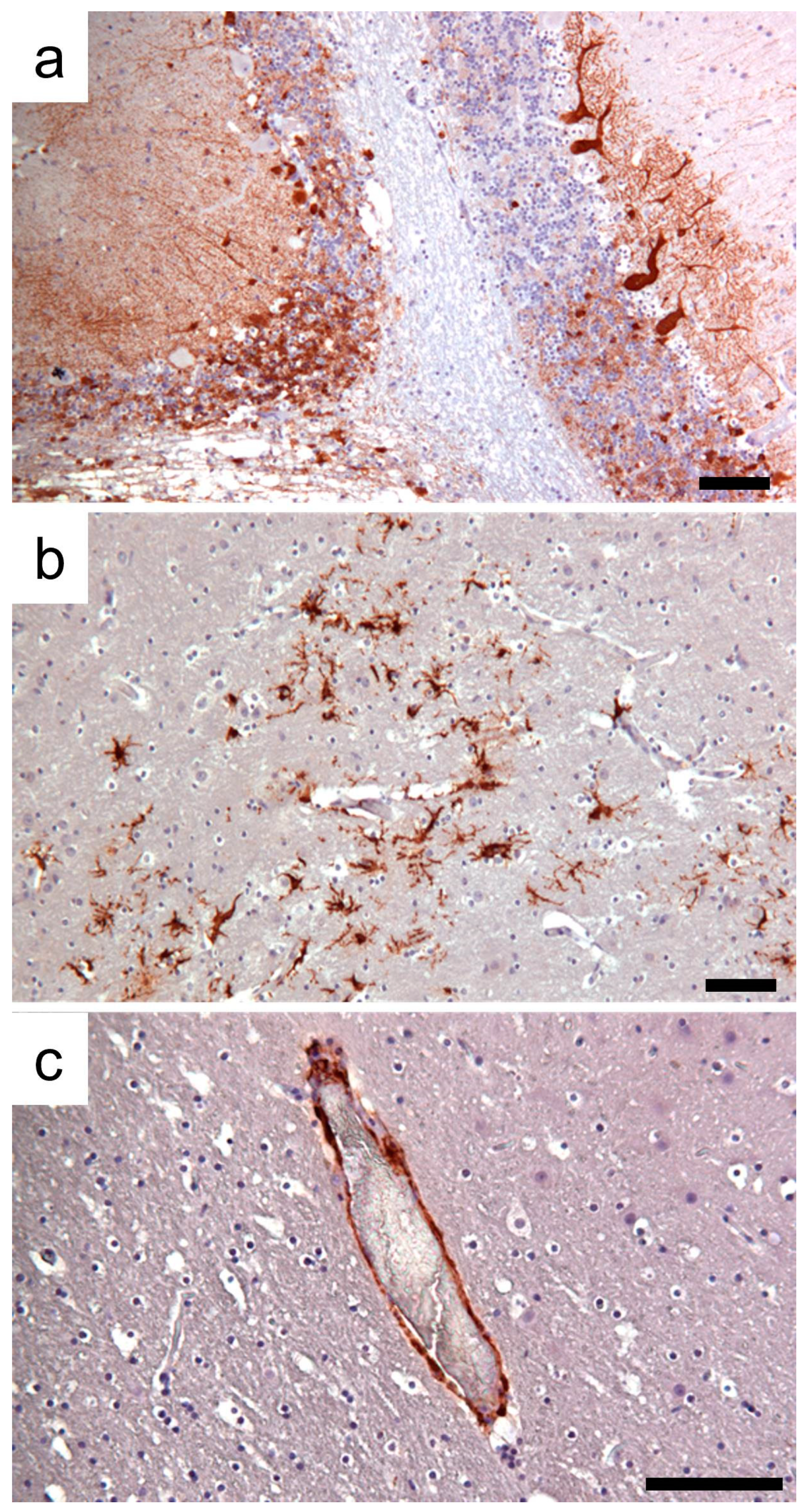
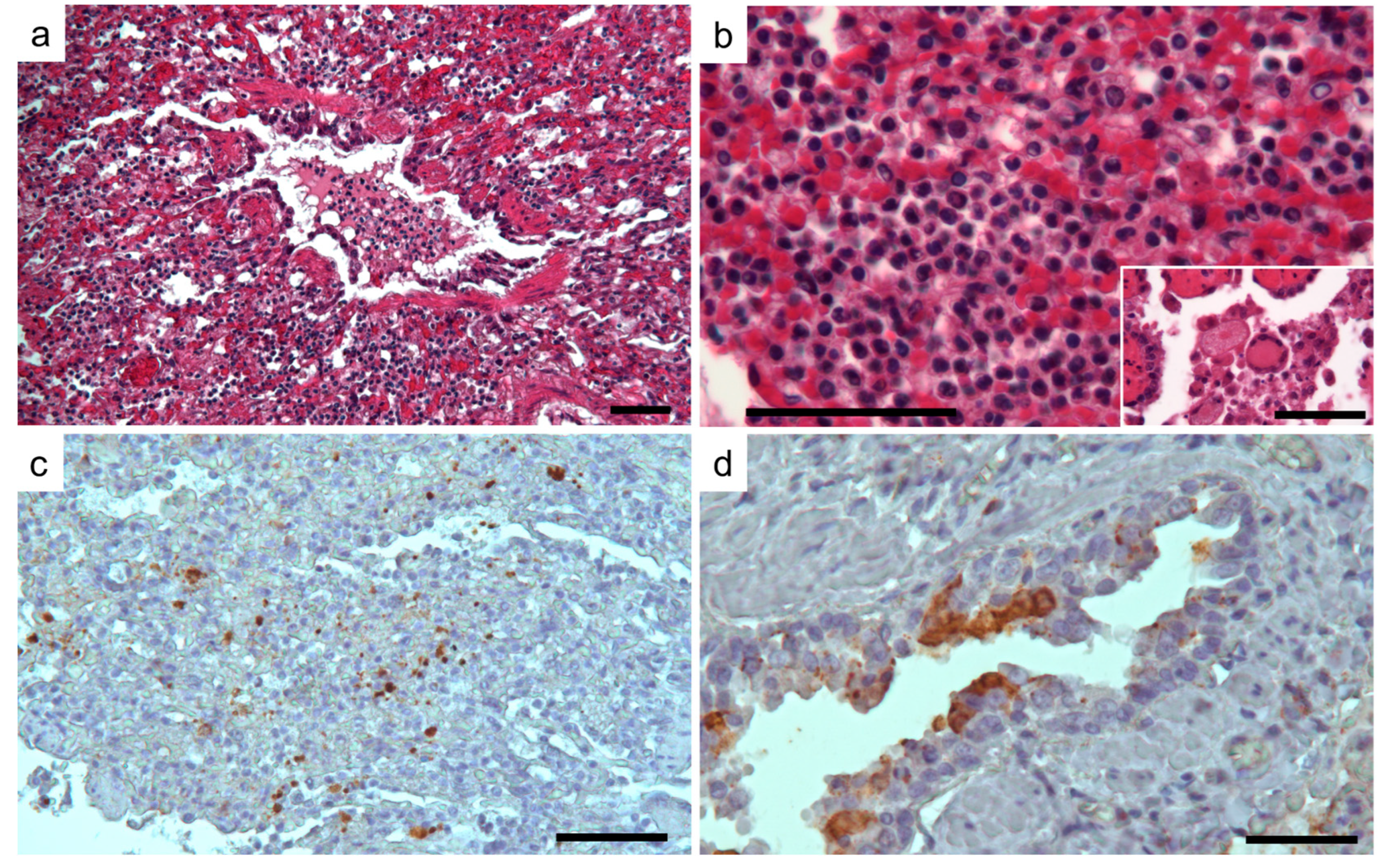
3.1. Clinic, Pathology, and Immunohistochemistry
3.2. Nucleic Acid Extraction, PCR Amplification
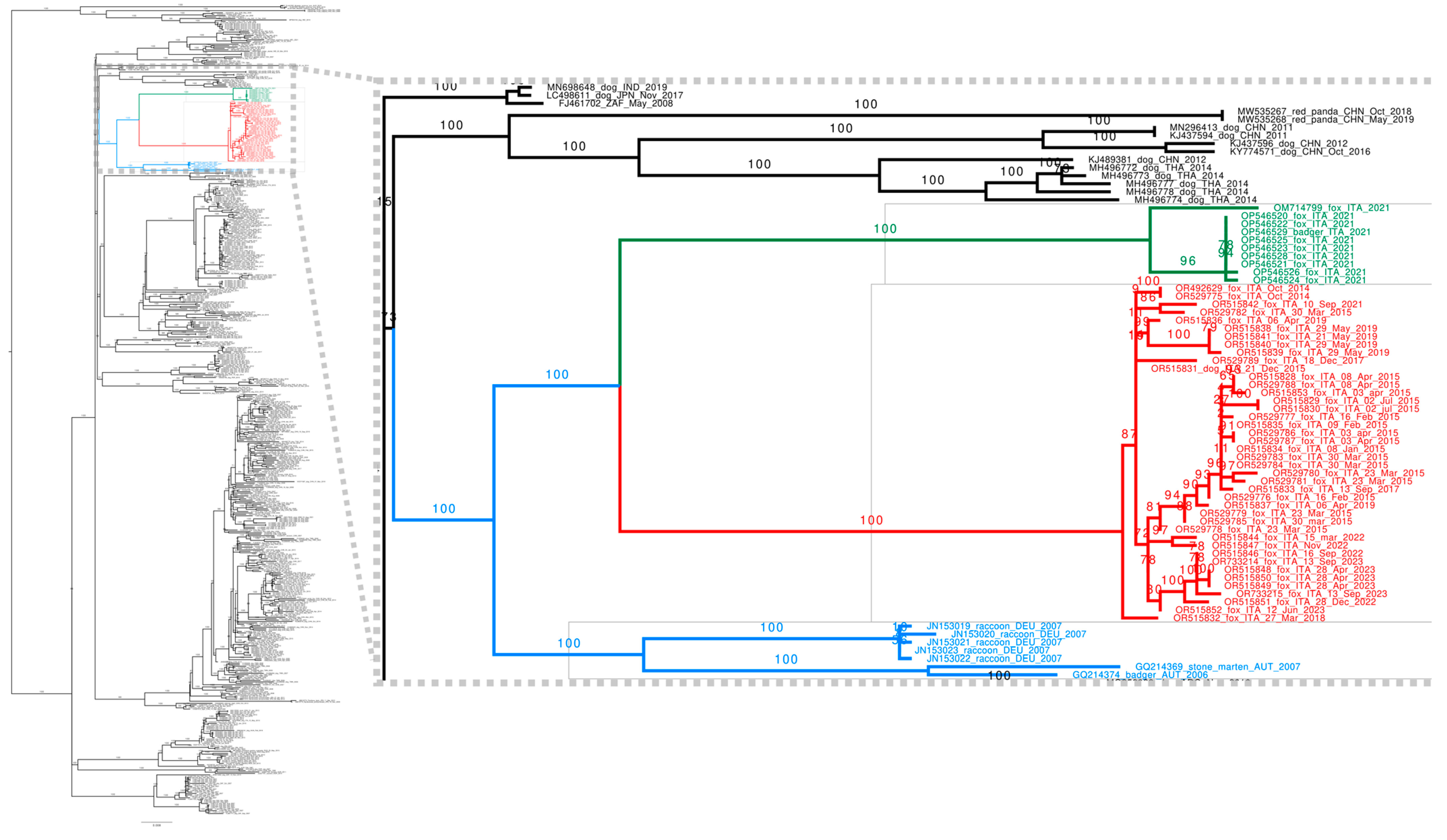
3.3. Sequencing and Phylogenetic Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Echeverry-Bonilla, D.F.; Buriticá-Gaviria, E.F.; Orjuela-Acosta, D.; Chinchilla-Cardenas, D.J.; Ruiz-Saenz, J. The First Report and Phylogenetic Analysis of Canine Distemper Virus in Cerdocyon thous from Colombia. Viruses 2022, 14, 1947. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gutierrez, M.; Ruiz-Saenz, J. Diversity of susceptible hosts in canine distemper virus infection: A systematic review and data synthesis. BMC Vet. Res. 2016, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Soutyrina, S.V.; Seryodkin, I.V. Canine distemper virus as a threat to wild tigers in Russia and across their range. Integr. Zool. 2015, 10, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.S.; Thorne, E.T.; Appel, M.J.; Belitsky, D.W. Canine distemper in black-footed ferrets (Mustela nigripes) from Wyoming. J. Wildl. Dis. 1988, 24, 385–398. [Google Scholar] [CrossRef]
- Balboni, A.; Savini, F.; Scagliarini, A.; Berti, E.; Naldi, M.; Urbani, L.; Fontana, M.C.; Carra, E.; Gibelli, L.R.M.; Gobbo, F.; et al. Natural distemper infection in stone martens (Martes foina): From infection to neutralizing antibodies. Res. Vet. Sci. 2021, 138, 196–200. [Google Scholar] [CrossRef]
- Kennedy, J.M.; Earle, J.A.; Omar, S.; Abdullah, H.A.; Nielsen, O.; Roelke-Parker, M.E.; Cosby, S.L. Canine and phocine distemper viruses: Global spread and genetic basis of jumping species barriers. Viruses 2019, 11, 944. [Google Scholar] [CrossRef]
- Loots, A.K.; Mitchell, E.; Dalton, D.L.; Kotzé, A.; Venter, E.H. Advances in canine distemper virus pathogenesis research: A wildlife perspective. J. Gen. Virol. 2017, 98, 311–321. [Google Scholar] [CrossRef]
- Origgi, F.C.; Plattet, P.; Sattler, U.; Robert, N.; Casaubon, J.; Mavrot, F.; Pewsner, M.; Wu, N.; Giovannini, S.; Oevermann, A.; et al. Emergence of canine distemper virus strains with modified molecular signature and enhanced neuronal tropism leading to high mortality in wild carnivores. Vet. Pathol. 2012, 49, 913–929. [Google Scholar] [CrossRef]
- Pope, J.P.; Miller, D.L.; Riley, M.C.; Anis, E.; Wilkes, R.P. Characterization of a novel canine distemper virus causing disease in wildlife. J. Vet. Diagn. 2016, 28, 506–513. [Google Scholar] [CrossRef]
- Sakai, K.; Nagata, N.; Ami, Y.; Seki, F.; Suzaki, Y.; Iwata-Yoshikawa, N.; Suzuki, T.; Fukushi, S.; Mizutani, T.; Yoshikawa, T.; et al. Lethal canine distemper virus outbreak in cynomolgus monkeys in Japan in 2008. J. Virol 2013, 87, 1105–1111. [Google Scholar] [CrossRef]
- Sun, Z.; Li, A.; Ye, H.; Shi, Y.; Hu, Z.; Zeng, L. Natural infection with canine distemper virus in hand-feeding Rhesus monkeys in China. Vet. Microbiol. 2010, 141, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Oleaga, A.; Blanco Vázquez, C.; Royo, L.J.; Doria Barral, T.; Bonnaire, D.; Armenteros, J.A.; Rabanal, B.; Gortázar, C.; Balseiro, A. Canine distemper virus in wildlife in south-western Europe. Transbound. Emerg. Dis. 2022, 69, e473–e485. [Google Scholar] [CrossRef] [PubMed]
- Blancou, J. Dog distemper: Imported into Europe from South America? Hist. Med. Vet. 2004, 29, 35–41. [Google Scholar] [PubMed]
- Rendon-Marin, S.; da Fontoura Budaszewski, R.; Canal, C.W.; Ruiz-Saenz, J. Tropism and molecular pathogenesis of canine distemper virus. Virol. J. 2019, 16, 30. [Google Scholar] [CrossRef]
- Carré, H. Sur la maladie des jeunes chiens. C. r. Acad. Sci. 1905, 140, 1489–1491. [Google Scholar]
- Panzera, Y.; Sarute, N.; Iraola, G.; Hernandez, M.; Perez, R. Molecular phylogeography of canine distemper virus: Geographic origin and global spreading. Mol. Phylogenet Evol. 2015, 92, 147–154. [Google Scholar] [CrossRef]
- Ndiana, L.A.; Lanave, G.; Desario, C.; Odigie, A.E.; Madubuike, K.G.; Lucente, M.S.; Ezeifeka, C.A.; Patruno, G.; Lorusso, E.; Elia, G.; et al. Detection of Selected Canine Viruses in Nigerian Free-Ranging Dogs Traded for Meat Consumption. Animals 2023, 13, 1119. [Google Scholar] [CrossRef]
- von Messling, V.; Zimmer, G.; Herrler, G.; Haas, L.; Cattaneo, R. The hemagglutinin of canine distemper virus determines tropism and cytopathogenicity. J. Virol. 2001, 75, 6418–6427. [Google Scholar] [CrossRef]
- Trogu, T.; Canziani, S.; Salvato, S.; Bianchi, A.; Bertoletti, I.; Gibelli, L.R.; Alborali, G.L.; Barbieri, I.; Gaffuri, A.; Sala, G.; et al. Canine Distemper Outbreaks in Wild Carnivores in Northern Italy. Viruses 2021, 13, 99. [Google Scholar] [CrossRef]
- Mira, F.; Purpari, G.; Di Bella, S.; Vicari, D.; Schirò, G.; Di Marco, P.; Macaluso, G.; Battilani, M.; Guercio, A. Update on canine distemper virus (CDV) strains of Arctic-like lineage detected in dogs in Italy. Vet. Ital. 2018, 54, 225–236. [Google Scholar]
- Monne, I.; Fusaro, A.; Valastro, V.; Citterio, C.; Dalla Pozza, M.; Obber, F.; Trevisiol, K.; Cova, M.; De Benedictis, P.; Bregoli, M.; et al. Distinct CDV genotype causing a major epidemic in Alpine wildlife. Vet. Microbiol. 2011, 150, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, A.; Savini, G. Old diseases for new nightmares: Distemper strikes back in Italy. Vet. Ital. 2014, 50, 151–154. [Google Scholar] [PubMed]
- Bianco, A.; Zecchin, B.; Fusaro, A.; Schivo, A.; Ormelli, S.; Bregoli, M.; Citterio, C.V.; Obber, F.; Dellamaria, D.; Trevisiol, K.; et al. Two waves of canine distemper virus showing different spatio-temporal dynamics in Alpine wildlife (2006–2018). Infect. Genet. Evol. 2020, 84, 104359. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, D.; Lorusso, A.; Di Francesco, C.E.; Gentile, L.; Di Pirro, V.; Bellacicco, A.L.; Giovannini, A.; Di Francesco, G.; Marruchella, G.; Marsilio, F.; et al. Arctic lineage-canine distemper virus as a cause of death in Apennine wolves (Canis lupus) in Italy. PLoS ONE 2014, 9, e82356. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, D.; Di Francesco, G.; Zaccaria, G.; Malatesta, D.; Brugnola, L.; Marcacci, M.; Portanti, O.; De Massis, F.; Savini, G.; Teodori, L.; et al. Lethal distemper in badgers (Meles meles) following epidemic in dogs and wolves. Infect. Genet. Evol. 2016, 46, 130–137. [Google Scholar] [CrossRef]
- Trogu, T.; Castelli, A.; Canziani, S.; Tolini, C.; Carrera, M.; Sozzi, E.; Lelli, D.; Tosi, G.; Fiorentini, L.; Di Donato, A.; et al. Detection and Molecular Characterization of Canine Distemper Virus in Wildlife from Northern Italy. Pathogens 2022, 11, 1557. [Google Scholar] [CrossRef]
- SardegnaForeste. Available online: https://www.sardegnaforeste.it/fauna/volpe (accessed on 12 February 2024).
- Frölich, K.; Czupalla, O.; Haas, L.; Hentschke, J.; Dedek, J.; Fickel, J. Epizootiological investigations of canine distemper virus in free-ranging carnivores from Germany. Vet. Microbiol. 2000, 74, 283–292. [Google Scholar] [CrossRef]
- Müller, A.; Silva, E.; Santos, N.; Thompson, G. Domestic dog origin of canine distemper virus in free-ranging wolves in Portugal as revealed by hemagglutinin gene characterization. J. Wildl. Dis. 2011, 47, 725–729. [Google Scholar] [CrossRef]
- Beineke, A.; Baumgärtner, W.; Wohlsein, P. Cross-species transmission of canine distemper virus—An update. One Health 2015, 1, 49–59. [Google Scholar] [CrossRef]
- Nouvellet, P.; Donnelly, C.A.; De Nardi, M.; Rhodes, C.J.; De Benedictis, P.; Citterio, C.; Obber, F.; Lorenzetto, M.; Dalla Pozza, M.; Cauchemez, S.; et al. Rabies and canine distemper virus epidemics in the red fox population of northern Italy (2006–2010). PLoS ONE 2013, 8, e61588. [Google Scholar] [CrossRef]
- Liao, P.; Guo, L.; Wen, Y.; Yang, Y.; Cheng, S. Phylogenetic features of hemagglutin gene in canine distemper virus strains from different genetic lineages. Int. J. Clin. Exp. Med. 2015, 8, 6607–6612. [Google Scholar]
- Senda, M.; Parrish, C.R.; Harasawa, R.; Gamoh, K.; Muramatsu, M.; Hirayama, N.; Itoh, O. Detection by PCR of wild-type canine parvovirus which contaminates dog vaccines. J. Clin. Microbiol. 1995, 33, 110–113. [Google Scholar] [CrossRef]
- Drzewnioková, P.; Festa, F.; Panzarin, V.; Lelli, D.; Moreno, A.; Zecchin, B.M.; De Benedictis, P.; Leopardi, S. Best Molecular Tools to Investigate Coronavirus Diversity in Mammals: A Comparison. Viruses 2021, 13, 1975. [Google Scholar] [CrossRef]
- Stoddard, R.A.; Gee, J.E.; Wilkins, P.P.; McCaustland, K.; Hoffmaster, A.R. Detection of pathogenic Leptospira spp. Through TaqMan polymerase chain reaction targeting LipL32 gene. Diagn. Microbiol. Infect. Dis. 2009, 64, 247–255. [Google Scholar] [CrossRef]
- Frisk, A.L.; König, M.; Moritz, A.; Baumgärtner, W. Detection of Canine Distemper Virus Nucleoprotein RNA by Reverse Transcription-PCR Using Serum, Whole Blood, and Cerebrospinal Fluid from Dogs with Distemper. J. Clin. Microbiol. 1999, 37, 3634–3643. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef]
- Viana, M.; Cleaveland, S.; Matthiopoulos, J.; Halliday, J.; Packer, C.; Craft, M.E.; Hampson, K.; Czupryna, A.; Dobson, A.P.; Dubovi, E.J.; et al. Dynamics of a morbillivirus at the domestic-wildlife interface: Canine distemper virus in domestic dogs and lions. Proc. Natl. Acad. Sci. USA 2015, 112, 1464–1469. [Google Scholar] [CrossRef]
- Guercio, A.; Mira, F.; Di Bella, S.; Gucciardi, F.; Lastra, A.; Purpari, G.; Castronovo, C.; Pennisi, M.; Di Marco Lo Presti, V.; Rizzo, M.; et al. Biomolecular Analysis of Canine Distemper Virus Strains in Two Domestic Ferrets (Mustela putorius furo). Vet. Sci. 2023, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Alfano, F.; Lanave, G.; Lucibelli, M.G.; Miletti, G.; D’Alessio, N.; Gallo, A.; Auriemma, C.; Amoroso, M.G.; Lucente, M.S.; De Carlo, E.; et al. Canine Distemper Virus in Autochtonous and Imported Dogs, Southern Italy (2014–2021). Animals 2022, 12, 2852. [Google Scholar] [CrossRef] [PubMed]
- Martella, V.; Cirone, F.; Elia, G.; Lorusso, E.; Decaro, N.; Campolo, M.; Desario, C.; Lucente, M.S.; Bellacicco, A.L.; BlixenkroneMøller, M.; et al. Heterogeneity within the hemagglutinin genes of canine distemper virus (CDV) strains detected in Italy. Vet. Microbiol. 2006, 116, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Martella, V.; Pratelli, A.; Cirone, F.; Zizzo, N.; Decaro, N.; Tinelli, A.; Foti, M.; Buonavoglia, C. Detection and genetic characterization of canine distemper virus (CDV) from free-ranging red foxes in Italy. Mol. Cell Probes 2002, 16, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, X.; Zhai, J.; Zhang, P.; Irwin, D.M.; Shen, X.; Chen, W.; Shen, Y. A new canine distemper virus lineage identified from red pandas in China. Transbound. Emerg. Dis. 2021, 69, e944–e952. [Google Scholar] [CrossRef]
- Ricci, I.; Cersini, A.; Manna, G.; Marcario, G.A.; Conti, R.; Brocherel, G.; Grifoni, G.; Eleni, C.; Scicluna, M.T. A Canine Distemper Virus Retrospective Study Conducted from 2011 to 2019 in Central Italy (Latium and Tuscany Regions). Viruses 2021, 13, 272. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Shaw, M.A.; Goodman, S.J. Pathogen evolution and disease emergence in carnivores. Proc. Biol. Sci. 2007, 274, 3165–3174. [Google Scholar] [CrossRef]



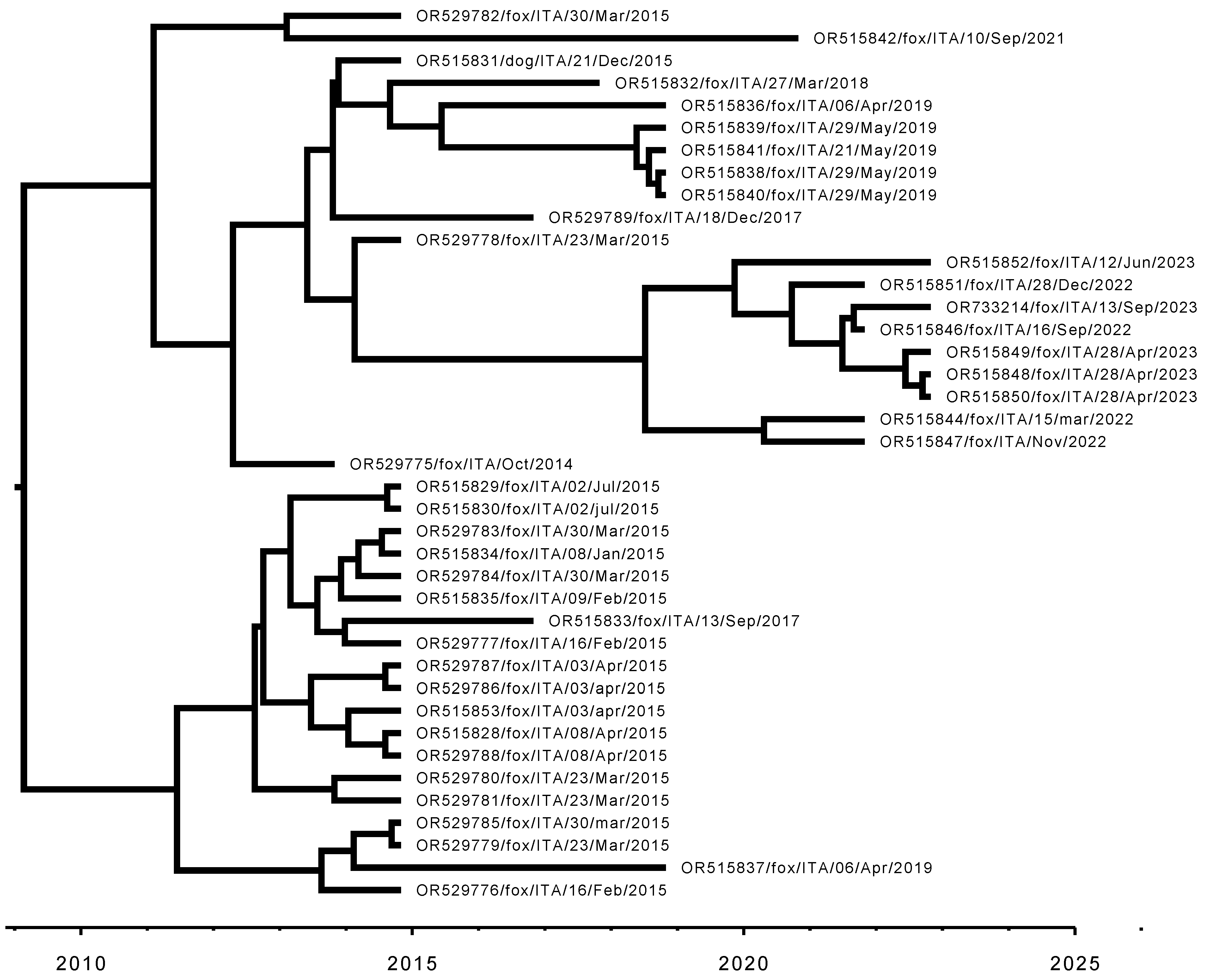

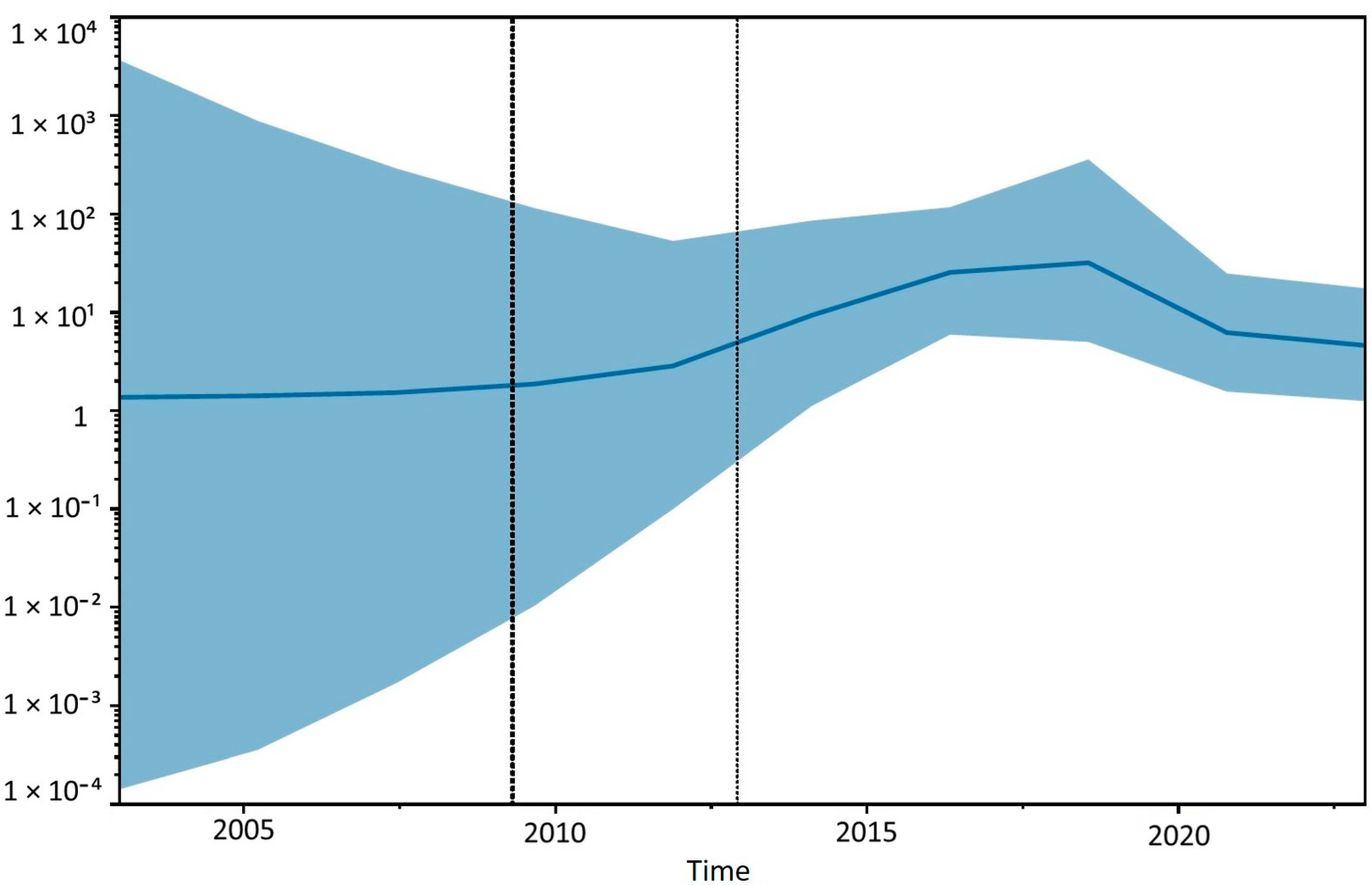
| Strain ID | Sample | Accession Number | Country | Collection Date | Host |
|---|---|---|---|---|---|
| Sardinian 1 | Brain | OR492629 | Italy | 3 October 2014 | Fox |
| Sardinian 2 | Spleen | OR529775 | Italy | 3 October 2014 | Fox |
| Sardinian 3 | Brain | OR529776 | Italy | 16 February 2015 | Fox |
| Sardinian 4 | Lung | OR529777 | Italy | 16 February 2015 | Fox |
| Sardinian 5 | Lung | OR529778 | Italy | 23 March 2015 | Fox |
| Sardinian 6 | Brain | OR529779 | Italy | 23 March 2015 | Fox |
| Sardinian 7 | Brain | OR529780 | Italy | 23 March 2015 | Fox |
| Sardinian 8 | Lung | OR529781 | Italy | 23 March 2015 | Fox |
| Sardinian 9 | Brain | OR529782 | Italy | 30 March 2015 | Fox |
| Sardinian 10 | Brain | OR529783 | Italy | 30 March 2015 | Fox |
| Sardinian 11 | Lung | OR529784 | Italy | 30 March 2015 | Fox |
| Sardinian 12 | Eyelid | OR529785 | Italy | 30 March 2015 | Fox |
| Sardinian 13 | Brain | OR529786 | Italy | 3 April 2015 | Fox |
| Sardinian 14 | Brain | OR529787 | Italy | 3 April 2015 | Fox |
| Sardinian 15 | Brain | OR529788 | Italy | 8 April 2015 | Fox |
| Sardinian 16 | Lung | OR515828 | Italy | 8 April 2015 | Fox |
| Sardinian 17 | Brain | OR515829 | Italy | 2 July 2015 | Fox |
| Sardinian 18 | Lung | OR515830 | Italy | 2 July 2015 | Fox |
| Sardinian 19 | Brain | OR515831 | Italy | 21 December 2015 | Dog |
| Sardinian 20 | Brain | OR515832 | Italy | 27 March 2018 | Fox |
| Sardinian 21 | Brain | OR515833 | Italy | 13 September 2017 | Fox |
| Sardinian 22 | Brain | OR529789 | Italy | 18 December 2017 | Fox |
| Sardinian 23 | Brain | OR515834 | Italy | 8 January 2015 | Fox |
| Sardinian 24 | Lung | OR515835 | Italy | 9 February 2015 | Fox |
| Sardinian 25 | Brain | OR515836 | Italy | 6 April 2019 | Fox |
| Sardinian 26 | Lung | OR515837 | Italy | 6 April 2019 | Fox |
| Sardinian 27 | Lung | OR515838 | Italy | 29 May 2019 | Fox |
| Sardinian 28 | Brain | OR515839 | Italy | 29 May 2019 | Fox |
| Sardinian 29 | Eyelid | OR515840 | Italy | 29 May 2019 | Fox |
| Sardinian 30 | Lung | OR515841 | Italy | 21 May 2019 | Fox |
| Sardinian 31 | Spleen | OR515842 | Italy | 10 September 2021 | Fox |
| Sardinian 33 | Brain | OR515844 | Italy | 15 March 2022 | Fox |
| Sardinian 35 | Lung | OR515846 | Italy | 16 September 2022 | Fox |
| Sardinian 36 | Brain | OR515847 | Italy | 28 November 2022 | Fox |
| Sardinian 37 | Brain | OR515848 | Italy | 28 April 2023 | Fox |
| Sardinian 38 | Lung | OR515849 | Italy | 28 April 2023 | Fox |
| Sardinian 39 | Eyelid | OR515850 | Italy | 28 April 2023 | Fox |
| Sardinian 40 | Brain | OR515851 | Italy | 28 December 2022 | Fox |
| Sardinian 41 | Brain | OR515852 | Italy | 12 June 2023 | Fox |
| Sardinian 43 | Eyelid | OR515853 | Italy | 3 April 2015 | Fox |
| Sardinian 44 | Brain | OR733214 | Italy | 13 September 2023 | Fox |
| Sardinian 45 | Lung | OR733215 | Italy | 13 September 2023 | Fox |
| Primer Set | Primer Sequence | Size | References | Annealing Temperature (C°) |
|---|---|---|---|---|
| 1st PCR: gF6708 | 5′ TCTAACCAGATCCTTGAGAC 3′ | 926 bp | This study | 56° |
| 1st PCR: gH7633rev | 5′ TGGTGGGAATATGTCACCTCT 3′ | |||
| 2nd PCR: CDVH 388 for | 5′ GAATTCGACTTCCGCGATCTCC 3′ | 968 bp | This study | 50° |
| 2nd PCR: CDVH 1335 rev | 5′ ATGGTAAGCCATCCGGAGTTC 3′ | |||
| 3rd PCR: GH 8360 for | 5′ GGTCCGTTATACTGAATGG 3′ | 792 bp | This study | 53° |
| 3rd PCR: gL 9151 rev | 5′ AGGAGCTGATAGTTATGTC 3′ |
| Amino Acid Divergence | |||
| Sardinia | Central Italy | Central Europe | |
| Sardinia | 4.73% | 5.52% | |
| Central Italy | 5.34% | ||
| Central Europe | |||
| Nucleotide Divergence | |||
| Sardinia | Central Italy | Central Europe | |
| Sardinia | 5.03% | ||
| Central Italy | 5.20% | ||
| Central Europe | 5.01% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coradduzza, E.; Stefania, F.M.; Pintus, D.; Ferretti, L.; Ledda, A.; Chessa, G.S.; Rocchigiani, A.M.; Lostia, G.; Rossi, R.; Cancedda, M.G.; et al. Canine Distemper Virus in Sardinia, Italy: Detection and Phylogenetic Analysis in Foxes. Animals 2024, 14, 3134. https://doi.org/10.3390/ani14213134
Coradduzza E, Stefania FM, Pintus D, Ferretti L, Ledda A, Chessa GS, Rocchigiani AM, Lostia G, Rossi R, Cancedda MG, et al. Canine Distemper Virus in Sardinia, Italy: Detection and Phylogenetic Analysis in Foxes. Animals. 2024; 14(21):3134. https://doi.org/10.3390/ani14213134
Chicago/Turabian StyleCoradduzza, Elisabetta, Fiori Mariangela Stefania, Davide Pintus, Luca Ferretti, Alice Ledda, Gian Simone Chessa, Angela Maria Rocchigiani, Giada Lostia, Renata Rossi, Maria Giovanna Cancedda, and et al. 2024. "Canine Distemper Virus in Sardinia, Italy: Detection and Phylogenetic Analysis in Foxes" Animals 14, no. 21: 3134. https://doi.org/10.3390/ani14213134
APA StyleCoradduzza, E., Stefania, F. M., Pintus, D., Ferretti, L., Ledda, A., Chessa, G. S., Rocchigiani, A. M., Lostia, G., Rossi, R., Cancedda, M. G., Macciocu, S., Cherchi, M., Denurra, D., Pintore, A., Bechere, R., Pudda, F., Muzzeddu, M., Dettori, M. A., Ruiu, A., ... Puggioni, G. (2024). Canine Distemper Virus in Sardinia, Italy: Detection and Phylogenetic Analysis in Foxes. Animals, 14(21), 3134. https://doi.org/10.3390/ani14213134





