First Investigation of the Spring Dietary Composition of Siberian Musk Deer (Moschus moschiferus) Using Next-Generation Sequencing
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
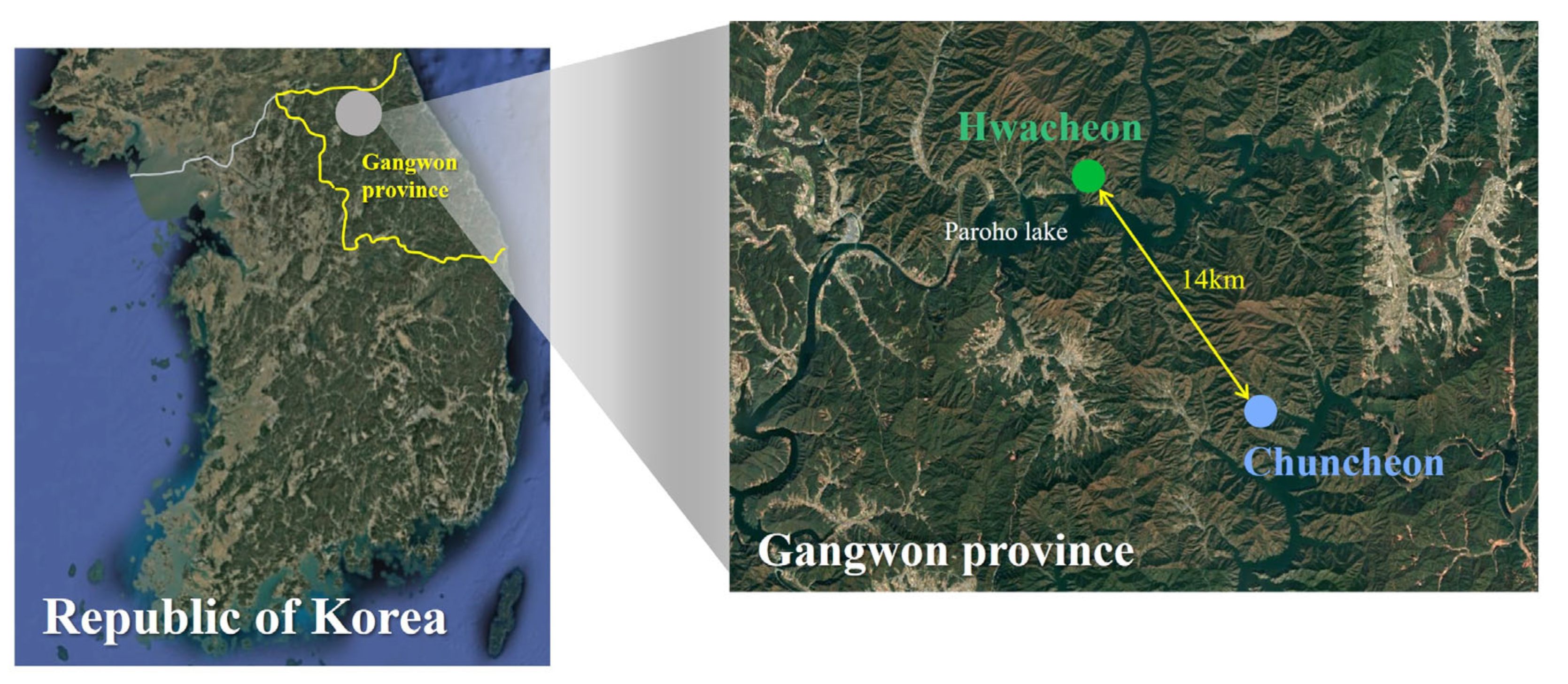
2.1. Sample Collection
2.2. Study Area
2.3. DNA Extraction and Sequencing
2.4. Operational Taxonomic Unit and Taxonomy Assignments
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Groves, C.P. Family Moschidae (musk deer). In Handbook of the Mammals of the World: Vol. 2: Hoofed Mammals; Wilson, D.E., Mittermeier, R.A., Eds.; Lynx Edicions: Barcelona, Spain, 2011; pp. 336–348. [Google Scholar]
- Tsendjav, D. Mongolian Musk Deer (Moschus moschiferus Linnaeus, 1758); Jinst Cargana Co., Ltd.: Ulaanbaatar, Mongolia, 2002. [Google Scholar]
- Yi, L.; Dalai, M.; Su, R.; Lin, W.; Erdenedalai, M.; Luvsantseren, B.; Chimedtseren, C.; Wang, Z.; Hasi, S. Whole-Genome Sequencing of Wild Siberian Musk Deer (Moschus moschiferus) Provides Insights into Its Genetic Features. BMC Genom. 2020, 21, 108. [Google Scholar] [CrossRef]
- Sharief, A.; Joshi, B.D.; Kumar, V.; Singh, H.; Singh, V.K.; Dar, S.A.; Graham, C.; Ramesh, C.; Quyoom, I.; Thakur, M.; et al. Empirical Data Suggest That the Kashmir Musk Deer (Moschus cupreus, Grubb 1982) Is the One Musk Deer Distributed in the Western Himalayas: An Integration of Ecology, Genetics and Geospatial Modelling Approaches. Biology 2023, 12, 786. [Google Scholar] [CrossRef]
- Nyambayar, B.; Mix, H.; Tsytsulina, K. Moschus moschiferus The IUCN Red List of Threatened Species. 2015: E.T13897A61977573. Available online: https://dx.doi.org/10.2305/IUCN.UK.2015-2.RLTS.T13897A61977573.en (accessed on 7 October 2024).
- Lee, S.-D.; Miller-Rushing, A.J. Degradation, Urbanization, and Restoration: A Review of the Challenges and Future of Conservation on the Korean Peninsula. Biol. Conserv. 2014, 176, 262–276. [Google Scholar] [CrossRef]
- Jo, Y.-S.; Baccus, J.T.; Koprowski, J. Mammals of Korea; National Institute of Biological Resources: Incheon, Republic of Korea, 2018; p. 291. ISBN 978-89-6811-369-7. [Google Scholar]
- Green, M.J.B. Diet Composition and Quality in Himalayan Musk Deer Based on Fecal Analysis. J. Wildl. Manag. 1987, 51, 880. [Google Scholar] [CrossRef]
- Sathyakumar, S.; Gopal, S.R.; Johnsingh, A.J.T. The Musk Deer. Sanctuary Asia 1992, 12, 52–57. [Google Scholar]
- Shrestha, B.B. Communal Pellet Deposition Sites of Himalayan Musk Deer (Moschus chrysogaster) and Associated Vegetation Composition. Master’s Thesis, Norwegian University of Life Sciences, Ås, Norway, 2012. [Google Scholar]
- Wang, W.; He, L.; Liu, B.; Li, L.; Wei, N.; Zhou, R.; Qi, L.; Liu, S.; Hu, D. Feeding Performance and Preferences of Captive Forest Musk Deer While on a Cafeteria Diet. Folia Zool. 2015, 64, 151–160. [Google Scholar] [CrossRef]
- Bell, R.H. A Grazing Ecosystem in the Serengeti. Sci. Am. 1971, 225, 86–93. [Google Scholar] [CrossRef]
- Jarman, P. The Social Organization of Antelope in Relation to Their Ecology. Behaviour 1974, 48, 215–267. [Google Scholar] [CrossRef]
- Hofmann, R.R. Evolutionary Steps of Ecophysiological Adaptation and Diversification of Ruminants: A Comparative View of Theirdigestive System. Oecologia 1989, 78, 443–457. [Google Scholar] [CrossRef]
- Shan, J.H. Food-Habits of Musk Deer (Moschus moschiferus) in Tonghe Forest Area of the Lesser Khingnan Mountains. Master’s Thesis, College of Wildlife Conservation and Utilization, Northeast Forestry University, Harbin, China, 2005. [Google Scholar]
- Zhang, L.B. On the Diet Composition of Musk Deer in Feng County, Shanxi Province. Master’s Thesis, Department of Life Science, East China Normal University, Shanghai, China, 2008. [Google Scholar]
- Hao, Y.H.; An, W.S.; Whang, Z.H.; Hao, Y.F.; Meng, X.L. The Feeding Study of Siberian Musk Deer (Moschus moschiferus). Chin. J. Zool. 1994, 1, 46–50. [Google Scholar]
- Domanov, T.A. Musk Deer Moschus moschiferus Nutrition in the Tukuringra Mountain Range, Russian Far East, during the Snow Season. Russ. J. Thériol. 2013, 12, 91–97. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, R.; He, L.; Liu, S.; Zhou, J.; Qi, L.; Li, L.; Hu, D. The Progress in Nutrition Research of Musk Deer: Implication for Conservation. Appl. Anim. Behav. Sci. 2015, 172, 1–8. [Google Scholar] [CrossRef]
- Khadka, K.K.; Singh, N.; Magar, K.T.; James, D.A. Dietary Composition, Breadth, and Overlap between Seasonally Sympatric Himalayan Musk Deer and Livestock: Conservation Implications. J. Nat. Conserv. 2017, 38, 30–36. [Google Scholar] [CrossRef]
- Biffi, M.; Gillet, F.; Laffaille, P.; Colas, F.; Aulagnier, S.; Blanc, F.; Galan, M.; Tiouchichine, M.-L.; Némoz, M.; Buisson, L.; et al. Novel Insights into the Diet of the Pyrenean Desman (Galemys pyrenaicus) Using Next-Generation Sequencing Molecular Analyses. J. Mammal. 2017, 98, 1497–1507. [Google Scholar] [CrossRef]
- Pompanon, F.; Deagle, B.E.; Symondson, W.O.C.; Brown, D.S.; Jarman, S.N.; Taberlet, P. Who Is Eating What: Diet Assessment Using Next Generation Sequencing. Mol. Ecol. 2012, 21, 1931–1950. [Google Scholar] [CrossRef]
- Gillet, F.; Tiouchichine, M.-L.; Galan, M.; Blanc, F.; Némoz, M.; Aulagnier, S.; Michaux, J.R. A New Method to Identify the Endangered Pyrenean Desman (Galemys pyrenaicus) and to Study Its Diet, Using Next Generation Sequencing from Faeces. Mamm. Biol. Z. für Säugetierkunde 2015, 80, 505–509. [Google Scholar] [CrossRef]
- Lee, S.-K.; Woo, C.; Lee, E.J.; Yamamoto, N. Using High-Throughput Sequencing to Investigate the Dietary Composition of the Korean Water Deer (Hydropotes inermis argyropus): A Spatiotemporal Comparison. Sci. Rep. 2022, 12, 22271. [Google Scholar] [CrossRef]
- Kumari, P.; Dong, K.; Eo, K.Y.; Lee, W.-S.; Kimura, J.; Yamamoto, N. DNA Metabarcoding-Based Diet Survey for the Eurasian Otter (Lutra lutra): Development of a Eurasian Otter-Specific Blocking Oligonucleotide for 12S rRNA Gene Sequencing for Vertebrates. PLoS ONE 2019, 14, e0226253. [Google Scholar] [CrossRef]
- Tingga, R.C.T.; Liam, J.; Deli, B.; Anuar, M.L.; Ampeng, A.; Md-Zain, B.M.; Tingga, R.C.T.; Md-Zain, B.M. First DNA Metabarcoding Diet Assessment on the Critically Endangered Tricolour Langur, Presbytis chrysomelas cruciger. Biodivers. Data J. 2024, 12, e124990. [Google Scholar] [CrossRef]
- Nichols, R.V.; Åkesson, M.; Kjellander, P. Diet Assessment Based on Rumen Contents: A Comparison between DNA Metabarcoding and Macroscopy. PLoS ONE 2016, 11, e0157977. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, S.; Zhao, X.; Li, C.; Gong, M. Advances and Limitations of Next Generation Sequencing in Animal Diet Analysis. Genes 2021, 12, 1854. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.B.; Emmons, C.K.; Ford, M.J.; Everett, M.; Parsons, K.; Park, L.K.; Hempelmann, J.; Doornik, D.M.V.; Schorr, G.S.; Jacobsen, J.K.; et al. Endangered Predators and Endangered Prey: Seasonal Diet of Southern Resident Killer Whales. PLoS ONE 2021, 16, e0247031. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, Z.; Tan, M.; Wang, Y.; Dai, W.; Ge, J.; Feng, L. Molecular Dietary Analysis of Three Sympatric Mustelidae in Northeast China. Animals 2022, 12, 3290. [Google Scholar] [CrossRef]
- Schumm, Y.R.; Masello, J.F.; Vreugdenhil-Rowlands, J.; Fischer, D.; Hillerich, K.; Quillfeldt, P. Diet Composition of Wild Columbiform Birds: Next-Generation Sequencing of Plant and Metazoan DNA in Faecal Samples. Sci. Nat. 2023, 110, 38. [Google Scholar] [CrossRef]
- Zaitsev, V.A.; Maksimova, D.A.; Smirnov, Y.V.; Belotelov, N.V. Home Range Use by a Male Siberian Musk Deer (Moschus moschiferus L.) in Central Sikhote-Alin. Biol. Bull. 2021, 48, 1631–1649. [Google Scholar] [CrossRef]
- Cheng, T.; Xu, C.; Lei, L.; Li, C.; Zhang, Y.; Zhou, S. Barcoding the Kingdom Plantae: New PCR Primers for ITS Regions of Plants with Improved Universality and Specificity. Mol. Ecol. Resour. 2016, 16, 138–149. [Google Scholar] [CrossRef]
- Dubois, B.; Debode, F.; Hautier, L.; Hulin, J.; Martin, G.S.; Delvaux, A.; Janssen, E.; Mingeot, D. A Detailed Workflow to Develop QIIME2-Formatted Reference Databases for Taxonomic Analysis of DNA Metabarcoding Data. BMC Genom. Data 2022, 23, 53. [Google Scholar] [CrossRef]
- Zhixiao, L.; Helin, S. Effect of Habitat Fragmentation and Isolation on the Population of Alpine Musk Deer. Russ. J. Ecol. 2002, 33, 121–124. [Google Scholar] [CrossRef]
- Woo, H.C. The Report of the Rare and Endangered Species of Mammals in Korea. Bull. Korean Assoc. Conserv. Nat. 1990, 10, 5–27. [Google Scholar]

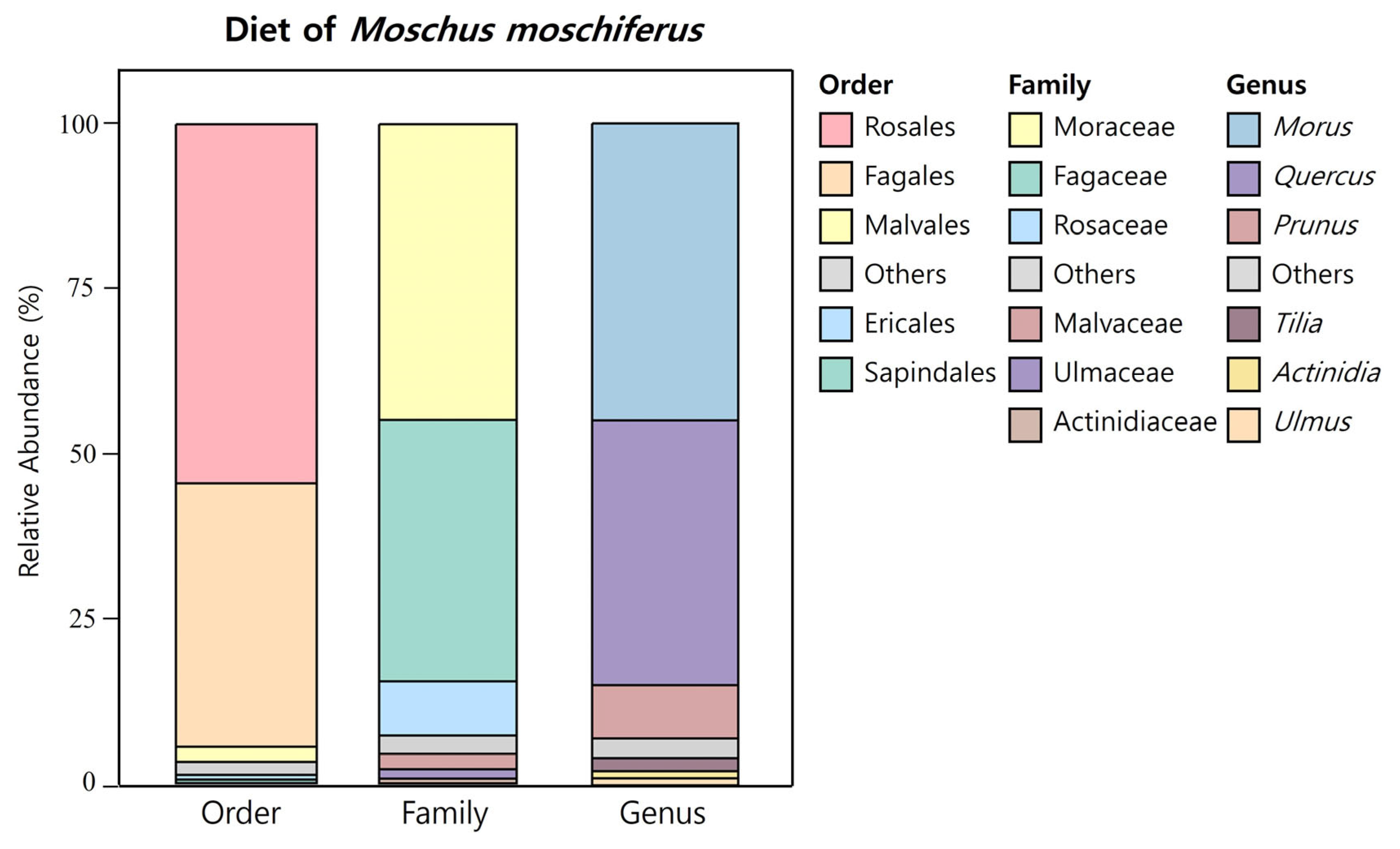
| Order | Family | Genus | Relative Abundance (%) | Growth Form | |
|---|---|---|---|---|---|
| Rosales | Moraceae | Morus | 44.94 | Woody | Tree |
| Fagales | Fagaceae | Quercus | 39.74 | Woody | Tree |
| Rosales | Rosaceae | Prunus | 7.83 | Woody | Tree/Shrub * |
| Malavales | Malvaceae | Tilia | 2.19 | Woody | Tree |
| Rosales | Ulmaceae | Ulmus | 1.38 | Woody | Tree |
| Ericales | Actinidiaceae | Actinidia | 0.71 | Woody | Vine |
| Sapindales | Sapindaceae | Acer | 0.40 | Woody | Tree |
| Pinales | Pinaceae | Pinus | 0.39 | Woody | Tree |
| Pottiales | Pottiaceae | Chionoloma | 0.28 | Mosses | - |
| Rosales | Rosaceae | Rubus | 0.32 | Woody | Shrub |
| Santalales | Viscaceae | Viscum | 0.25 | Woody | Parasitic shrub |
| Lamiales | Lamiaceae | Clerodendrum | 0.21 | Woody | Tree/Shrub * |
| Fagales | Betulaceae | Alnus | 0.17 | Woody | Tree/Shrub * |
| Fabales | Fabaceae | Pueraria | 0.13 | Woody | Vine |
| Fagales | Betulaceae | Betula | 0.11 | Woody | Tree |
| Fagales | Juglandaceae | Juglans | 0.05 | Woody | Tree |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.; Kim, A.; Lee, J.-M.; Kim, A.-Y.; Lee, Y.; Jo, Y.; Kim, K.; Do, K.-H.; Seo, K.-W.; Yoon, K.-B.; et al. First Investigation of the Spring Dietary Composition of Siberian Musk Deer (Moschus moschiferus) Using Next-Generation Sequencing. Animals 2024, 14, 3662. https://doi.org/10.3390/ani14243662
Kim N, Kim A, Lee J-M, Kim A-Y, Lee Y, Jo Y, Kim K, Do K-H, Seo K-W, Yoon K-B, et al. First Investigation of the Spring Dietary Composition of Siberian Musk Deer (Moschus moschiferus) Using Next-Generation Sequencing. Animals. 2024; 14(24):3662. https://doi.org/10.3390/ani14243662
Chicago/Turabian StyleKim, Nari, Areum Kim, Je-Min Lee, Ah-Young Kim, Yujin Lee, Yeonghoon Jo, Kiyoon Kim, Kyung-Hyo Do, Kwang-Won Seo, Kwang-Bae Yoon, and et al. 2024. "First Investigation of the Spring Dietary Composition of Siberian Musk Deer (Moschus moschiferus) Using Next-Generation Sequencing" Animals 14, no. 24: 3662. https://doi.org/10.3390/ani14243662
APA StyleKim, N., Kim, A., Lee, J.-M., Kim, A.-Y., Lee, Y., Jo, Y., Kim, K., Do, K.-H., Seo, K.-W., Yoon, K.-B., & Jeong, D.-H. (2024). First Investigation of the Spring Dietary Composition of Siberian Musk Deer (Moschus moschiferus) Using Next-Generation Sequencing. Animals, 14(24), 3662. https://doi.org/10.3390/ani14243662






