The Immune Contexture in Canine Anal Sac Adenocarcinoma: Immunohistochemical Quantification of Tumor-Infiltrating Lymphocytes and Tumor-Associated Macrophages with Image Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Immunohistochemical Methods
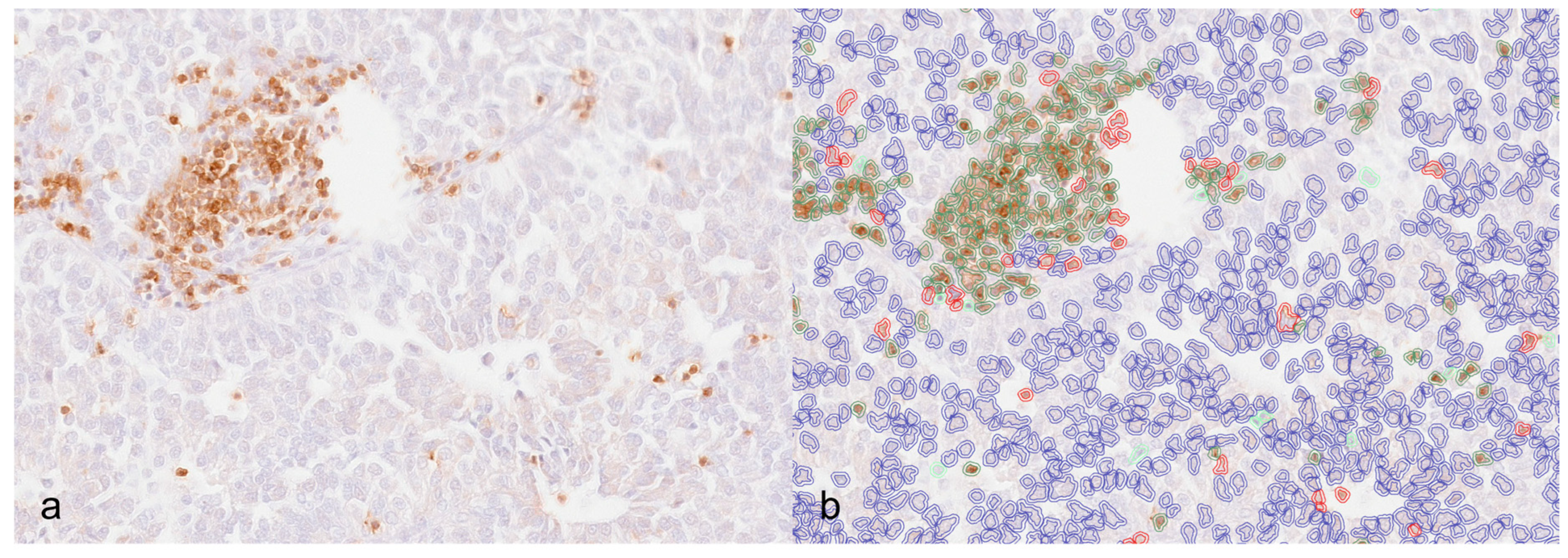
2.3. Digital Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients Data
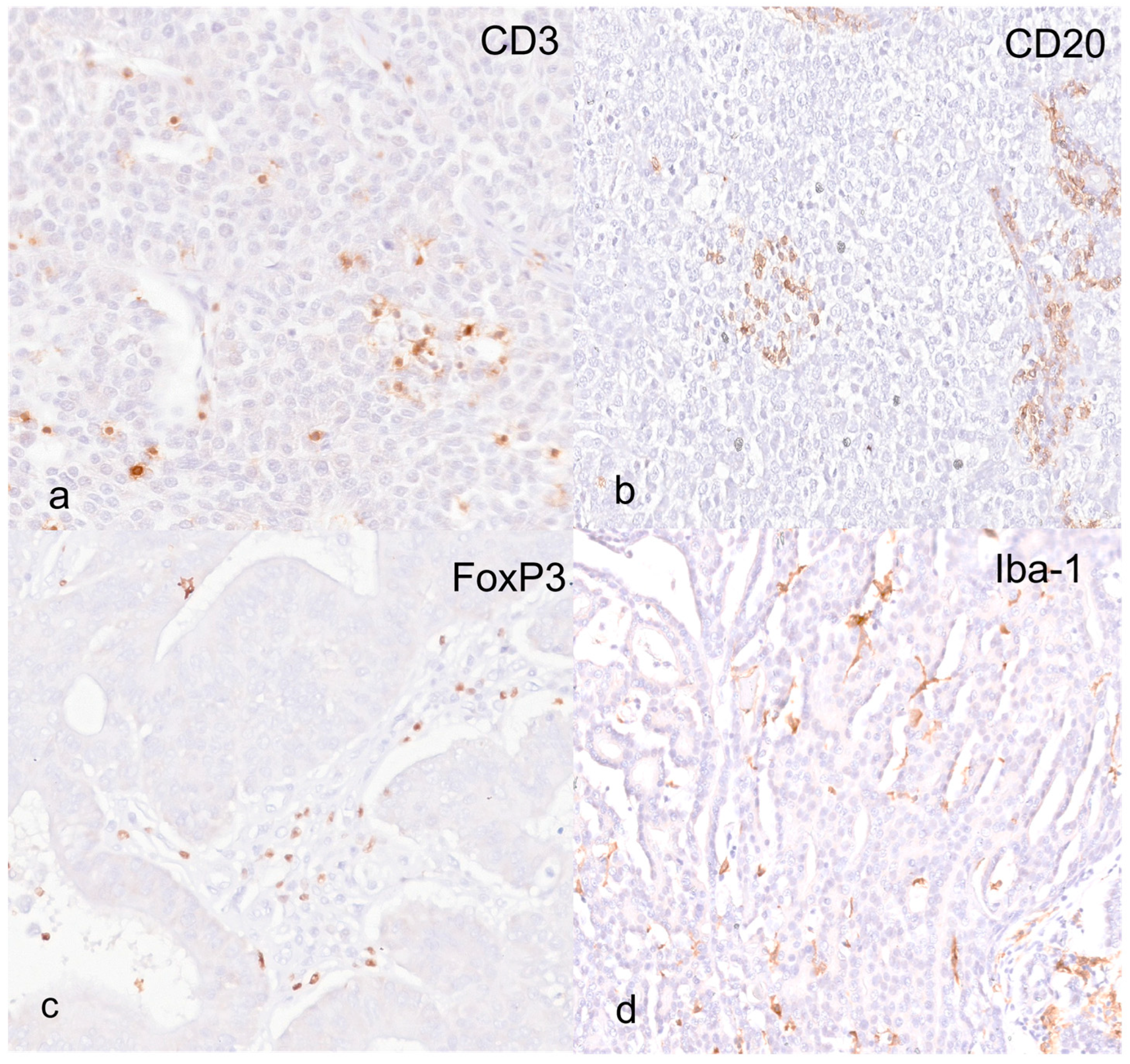
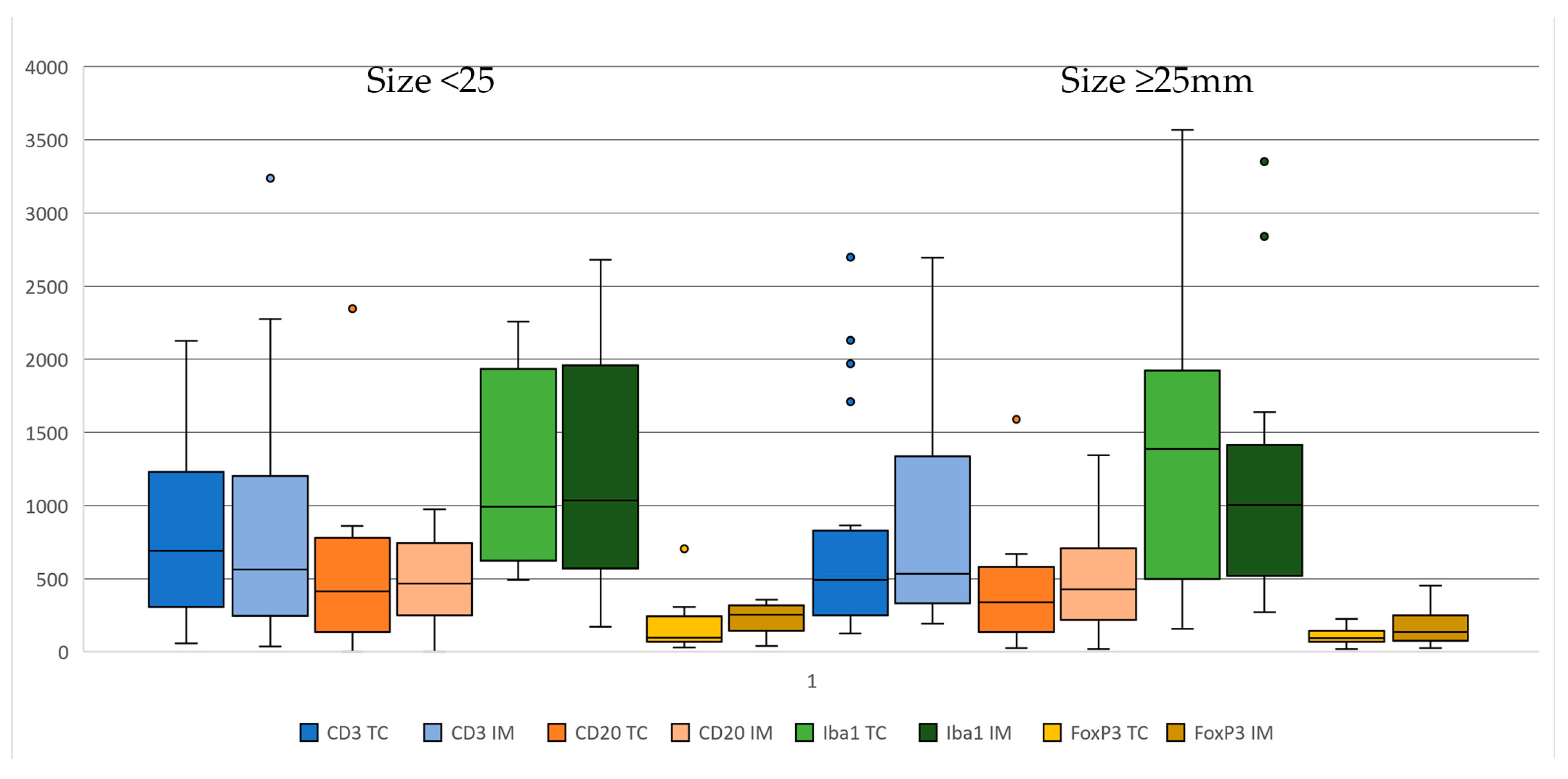
3.2. Immunohistochemistry and Digital Image Analysis
3.3. Clinical/Immunohistochemical Associations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polton, G.A.; Brearley, M.J. Clinical Stage, Therapy, and Prognosis in Canine Anal Sac Gland Carcinoma. J. Vet. Intern. Med. 2007, 21, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Pradel, J.; Berlato, D.; Dobromylskyj, M.; Rasotto, R. Prognostic Significance of Histopathology in Canine Anal Sac Gland Adenocarcinomas: Preliminary Results in a Retrospective Study of 39 Cases. Vet. Comp. Oncol. 2018, 16, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Morello, E.M.; Cino, M.; Giacobino, D.; Nicoletti, A.; Iussich, S.; Buracco, P.; Martano, M. Prognostic Value of Ki67 and Other Clinical and Histopathological Factors in Canine Apocrine Gland Anal Sac Adenocarcinoma. Animals 2021, 11, 1649. [Google Scholar] [CrossRef]
- Wong, H.; Byrne, S.; Rasotto, R.; Drees, R.; Taylor, A.; Priestnall, S.L.; Leo, C. A Retrospective Study of Clinical and Histopathological Features of 81 Cases of Canine Apocrine Gland Adenocarcinoma of the Anal Sac: Independent Clinical and Histopathological Risk Factors Associated with Outcome. Animals 2021, 11, 3327. [Google Scholar] [CrossRef]
- Williams, L.E.; Gliatto, J.M.; Dodge, R.K.; Johnson, J.L.; Gamblin, R.M.; Thamm, D.H.; Lana, S.E.; Szymkowski, M.; Moore, A.S. Carcinoma of the Apocrine Glands of the Anal Sac in Dogs: 113 Cases (1985–1995). J. Am. Vet. Med. Assoc. 2003, 223, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Schlag, A.N.; Johnson, T.; Vinayak, A.; Kuvaldina, A.; Skinner, O.T.; Wustefeld-Janssens, B.G. Comparison of Methods to Determine Primary Tumour Size in Canine Apocrine Gland Anal Sac Adenocarcinoma. J. Small Anim. Pract. 2020, 61, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.E.; Wustefeld-Janssens, B.G. A Relatively High Proportion of Dogs with Small Apocrine Gland Anal Sac Adenocarcinoma (AGASACA) Primary Tumours Present with Locoregional Lymph Node Metastasis. Vet. Comp. Oncol. 2023, 21, 327–331. [Google Scholar] [CrossRef]
- Tanis, J.-B.; Simlett-Moss, A.B.; Ossowksa, M.; Maddox, T.W.; Guillem, J.; Lopez-Jimenez, C.; Polton, G.; Rachel, B.; Finotello, R. Canine Anal Sac Gland Carcinoma with Regional Lymph Node Metastases Treated with Sacculectomy and Lymphadenectomy: Outcome and Possible Prognostic Factors. Vet. Comp. Oncol. 2021, 20, 276–292. [Google Scholar] [CrossRef] [PubMed]
- De Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical Roles of the Immune System during Cancer Development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef]
- Barnes, T.A.; Amir, E. HYPE or HOPE: The Prognostic Value of Infiltrating Immune Cells in Cancer. Br. J. Cancer 2017, 117, 451–460. [Google Scholar] [CrossRef]
- Kwak, Y.; Koh, J.; Kim, D.-W.; Kang, S.-B.; Kim, W.H.; Lee, H.S. Immunoscore Encompassing CD3+ and CD8+ T Cell Densities in Distant Metastasis Is a Robust Prognostic Marker for Advanced Colorectal Cancer. Oncotarget 2016, 7, 81778–81790. [Google Scholar] [CrossRef] [PubMed]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-Infiltrating Lymphocytes in the Immunotherapy Era. Cell Mol. Immunol. 2021, 18, 842–859. [Google Scholar] [CrossRef]
- Tiwari, A.; Oravecz, T.; Dillon, L.A.; Italiano, A.; Audoly, L.; Fridman, W.H.; Clifton, G.T. Towards a Consensus Definition of Immune Exclusion in Cancer. Front. Immunol. 2023, 14, 1084887. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Coupland, S.E.; Aittokallio, T.; Figueiredo, C.R. Resistance to Immune Checkpoint Therapies by Tumour-Induced T-Cell Desertification and Exclusion: Key Mechanisms, Prognostication and New Therapeutic Opportunities. Br. J. Cancer 2023, 129, 1212–1224. [Google Scholar] [CrossRef]
- Minoli, L.; Licenziato, L.; Kocikowski, M.; Cino, M.; Dziubek, K.; Iussich, S.; Fanelli, A.; Morello, E.; Martano, M.; Hupp, T.; et al. Development of Monoclonal Antibodies Targeting Canine PD-L1 and PD-1 and Their Clinical Relevance in Canine Apocrine Gland Anal Sac Adenocarcinoma. Cancers 2022, 14, 6188. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Angell, H.; Galon, J. From the Immune Contexture to the Immunoscore: The Role of Prognostic and Predictive Immune Markers in Cancer. Curr. Opin. Immunol. 2013, 25, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Porcellato, I.; Silvestri, S.; Menchetti, L.; Recupero, F.; Mechelli, L.; Sforna, M.; Iussich, S.; Bongiovanni, L.; Lepri, E.; Brachelente, C. Tumour-Infiltrating Lymphocytes in Canine Melanocytic Tumours: An Investigation on the Prognostic Role of CD3+ and CD20+ Lymphocytic Populations. Vet. Comp. Oncol. 2020, 18, 370–380. [Google Scholar] [CrossRef]
- Stevenson, V.B.; Perry, S.N.; Todd, M.; Huckle, W.R.; LeRoith, T. PD-1, PD-L1, and PD-L2 Gene Expression and Tumor Infiltrating Lymphocytes in Canine Melanoma. Vet. Pathol. 2021, 58, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Porcellato, I.; Brachelente, C.; De Paolis, L.; Menchetti, L.; Silvestri, S.; Sforna, M.; Vichi, G.; Iussich, S.; Mechelli, L. FoxP3 and IDO in Canine Melanocytic Tumors. Vet. Pathol. 2019, 56, 189–199. [Google Scholar] [CrossRef]
- Yasumaru, C.C.; Xavier, J.G.; Strefezzi, R.D.F.; Salles-Gomes, C.O.M. Intratumoral T-Lymphocyte Subsets in Canine Oral Melanoma and Their Association with Clinical and Histopathological Parameters. Vet. Pathol. 2021, 58, 491–502. [Google Scholar] [CrossRef]
- Bertola, L.; Pellizzoni, B.; Giudice, C.; Grieco, V.; Ferrari, R.; Chiti, L.E.; Stefanello, D.; Manfredi, M.; De Zani, D.; Recordati, C. Tumor-Associated Macrophages and Tumor-Infiltrating Lymphocytes in Canine Cutaneous and Subcutaneous Mast Cell Tumors. Vet. Pathol. 2024, 61, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.I.; Pires, I.; Prada, J.; Gregório, H.; Lobo, L.; Queiroga, F.L. Intratumoral FoxP3 Expression Is Associated with Angiogenesis and Prognosis in Malignant Canine Mammary Tumors. Vet. Immunol. Immunopathol. 2016, 178, 1–9. [Google Scholar] [CrossRef]
- Estrela-Lima, A.; Araújo, M.S.S.; Costa-Neto, J.M.; Teixeira-Carvalho, A.; Barrouin-Melo, S.M.; Cardoso, S.V.; Martins-Filho, O.A.; Serakides, R.; Cassali, G.D. Immunophenotypic Features of Tumor Infiltrating Lymphocytes from Mammary Carcinomas in Female Dogs Associated with Prognostic Factors and Survival Rates. BMC Cancer 2010, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.N.; dos Reis, D.C.; Salgado, B.S.; Cassali, G.D. Clinical Significance and Prognostic Role of Tumor-Associated Macrophages Infiltration According to Histologic Location in Canine Mammary Carcinomas. Res. Vet. Sci. 2021, 135, 329–334. [Google Scholar] [CrossRef]
- Finotello, R.; Whybrow, K.; Scarin, G.; Ressel, L. Correlation between Tumour Associated Macrophage (Tam) Infiltration and Mitotic Activity in Canine Soft Tissue Sarcomas. Animals 2021, 11, 684. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of Cancer Immunity and the Cancer-Immune Set Point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Vázquez, S.; Vallejo, R.; Espinosa, J.; Arteche, N.; Vega, J.A.; Pérez, V. Immunohistochemical Characterization of Tumor-Associated Macrophages in Canine Lymphomas. Animals 2021, 11, 2301. [Google Scholar] [CrossRef] [PubMed]
- Raposo, T.; Gregório, H.; Pires, I.; Prada, J.; Queiroga, F.L. Prognostic Value of Tumour-Associated Macrophages in Canine Mammary Tumours. Vet. Comp. Oncol. 2014, 12, 10–19. [Google Scholar] [CrossRef]
- Yokota, S.; Kaji, K.; Yonezawa, T.; Momoi, Y.; Maeda, S. CD204+ Tumor-Associated Macrophages Are Associated with Clinical Outcome in Canine Pulmonary Adenocarcinoma and Transitional Cell Carcinoma. Vet. J. 2023, 296–297, 105992. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, D.; Tsun, A.; Li, B. FOXP3+ Regulatory T Cells and Their Functional Regulation. Cell Mol. Immunol. 2015, 12, 558–565. [Google Scholar] [CrossRef]
- Ferreira, F.C.; Dos, M.; Pires, A.; Piersigilli, A.; Huckle, W.R. Canine Melanoma: A Review of Diagnostics and Comparative Mechanisms of Disease and Immunotolerance in the Era of the Immunotherapies. Front. Vet. Sci. 2023, 9, 1046636. [Google Scholar]


| Antibody | Manufacturer/Code | Antigen Retrieval | Dilution |
|---|---|---|---|
| CD3 | Dako, Glostrup, Denmark)/F7.2.38 | EDTA pH 8.00 | 1:200 |
| CD20 | Invitrogen, Rockford, IL, USA/PAS16701 | Citrate pH 6.00 | 1:1000 |
| FoxP3 | eBioscience, Invitrogen, Carlsbad, CA, USA/FJK-16S | Citrate pH 6.00 | 1:1000 |
| Iba-1 | Novus Biological, Centennial, CO, USA/NB100-1028 | Citrate pH 6.00 | 1:200 |
| Tumor Core (Cells/mm2) Median (Range) | Invasive Margin (Cells/mm2) Median (Range) | |||||
|---|---|---|---|---|---|---|
| Total | IE | S | Total | IE | S | |
| CD3+ | 521 (57–2696) | 47.5 (0–1882) | 353.5 (2–2693) | 534 (38–3238) | 51.5 (5–25–2949) | 347 (9–2176) |
| FoxP3+ | 93 (17–705) | 7.50 (0–127) | 75 (12–627) | 172 (25–453) | 19 (0–188) | 138 (24–374) |
| CD20+ | 339 (0–2346) | 1 (0–269) | 342 (23–2346) | 428 (0–1342) | 3 (0–387) | 467 (19–1117) |
| Iba-1 | 1089 (156–3569) | 92 (0–2956) | 881 (150–2751) | 1004 (173–3350) | 24 (0–1229) | 781 (173–2588) |
| Immune Cell | Metastasis at Diagnosis Cells/mm2 [Range] | |||
|---|---|---|---|---|
| No | Yes | p-Value | ||
| IE-CD3+ | CT | 121.50 [19.00–1882.00] | 10.00 [0.00–1310.00] | 0.016 |
| IM | 174.00 [6.00–2030.00] | 9.00 [0.00–2949.00] | 0.008 | |
| IE-Iba-1 | CT | 8.00 [0.00–267.00] | 198.50 [0.00–2956.00] | 0.070 |
| IM | 8.50 [0.00–293.00] | 50.00 [0.00–1229.00] | 0.048 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacci, B.; Brunetti, B.; Maino, C.; Martinoli, G.; Bacon, N.J.; Avallone, G. The Immune Contexture in Canine Anal Sac Adenocarcinoma: Immunohistochemical Quantification of Tumor-Infiltrating Lymphocytes and Tumor-Associated Macrophages with Image Analysis. Animals 2024, 14, 3696. https://doi.org/10.3390/ani14243696
Bacci B, Brunetti B, Maino C, Martinoli G, Bacon NJ, Avallone G. The Immune Contexture in Canine Anal Sac Adenocarcinoma: Immunohistochemical Quantification of Tumor-Infiltrating Lymphocytes and Tumor-Associated Macrophages with Image Analysis. Animals. 2024; 14(24):3696. https://doi.org/10.3390/ani14243696
Chicago/Turabian StyleBacci, Barbara, Barbara Brunetti, Cristiano Maino, Ginevra Martinoli, Nick J. Bacon, and Giancarlo Avallone. 2024. "The Immune Contexture in Canine Anal Sac Adenocarcinoma: Immunohistochemical Quantification of Tumor-Infiltrating Lymphocytes and Tumor-Associated Macrophages with Image Analysis" Animals 14, no. 24: 3696. https://doi.org/10.3390/ani14243696
APA StyleBacci, B., Brunetti, B., Maino, C., Martinoli, G., Bacon, N. J., & Avallone, G. (2024). The Immune Contexture in Canine Anal Sac Adenocarcinoma: Immunohistochemical Quantification of Tumor-Infiltrating Lymphocytes and Tumor-Associated Macrophages with Image Analysis. Animals, 14(24), 3696. https://doi.org/10.3390/ani14243696







