STC2 Inhibits Hepatic Lipid Synthesis and Correlates with Intramuscular Fatty Acid Composition, Body Weight and Carcass Traits in Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Bioinformatics Analysis and SNP Site Screening
2.3. Phenotype Data Collection
2.4. Vector Construction and Small Interfering RNA (siRNA) Synthesis
2.5. Culture and Treatment of Chicken Leghorn Male Hepatoma (LMH) Cell Line
2.6. RNA Exaction, cDNA Synthesis and Quantitative Real-Time PCR (qRT-PCR)
2.7. Detection of Intracellular Triglycerides and Cholesterol
2.8. Oil Red O Staining
2.9. Statistical Analysis
3. Results
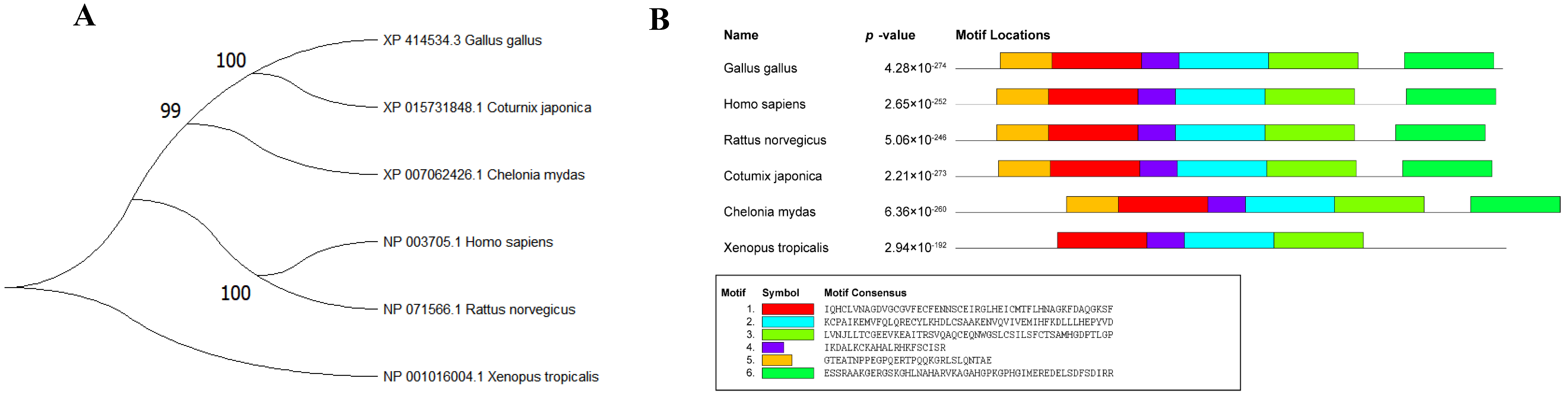
3.1. Phylogenetic Tree Construction and Conserved Motif Analysis of Different Species of STC2
3.2. Association Analysis between Polymorphism in STC2 Gene and Chicken Growth and Carcass Traits
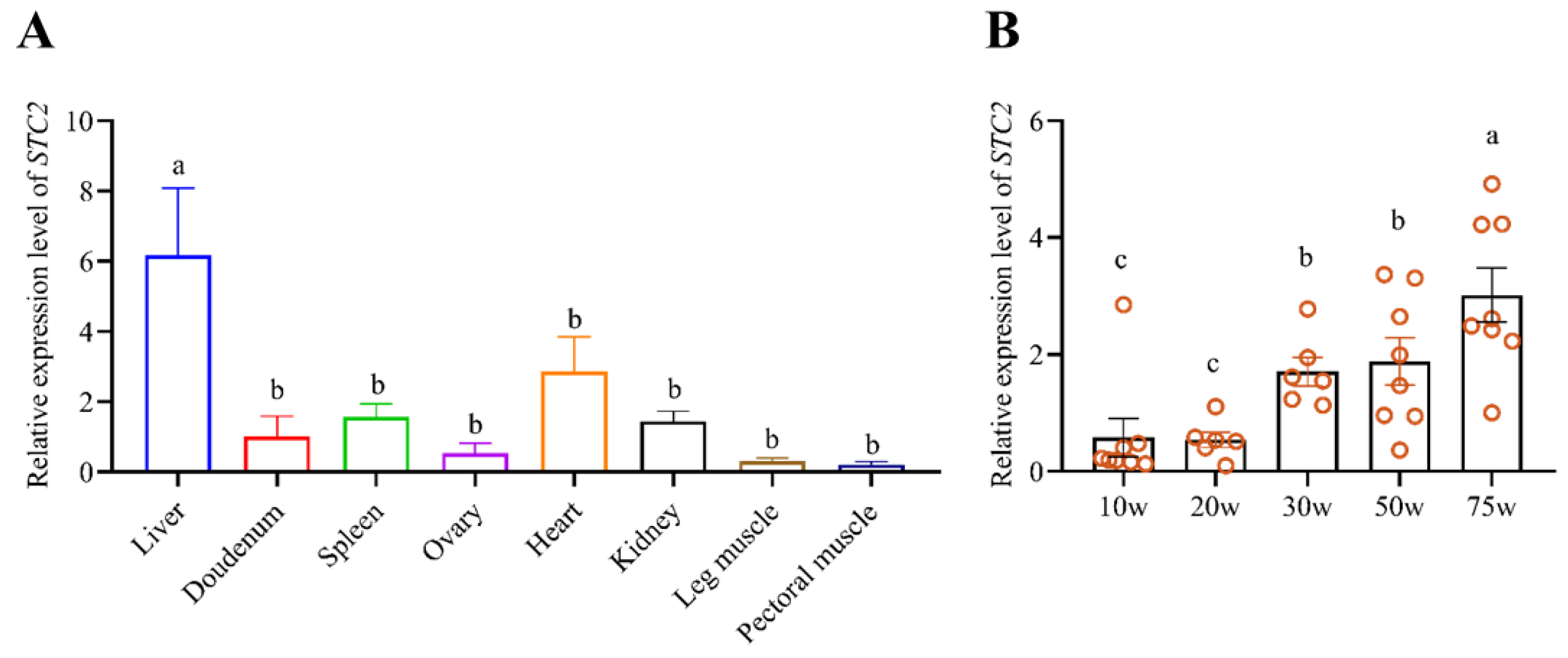
3.3. Expression Pattern of the Chicken STC2 Gene
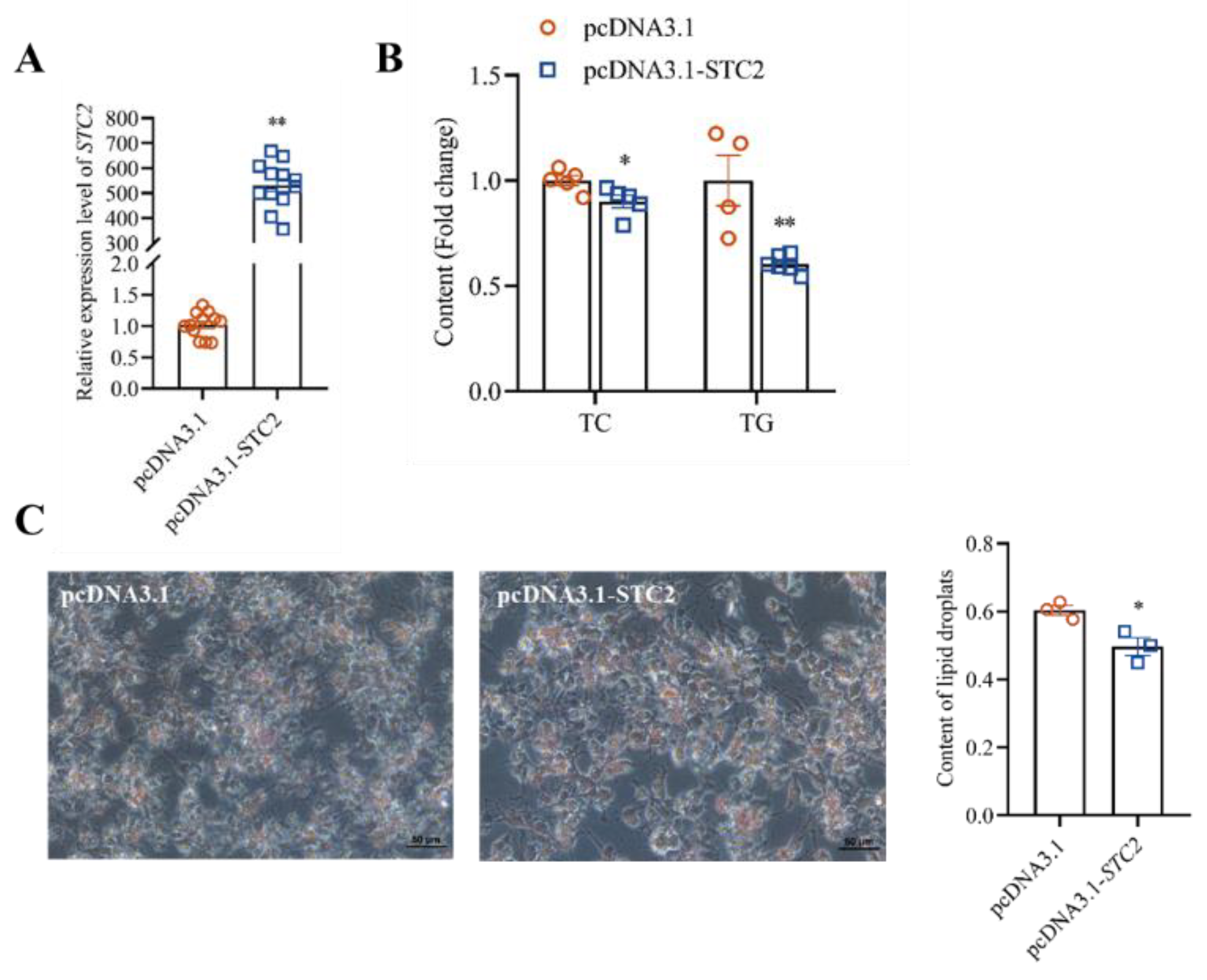
3.4. STC2 Gene Inhibits the Synthesis of TG and TC in LMH Cells
3.5. Correlation Analysis of STC2 Gene Expression with Lipid Molecules
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leveille, G.A. In vitro hepatic lipogenesis in the hen and chick. Comp. Biochem. Physiol. 1969, 28, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Wassie, T.; Cheng, B.; Zhou, T.; Gao, L.; Lu, Z.; Wang, J.; Mulu, B.; Taye, M.; Wu, X. Enteromorpha polysaccharide and yeast glycoprotein mixture improves growth, antioxidant activity, serum lipid profile and regulates lipid metabolism in broiler chickens. Poult. Sci. 2022, 101, 102064. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Wu, T.; Fu, Q.; Wang, P.; Zhao, Y.; Li, Y.; Xiao, H.; Zhou, L.; Song, Z. Effects of Dietary Inclusion of β-Hydroxy-β-Methylbutyrate on Growth Performance, Fat Deposition, Bile Acid Metabolism, and Gut Microbiota Function in High-Fat and High-Cholesterol Diet-Challenged Layer Chickens. Curr. Issues Mol. Biol. 2022, 44, 3413–3427. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Akiba, Y.; Chida, Y.; Takahashi, K. Lipoprotein hydrolysis and fat accumulation in chicken adipose tissues are reduced by chronic administration of lipoprotein lipase monoclonal antibodies. Poult. Sci. 1999, 78, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zheng, M.; Zhao, G.; Liu, R.; Wen, J. Identification of differentially expressed genes and pathways for intramuscular fat metabolism between breast and thigh tissues of chickens. BMC Genom. 2018, 19, 55. [Google Scholar] [CrossRef]
- Tunim, S.; Phasuk, Y.; Aggrey, S.E.; Duangjinda, M. Increasing Fat Deposition Via Upregulates the Transcription of Peroxisome Proliferator-Activated Receptor Gamma in Native Crossbred Chickens. Animals 2021, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Giannenas, I.; Skoufos, I.; Wang, J.; Zhu, W. The effects of plant extracts on lipid metabolism of chickens—A review. Anim. Biosci. 2023, 36, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.Y.; Cui, X.Y.; Li, L.; Fan, Q.L.; Lin, X.J.; Wang, Y.B.; Jiang, Z.Y.; Jiang, S.Q. Effects of dietary incorporation of linseed oil with soybean isoflavone on fatty acid profiles and lipid metabolism-related gene expression in breast muscle of chickens. Animal 2020, 14, 2414–2422. [Google Scholar] [CrossRef]
- Lin, C.W.; Huang, T.W.; Peng, Y.J.; Lin, Y.Y.; Mersmann, H.J.; Ding, S.T. A novel chicken model of fatty liver disease induced by high cholesterol and low choline diets. Poult. Sci. 2021, 100, 100869. [Google Scholar] [CrossRef]
- Chokeshaiusaha, K.; Sananmuang, T.; Puthier, D.; Nguyen, C. Cross-species analysis of differential transcript usage in humans and chickens with fatty liver disease. Vet. World 2023, 16, 1964–1973. [Google Scholar] [CrossRef]
- Flees, J.; Rajaei-Sharifabadi, H.; Greene, E.; Beer, L.; Hargis, B.M.; Ellestad, L.; Porter, T.; Donoghue, A.; Bottje, W.G.; Dridi, S. Effect of Morinda citrifolia (Noni)-Enriched Diet on Hepatic Heat Shock Protein and Lipid Metabolism-Related Genes in Heat Stressed Broiler Chickens. Front. Physiol. 2017, 8, 919. [Google Scholar] [CrossRef]
- Charpentier, A.H.; Bednarek, A.K.; Daniel, R.L.; Hawkins, K.A.; Laflin, K.J.; Gaddis, S.; MacLeod, M.C.; Aldaz, C.M. Effects of estrogen on global gene expression: Identification of novel targets of estrogen action. Cancer Res. 2000, 60, 5977–5983. [Google Scholar] [PubMed]
- Wu, Z.; Cheng, H.; Liu, J.; Zhang, S.; Zhang, M.; Liu, F.; Li, Y.; Huang, Q.; Jiang, Y.; Chen, S.; et al. The Oncogenic and Diagnostic Potential of Stanniocalcin 2 in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2022, 9, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, X.; Fang, Z.; Pan, Z. Effect of STC2 gene silencing on colorectal cancer cells. Mol. Med. Rep. 2019, 20, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, Q.; Li, D.; Lv, L.; Li, Y.; Wu, Z. The significance of Stanniocalcin 2 in malignancies and mechanisms. Bioengineered 2021, 12, 7276–7285. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Guo, C.; Chen, C.; Zhang, Y. Characterization of cellular senescence patterns predicts the prognosis and therapeutic response of hepatocellular carcinoma. Front. Mol. Biosci. 2022, 9, 1100285. [Google Scholar] [CrossRef]
- Hou, J.; Wang, Z.; Xu, H.; Yang, L.; Yu, X.; Yang, Z.; Deng, Y.; Meng, J.; Feng, Y.; Guo, X.; et al. Stanniocalicin 2 suppresses breast cancer cell migration and invasion via the PKC/claudin-1-mediated signaling. PLoS ONE 2015, 10, e0122179. [Google Scholar] [CrossRef]
- Roche, F.P.; Pietilä, I.; Kaito, H.; Sjöström, E.O.; Sobotzki, N.; Noguer, O.; Skare, T.P.; Essand, M.; Wollscheid, B.; Welsh, M.; et al. Leukocyte Differentiation by Histidine-Rich Glycoprotein/Stanniocalcin-2 Complex Regulates Murine Glioma Growth through Modulation of Antitumor Immunity. Mol. Cancer Ther. 2018, 17, 1961–1972. [Google Scholar] [CrossRef]
- Watanabe, T.; Shiozawa, M.; Kimura, Y.; Hiroshima, Y.; Hashimoto, I.; Komori, K.; Watanabe, H.; Kano, K.; Fujikawa, H.; Aoyama, T.; et al. Clinical Significance of Stanniocalcin2 mRNA Expression in Patients with Colorectal Cancer. Anticancer Res. 2021, 41, 2117–2122. [Google Scholar] [CrossRef]
- Chang, A.C.; Hook, J.; Lemckert, F.A.; McDonald, M.M.; Nguyen, M.A.; Hardeman, E.C.; Little, D.G.; Gunning, P.W.; Reddel, R.R. The murine stanniocalcin 2 gene is a negative regulator of postnatal growth. Endocrinology 2008, 149, 2403–2410. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhao, J.; Shi, G.; Liu, X.; Xiong, X.; Li, X.; Zhang, H.; Ma, Q.; Lu, Y. Stanniocalcin2 acts as an anorectic factor through activation of STAT3 pathway. Oncotarget 2017, 8, 91067–91075. [Google Scholar] [CrossRef] [PubMed]
- Ail, D.; Samardzija, M.; Chang, A.C.M.; Keck, J.; Reddel, R.R.; Grimm, C. Stanniocalcin2, but Not Stanniocalcin1, Responds to Hypoxia in a HIF1-Dependent Manner in the Retina. Front. Neurosci. 2022, 16, 882559. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhan, Y.; Zhang, S.; Liu, Z.; Qu, C. Research progress of STC2 in breast cancer. Biophys. Rep. 2021, 7, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Hjortebjerg, R.; Bojsen-Møller, K.N.; Søeby, M.; Oxvig, C.; Madsbad, S.; Frystyk, J. Metabolic improvement after gastric bypass correlates with changes in IGF-regulatory proteins stanniocalcin-2 and IGFBP-4. Metabolism 2021, 124, 154886. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jiao, Y.; Song, Y.; Liu, J.; Li, X.; Zhang, H.; Yang, J.; Lu, Y. Stanniocalcin 2 Ameliorates Hepatosteatosis Through Activation of STAT3 Signaling. Front. Physiol. 2018, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- Sarapio, E.; De Souza, S.K.; Model, J.F.A.; Trapp, M.; Da Silva, R.S.M. Stanniocalcin-1 and -2 effects on glucose and lipid metabolism in white adipose tissue from fed and fasted rats. Can. J. Physiol. Pharmacol. 2019, 97, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Khal De Souza, S.; Sarapio, E.; Lopes Vogt, E.; Schein, V.; Bandeira Fabres, R.; Felipe Argenta Model, J.; Vieira Lima, M.; Santos Rocha, D.; Silveira Martins Da Silva, R. Effects of stanniocalcin hormones on rat hepatic glucose homeostasis under fed and fasted conditions. Gen. Comp. Endocrinol. 2021, 302, 113661. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.D.; Thinakaran, G.; Elferink, C. Cinnabarinic Acid-Induced Stanniocalcin 2 Confers Cytoprotection against Alcohol-Induced Liver Injury. J. Pharmacol. Exp. Ther. 2022, 381, 1–11. [Google Scholar] [CrossRef]
- Patil, N.Y.; Friedman, J.E.; Joshi, A.D. Role of Hepatic Aryl Hydrocarbon Receptor in Non-Alcoholic Fatty Liver Disease. Receptors 2023, 2, 1–15. [Google Scholar] [CrossRef]
- Mittapalli, V.R.; Pröls, F.; Huang, R.; Christ, B.; Scaal, M. Avian stanniocalcin-2 is expressed in developing striated muscle and joints. Anat. Embryol. 2006, 211, 519–523. [Google Scholar] [CrossRef]
- Sah, N.; Kuehu, D.L.; Khadka, V.S.; Deng, Y.; Peplowska, K.; Jha, R.; Mishra, B. RNA sequencing-based analysis of the laying hen uterus revealed the novel genes and biological pathways involved in the eggshell biomineralization. Sci. Rep. 2018, 8, 16853. [Google Scholar] [CrossRef] [PubMed]
- NY/T 1871-2010; China’s Yellow-Feathered Broiler Rearing and Management Technical Regulations. China Agriculture Press: Beijing, China, 2010.
- Zhang, Y.; Wang, Y.; Li, Y.; Wu, J.; Wang, X.; Bian, C.; Tian, Y.; Sun, G.; Han, R.; Liu, X.; et al. Genome-wide association study reveals the genetic determinism of growth traits in a Gushi-Anka F(2) chicken population. Heredity 2021, 126, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Han, R.L.; Lan, X.Y.; Zhang, L.Z.; Ren, G.; Jing, Y.J.; Li, M.J.; Zhang, B.; Zhao, M.; Guo, Y.K.; Kang, X.T.; et al. A novel single-nucleotide polymorphism of the visfatin gene and its associations with performance traits in the chicken. J. Appl. Genet. 2010, 51, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, D.L.; Clayton, E.H.; Lamb, T.A.; van de Ven, R.J.; Refshauge, G.; Kerr, M.J.; Bailes, K.; Lewandowski, P.; Ponnampalam, E.N. The impact of supplementing lambs with algae on growth, meat traits and oxidative status. Meat Sci. 2014, 98, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Qin, P.; Zhang, K.; Wang, Y.; Guo, Y.; Cheng, Z.; Li, Z.; Tian, Y.; Kang, X.; Li, H.; et al. Integrated LC/MS-based lipidomics and transcriptomics analyses revealed lipid composition heterogeneity between pectoralis intramuscular fat and abdominal fat and its regulatory mechanism in chicken. Food Res. Int. 2023, 172, 113083. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, X.; Zhang, P.; Cao, Y.; Zhang, K.; Qin, P.; Guo, Y.; Li, Z.; Tian, Y.; Kang, X.; et al. ELOVL gene family plays a virtual role in response to breeding selection and lipid deposition in different tissues in chicken (Gallus gallus). BMC Genom. 2022, 23, 705. [Google Scholar] [CrossRef]
- Visweswaran, M.; Schiefer, L.; Arfuso, F.; Dilley, R.J.; Newsholme, P.; Dharmarajan, A. Wnt antagonist secreted frizzled-related protein 4 upregulates adipogenic differentiation in human adipose tissue-derived mesenchymal stem cells. PLoS ONE 2015, 10, e0118005. [Google Scholar] [CrossRef]
- Di Vincenzo, M.; Martino, M.; Lariccia, V.; Giancola, G.; Licini, C.; Di Benedetto, G.; Arnaldi, G.; Orciani, M. Mesenchymal Stem Cells Exposed to Persistently High Glucocorticoid Levels Develop Insulin-Resistance and Altered Lipolysis: A Promising In Vitro Model to Study Cushing’s Syndrome. Front. Endocrinol. 2022, 13, 816229. [Google Scholar] [CrossRef]
- Biggs, J.S.; Watkins, M.; Corneli, P.S.; Olivera, B.M. Defining a Clade by Morphological, Molecular and Toxinological Criteria: Distinctive Forms related to Conus praecellens A. Adams, 1854. Nautilus 2010, 124, 1–19. [Google Scholar]
- Tahiri, N.; Veriga, A.; Koshkarov, A.; Morozov, B. Invariant transformers of Robinson and Foulds distance matrices for Convolutional Neural Network. J. Bioinform. Comput. Biol. 2022, 20, 2250012. [Google Scholar] [CrossRef]
- Lipman, D.J.; Souvorov, A.; Koonin, E.V.; Panchenko, A.R.; Tatusova, T.A. The relationship of protein conservation and sequence length. BMC Evol. Biol. 2002, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Gao, C.; Liu, M.; Zhao, S. Evaluation of the genetic diversity of six Chinese indigenous chickens. Asian-Australas. J. Anim. Sci. 2020, 33, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.J.; Zody, M.C.; Eriksson, J.; Meadows, J.R.; Sherwood, E.; Webster, M.T.; Jiang, L.; Ingman, M.; Sharpe, T.; Ka, S.; et al. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature 2010, 464, 587–591. [Google Scholar] [CrossRef]
- Jepsen, M.R.; Kløverpris, S.; Mikkelsen, J.H.; Pedersen, J.H.; Füchtbauer, E.M.; Laursen, L.S.; Oxvig, C. Stanniocalcin-2 inhibits mammalian growth by proteolytic inhibition of the insulin-like growth factor axis. J. Biol. Chem. 2015, 290, 3430–3439. [Google Scholar] [CrossRef] [PubMed]
- Marouli, E.; Graff, M.; Medina-Gomez, C.; Lo, K.S.; Wood, A.R.; Kjaer, T.R.; Fine, R.S.; Lu, Y.; Schurmann, C.; Highland, H.M.; et al. Rare and low-frequency coding variants alter human adult height. Nature 2017, 542, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.H.; Hirschhorn, J.N.; Dauber, A. Insights and Implications of Genome-Wide Association Studies of Height. J. Clin. Endocrinol. Metab. 2018, 103, 3155–3168. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Cordero, A.I.; Gonzales, N.M.; Parker, C.C.; Sokolof, G.; Vandenbergh, D.J.; Cheng, R.; Abney, M.; Sko, A.; Douglas, A.; Palmer, A.A.; et al. Genome-wide Associations Reveal Human-Mouse Genetic Convergence and Modifiers of Myogenesis, CPNE1 and STC2. Am. J. Hum. Genet. 2019, 105, 1222–1236. [Google Scholar] [CrossRef]
- Ma, Z.; Li, H.; Zheng, H.; Jiang, K.; Yan, F.; Tian, Y.; Kang, X.; Wang, Y.; Liu, X. Hepatic ELOVL6 mRNA is regulated by the gga-miR-22-3p in egg-laying hen. Gene 2017, 623, 72–79. [Google Scholar] [CrossRef]
- Wan, X.; Yang, Z.; Ji, H.; Li, N.; Yang, Z.; Xu, L.; Yang, H.; Wang, Z. Effects of lycopene on abdominal fat deposition, serum lipids levels and hepatic lipid metabolism-related enzymes in broiler chickens. Anim. Biosci. 2021, 34, 385–392. [Google Scholar] [CrossRef]
- Zhao, L.; Cai, H.; Wu, Y.; Tian, C.; Wen, Z.; Yang, P. Severe choline deficiency induces alternative splicing aberrance in optimized duck primary hepatocyte cultures. Anim. Biosci. 2022, 35, 1787–1799. [Google Scholar] [CrossRef]
- Chen, W.; Ma, H.; Li, B.; Yang, F.; Xiao, Y.; Gong, Y.; Li, Z.; Li, T.; Zeng, Q.; Xu, K.; et al. Spatiotemporal Regulation of Circular RNA Expression during Liver Development of Chinese Indigenous Ningxiang Pigs. Genes 2022, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Luo, Y.; Wen, Z.; Dai, Y.; Zheng, C.; Zhu, X.; Qin, L.; Zhang, C.; Liang, H.; Zhang, J.; et al. GPX2 Gene Affects Feed Efficiency of Pigs by Inhibiting Fat Deposition and Promoting Muscle Development. Animals 2022, 12, 3528. [Google Scholar] [CrossRef] [PubMed]
- O’Hea, E.K.; Leveille, G.A. Lipid biosynthesis and transport in the domestic chick (Gallus domesticus). Comp. Biochem. Physiol. 1969, 30, 149–159. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Ou, K.; Wang, T.; Li, Z.; Tian, Y.; Wang, Y.; Kang, X.; Li, H.; Liu, X. Evolution, dynamic expression changes and regulatory characteristics of gene families involved in the glycerophosphate pathway of triglyceride synthesis in chicken (Gallus gallus). Sci. Rep. 2019, 9, 12735. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Xu, X.; He, S.; Zhang, J.; Wang, X.; Wu, P.; Liu, J.; Jiang, H.; Zheng, M.; Li, W.; et al. STC2 modulates ERK1/2 signaling to suppress adipogenic differentiation of human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2020, 524, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.F.; Chen, Y.P.; Jin, R.; Wang, C.; Wen, C.; Zhou, Y.M. Age-related changes in liver metabolism and antioxidant capacity of laying hens. Poult. Sci. 2021, 100, 101478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, E.; Peng, G.; Wang, Y.; Huang, F. Comprehensive Proteome and Acetyl-Proteome Atlas Reveals Hepatic Lipid Metabolism in Layer Hens with Fatty Liver Hemorrhagic Syndrome. Int. J. Mol. Sci. 2023, 24, 8491. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wang, C.; Sun, X.; Gao, S.; Liu, R.; Yang, X. Alterations in hepatic transcriptome and cecum microbiota underlying potential ways to prevent early fatty liver in laying hens. Poult. Sci. 2023, 102, 102593. [Google Scholar] [CrossRef]
- Cartoni Mancinelli, A.; Di Veroli, A.; Mattioli, S.; Cruciani, G.; Dal Bosco, A.; Castellini, C. Lipid metabolism analysis in liver of different chicken genotypes and impact on nutritionally relevant polyunsaturated fatty acids of meat. Sci. Rep. 2022, 12, 1888. [Google Scholar] [CrossRef]
- Momot, M.; Nogalski, Z.; Pogorzelska-Przybyłek, P.; Sobczuk-Szul, M. Influence of Genotype and Slaughter Age on the Content of Selected Minerals and Fatty Acids in the Longissimus Thoracis Muscle of Crossbred Bulls. Animals 2020, 10, 2004. [Google Scholar] [CrossRef]
- Maina, A.N.; Lewis, E.; Kiarie, E.G. Egg production, egg quality, and fatty acids profiles in eggs and tissues in Lohmann LSL lite hens fed algal oils rich in docosahexaenoic acid (DHA). Poult. Sci. 2023, 102, 102921. [Google Scholar] [CrossRef] [PubMed]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The seed of industrial hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zong, G.; Wu, Q.; Yun, H.; Niu, Z.; Zheng, H.; Zeng, R.; Sun, L.; Lin, X. Associations of plasma glycerophospholipid profile with modifiable lifestyles and incident diabetes in middle-aged and older Chinese. Diabetologia 2022, 65, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Khaza’ai, H.; Abed, Y.; Rahmat, A.; Ismail, P.; Ranneh, Y. Role of fish oil in human health and possible mechanism to reduce the inflammation. Inflammopharmacology 2015, 23, 79–89. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]




| Gene Name | Genebank ID | Sequence (5′ → 3′) | Product Size (bp) | Tm (°C) |
|---|---|---|---|---|
| STC2 | XM_414534.8 | F: ctagcgtttaaacttaagctt- ATGTGCGCGGGGCTCCGC | 963 | 60 |
| R: tgctggatatctgcagaattc- TCACAGAACGCAGCTAGACCTCC |
| Gene Name | Genebank ID | Sequence (5′ → 3′) | Product Size (bp) | Tm (°C) |
|---|---|---|---|---|
| STC2 | XM_414534.8 | F: CGGCTGTCCCTGCAGAACACA | 112 | 60 |
| R: AGCCTCGGATCTCACAAGAGT | ||||
| HMGCR | XM_046934671.1 | F: GCGAGGAGTGTCTATTCGCA | 167 | 60 |
| R: ATAGTGGTCCTGCTACGCCT | ||||
| SREBP1 | XM_046900546.1 | F: TTCTTCGTGGACGGGGATTG | 218 | 60 |
| R: AGCTGAAGGTACTCCAACGC | ||||
| SREBP2 | XM_040660556.2 | F: CACCTGTGGAACAGCCTCAA | 164 | 60 |
| R: GGTGAGGCATGGTAGGTCTC | ||||
| SQLE | NM_001194927.2 | F: CATCATGGGTCTCCGAAGGG | 152 | 60 |
| R: GCGGTGCATGAAGTTCCTTA | ||||
| FASN | NM_205155.4 | F: AGAGGCTTTGAAGCTCGGAC | 127 | 60 |
| R: GGTGCCTGAATACTTGGGCT | ||||
| ACACA | XM_046929960.1 | F: GCCTCCGAGAACCCAA | 128 | 60 |
| R: CCAGCAGTCTGAGCCACTA | ||||
| SCD | NM_204890.2 | F: CAAGTTCTCCGAGACGCATG | 178 | 60 |
| R: GGGCTTGTAGTATCTCCGCT | ||||
| LPIN1 | XM_004935771.2 | F: TAATGAGAGACAAGATGCCC | 164 | 60 |
| R: ATCTTTTATTCTGTTTGCCAT | ||||
| LPIN2 | NM_001006386.3 | F: CCACATCTCCAATACCCACT | 122 | 60 |
| R: AGTCTCTGTTTCCATAGCAT | ||||
| AGPAT2 | XM_001235299.4 | F: CACCGTCAAGAACATGAGGA | 165 | 60 |
| R: ACCTCCATCAGCCCCATCAT | ||||
| DGAT2 | XM_040661934.2 | F: ACTCCAAGCCCATCACCACT | 149 | 60 |
| R: CAACCCGAACCTGCCTTTGT | ||||
| ApoVLDLII | NM_205483.4 | F: CAGGGCATTGGTGATAGCTG | 162 | 60 |
| R: CCAGCTCTAGGGGACACC | ||||
| ApoB | NM_001044633.2 | F: ATGTTCAAAAGATGCGGCCC | 224 | 60 |
| R: GCATGGCTCTTCTCTCACTG | ||||
| MTTP | NM_001109784.3 | F: CAGGAGGGATGGAGTTCAGC | 166 | 60 |
| R: TGGTCACGGAATGCCTGAAA | ||||
| GAPDH | NM_204305.2 | F: AGAACATCATCCCAGCGT | 182 | 60 |
| R: AGCCTTCACTACCCTCTTG |
| Traits | Mean ± SD | p-Value | ||
|---|---|---|---|---|
| CC (n = 393) | CT (n = 239) | TT (n = 87) | ||
| Birth weight (g) | 30.391 ± 0.139 b | 31.223 ± 0.178 a | 31.157 ± 0.295 a | 4.56 × 10−4 |
| 2-week weight (g) | 119.717 ± 0.934 b | 126.224 ± 1.165 a | 128.158 ± 2.013 a | 2.00 × 10−6 |
| 4-week weight (g) | 312.854 ± 2.283 b | 333.460 ± 2.882 a | 336.756 ± 4.835 a | 2.32 × 10−9 |
| 6-week weight (g) | 541.944 ± 4.293 b | 583.958 ± 5.422 a | 596.016 ± 9.063 a | 1.16 × 10−11 |
| 8-week weight (g) | 791.321 ± 6.497 b | 850.956 ± 8.113 a | 836.937 ± 13.762 a | 3.53 × 10−8 |
| 10-week weight (g) | 1084.821 ± 8.054 b | 1145.940 ± 10.227 a | 1148.57 ± 16.947 a | 2.00 × 10−6 |
| 12-week weight (g) | 1321.621 ± 9.633 b | 1387.644 ± 12.315 a | 1401.965 ± 20.904 a | 7.00 × 10−6 |
| Carcass weight rate (%) | 89.581 ± 0.101 | 90.017 ± 0.129 | 90.032 ± 0.221 | 1.45 × 10−2 |
| Weight after shedding (g) | 1157.940 ± 8.480 b | 1222.700 ± 10.871 a | 1235.754 ± 18.029 a | 4.71 × 10−7 |
| Semievisceration weight (g) | 1070.511 ± 8.260 b | 1131.991 ± 10.611 a | 1145.031 ± 17.465 a | 8.67 × 10−7 |
| Semievisceration weight rate (%) | 81.086 ± 0.101 b | 81.700 ± 0.130 a | 81.767 ± 0.217 a | 1.60 × 10−4 |
| Evisceration weight (g) | 891.641 ± 7.127 b | 949.339 ± 9.154 a | 959.403 ± 15.099 a | 1.05 × 10−7 |
| Evisceration weight rate (%) | 67.556 ± 0.099 b | 68.415 ± 0.128 a | 68.468 ± 0.214 a | 4.30 × 10−8 |
| Abdominal fat weight (g) | 8.980 ± 0.585 | 7.153 ± 0.748 | 6.008 ± 1.244 | 3.65 × 10−2 |
| Abdominal fat percentage (%) | 1.006 ± 0.061 a | 0.735 ± 0.079 b | 0.625 ± 0.130 b | 3.56 × 10−3 |
| Liver weight rate (%) | 2.212 ± 0.016 a | 2.081 ± 0.020 b | 2.060 ± 0.034 b | 5.97 × 10−8 |
| breast muscle weight (g) | 67.778 ± 0.746 b | 73.787 ± 0.956 a | 72.929 ± 1.578 a | 1.00 × 10−6 |
| breast muscle weight rate (%) | 15.084 ± 0.090 b | 15.443 ± 0.117 a | 15.124 ± 0.190 a | 4.70 × 10−2 |
| Leg muscle weight (g) | 96.008 ± 0.871 b | 103.653 ± 1.104 a | 104.117 ± 1.821 a | 1.76 × 10−8 |
| Leg muscle weight rate (%) | 21.375 ± 0.077 b | 21.737 ± 0.099 a | 21.637 ± 0.161 a | 1.24 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Jia, Q.; Xing, Y.; Ma, C.; Guan, H.; Tian, W.; Kang, X.; Tian, Y.; Liu, X.; Li, H. STC2 Inhibits Hepatic Lipid Synthesis and Correlates with Intramuscular Fatty Acid Composition, Body Weight and Carcass Traits in Chickens. Animals 2024, 14, 383. https://doi.org/10.3390/ani14030383
Cao Y, Jia Q, Xing Y, Ma C, Guan H, Tian W, Kang X, Tian Y, Liu X, Li H. STC2 Inhibits Hepatic Lipid Synthesis and Correlates with Intramuscular Fatty Acid Composition, Body Weight and Carcass Traits in Chickens. Animals. 2024; 14(3):383. https://doi.org/10.3390/ani14030383
Chicago/Turabian StyleCao, Yuzhu, Qihui Jia, Yuxin Xing, Chenglin Ma, Hongbo Guan, Weihua Tian, Xiangtao Kang, Yadong Tian, Xiaojun Liu, and Hong Li. 2024. "STC2 Inhibits Hepatic Lipid Synthesis and Correlates with Intramuscular Fatty Acid Composition, Body Weight and Carcass Traits in Chickens" Animals 14, no. 3: 383. https://doi.org/10.3390/ani14030383
APA StyleCao, Y., Jia, Q., Xing, Y., Ma, C., Guan, H., Tian, W., Kang, X., Tian, Y., Liu, X., & Li, H. (2024). STC2 Inhibits Hepatic Lipid Synthesis and Correlates with Intramuscular Fatty Acid Composition, Body Weight and Carcass Traits in Chickens. Animals, 14(3), 383. https://doi.org/10.3390/ani14030383





