Status Quo of Feline Leukaemia Virus Infection in Turkish Cats and Their Antigenic Prevalence
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. Diagnosis of Viral Nucleic Acids by Polymerase Chain Reaction (PCR)
2.3. Sequencing of FeLV-Positive PCR Amplicons and Their Phylogenetic Analysis
2.4. Statistical Analysis on FeLV-Positive Cats
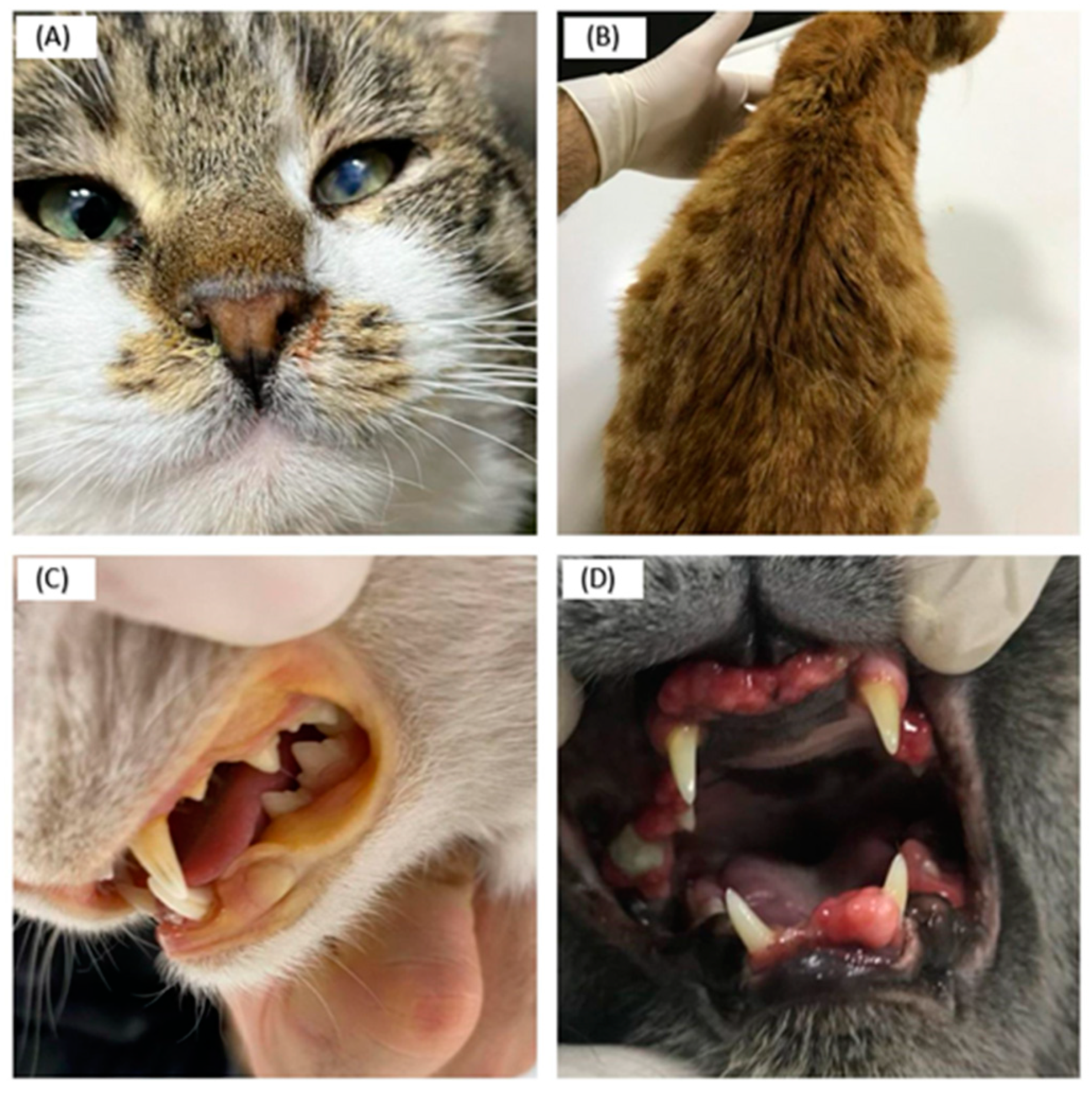
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgment
Conflicts of Interest
References
- Hoover, E.A.; Mullins, J.I. Feline leukemia virus infection and diseases. J. Am. Vet. Med. Assoc. 1991, 199, 1287–1297. [Google Scholar] [CrossRef]
- Miyazawa, T. Infections of feline leukemia virus and feline immunodeficiency virus. Front. Biosci.-Landmark 2002, 7, 504–518. [Google Scholar] [CrossRef]
- Benveniste, R.E.; Sherr, C.J.; Todaro, G.J. Evolution of type C viral genes: Origin of feline leukemia virus. Science 1975, 190, 886–888. [Google Scholar] [CrossRef]
- Stewart, H.; Jarrett, O.; Hosie, M.; Willett, B. Are endogenous feline leukemia viruses really endogenous? Vet. Immunol. Immunopathol. 2011, 143, 325–331. [Google Scholar] [CrossRef]
- Anai, Y.; Ochi, H.; Watanabe, S.; Nakagawa, S.; Kawamura, M.; Gojobori, T.; Nishigaki, K. Infectious endogenous retroviruses in cats and emergence of recombinant viruses. J. Virol. 2012, 86, 8634–8644. [Google Scholar] [CrossRef]
- Hardy, W.D., Jr.; Hess, P.W.; MacEwen, E.G.; McClelland, A.J.; Zuckerman, E.E.; Essex, M.; Cotter, S.M.; Jarrett, O. Biology of feline leukemia virus in the natural environment. Cancer Res. 1976, 36 Pt 2, 582–588. [Google Scholar] [PubMed]
- Krunic, M.; Ertl, R.; Hagen, B.; Sedlazeck, F.J.; Hofmann-Lehmann, R.; von Haeseler, A.; Klein, D. Decreased expression of endogenous feline leukemia virus in cat lymphomas: A case control study. BMC Vet. Res. 2015, 11, 90. [Google Scholar] [CrossRef]
- Benveniste, R.E.; Todaro, G.J. Segregation of RD-114 and FeLV-related sequences in crosses between domestic cat and leopard cat. Nature 1975, 257, 506–508. [Google Scholar] [CrossRef]
- Soe, L.H.; Devi, B.G.; Mullins, J.; Roy-Burman, P. Molecular cloning and characterization of endogenous feline leukemia virus sequences from a cat genomic library. J. Virol. 1983, 46, 829–840. [Google Scholar] [CrossRef]
- Soe, L.H.; Shimizu, R.W.; Landolph, J.R.; Roy-Burman, P. Molecular analysis of several classes of endogenous feline leukemia virus elements. J. Virol. 1985, 56, 701–710. [Google Scholar] [CrossRef]
- Koshy, R.; Gallo, R.; Wong-Staal, F. Characterization of the endogenous feline leukemia virus-related DNA sequences in cats and attempts to identify exogenous viral sequences in tissues of virus-negative leukemic animals. Virology 1980, 103, 434–445. [Google Scholar] [CrossRef]
- Roy-Burman, P. Endogenousenv elements: Partners in generation of pathogenic feline leukemia viruses. Virus Genes 1995, 11, 147–161. [Google Scholar] [CrossRef]
- Eggers, H.J. Principles of virology: Molecular biology pathogenesis and control of animal viruses. Int. J. Med. Microbiol. 2004, 294, 140. [Google Scholar]
- Lauring, A.S.; Anderson, M.M.; Overbaugh, J. Specificity in receptor usage by T-cell-tropic feline leukemia viruses: Implications for the in vivo tropism of immunodeficiency-inducing variants. J. Virol. 2001, 75, 8888–8898. [Google Scholar] [CrossRef]
- Chiu, E.S.; Hoover, E.A.; VandeWoude, S. A retrospective examination of feline leukemia subgroup characterization: Viral interference assays to deep sequencing. Viruses 2018, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Roca, A.L.; Pecon-Slattery, J.; O’Brien, S.J. Genomically intact endogenous feline leukemia viruses of recent origin. J. Virol. 2004, 78, 4370–4375. [Google Scholar] [CrossRef] [PubMed]
- Szilasi, A.; Dénes, L.; Jakab, C.; Erdélyi, I.; Resende, T.; Vannucci, F.; Csomor, J.; Mándoki, M.; Balka, G. In situ hybridization of feline leukemia virus in a primary neural B-cell lymphoma. J. Vet. Diagn. Investig. 2020, 32, 454–457. [Google Scholar] [CrossRef]
- Hofmann-Lehmann, R.; Cattori, V.; Tandon, R.; Boretti, F.S.; Meli, M.L.; Riond, B.; Pepin, A.C.; Willi, B.; Ossent, P.; Lutz, H. Vaccination against the feline leukaemia virus: Outcome and response categories and long-term follow-up. Vaccine 2007, 25, 5531–5539. [Google Scholar] [CrossRef]
- Miyake, A.; Watanabe, S.; Hiratsuka, T.; Ito, J.; Ngo, M.H.; Makundi, I.; Kawasaki, J.; Endo, Y.; Tsujimoto, H.; Nishigaki, K. Novel feline leukemia virus interference group based on the env gene. J. Virol. 2016, 90, 4832–4837. [Google Scholar] [CrossRef] [PubMed]
- Polani, S.; Roca, A.L.; Rosensteel, B.B.; Kolokotronis, S.-O.; Bar-Gal, G.K. Evolutionary dynamics of endogenous feline leukemia virus proliferation among species of the domestic cat lineage. Virology 2010, 405, 397–407. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Herring, E.; Troy, G.; Toth, T.; Forrester, S.; Weigt, L.; Herring, I. Detection of feline leukaemia virus in blood and bone marrow of cats with varying suspicion of latent infection. J. Feline Med. Surg. 2001, 3, 133–141. [Google Scholar] [CrossRef]
- Simons, F.A.; Vennema, H.; Rofina, J.E.; Pol, J.M.; Horzinek, M.C.; Rottier, P.J.; Egberink, H.F. A mRNA PCR for the diagnosis of feline infectious peritonitis. J. Virol. Methods 2005, 124, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Buonavoglia, C.; Martella, V.; Pratelli, A.; Tempesta, M.; Cavalli, A.; Buonavoglia, D.; Bozzo, G.; Elia, G.; Decaro, N.; Carmichael, L. Evidence for evolution of canine parvovirus type 2 in Italy. J. Gen. Virol. 2001, 82, 3021–3025. [Google Scholar] [CrossRef] [PubMed]
- Koç, B.T.; Oğuzoğlu, T.Ç. A phylogenetic study of Feline Immunodeficiency Virus (FIV) among domestic cats in Turkey. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101544. [Google Scholar] [CrossRef] [PubMed]
- Adıgüzel, E.; Erdem-Şahinkesen, E.; Koç, B.T.; Demirden, C.; Oğuzoğlu, T.Ç. The detection and full genomic characterization of domestic cat Orthohepadnaviruses from Türkiye. Vet. Med. Sci. 2023, 9, 1965–1972. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Koç, B.; Oğuzoğlu, T. Does endogenous feline leukemia virus occur as a risk factor?: A molecular characterization study from Türkiye: Molecular analysis of enFeLVs. J. Hell. Vet. Med. Soc. 2023, 74, 6093–6098. [Google Scholar] [CrossRef]
- De Almeida, N.R.; Danelli, M.G.; da Silva, L.H.; Hagiwara, M.K.; Mazur, C. Prevalence of feline leukemia virus infection in domestic cats in Rio de Janeiro. J. Feline Med. Surg. 2012, 14, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Studer, N.; Lutz, H.; Saegerman, C.; Gönczi, E.; Meli, M.L.; Boo, G.; Hartmann, K.; Hosie, M.J.; Moestl, K.; Tasker, S. Pan-European study on the prevalence of the feline leukaemia virus infection–reported by the European Advisory Board on Cat Diseases (ABCD Europe). Viruses 2019, 11, 993. [Google Scholar] [CrossRef] [PubMed]
- Boeke, J.D.; Stoye, J.P. Retrotransposons, endogenous retroviruses, and the evolution of retroelements. Sci. Rep. 2011, 13, 7380. [Google Scholar]
- Powers, J.A.; Chiu, E.S.; Kraberger, S.J.; Roelke-Parker, M.; Lowery, I.; Erbeck, K.; Troyer, R.; Carver, S.; VandeWoude, S. Feline leukemia virus (FeLV) disease outcomes in a domestic cat breeding colony: Relationship to endogenous FeLV and other chronic viral infections. J. Virol. 2018, 92, 18. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J.; Horzinek, M.; Schultz, R.; Squires, R. WSAVA Guidelines for the vaccination of dogs and cats. J. Small Anim. Pract. 2016, 57, E1. [Google Scholar] [CrossRef]
- Helfer-Hungerbuehler, A.K.; Spiri, A.M.; Riond, B.; Grest, P.; Boretti, F.S.; Hofmann-Lehmann, R. No benefit of therapeutic vaccination in clinically healthy cats persistently infected with feline leukemia virus. Vaccine 2015, 33, 1578–1585. [Google Scholar] [CrossRef]


| Total | Only FeLV | FeLV FCoV | FeLV FPV | FeLV FCoV FPV | FeLV DCH | FeLV DCH FCoV | FeLV DCH FPV | ||
|---|---|---|---|---|---|---|---|---|---|
| Breed | Tabby | 74 | 35 | 16 | 7 | 4 | 10 | 1 | 1 |
| British | 19 | 14 | 4 | 1 | 0 | 0 | 0 | 0 | |
| Scottish | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Siamese | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Persian | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |
| Mix | 11 | 9 | 0 | 1 | 0 | 1 | 0 | 0 | |
| p-value < 0.05 | |||||||||
| Sex | Female | 53 | 31 | 9 | 4 | 4 | 4 | 0 | 1 |
| Male | 59 | 33 | 13 | 5 | 0 | 7 | 1 | 0 | |
| p-value > 0.05 | |||||||||
| Age (in months) | 0–6 | 16 | 5 | 5 | 1 | 3 | 1 | 0 | 1 |
| 7–12 | 36 | 20 | 4 | 5 | 1 | 5 | 1 | 0 | |
| 13–48 | 45 | 28 | 11 | 2 | 0 | 4 | 0 | 0 | |
| >48 | 12 | 8 | 2 | 1 | 0 | 1 | 0 | 0 | |
| Undetermined | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| p-value > 0.05 | |||||||||
| Lifestyle | Indoor | 67 | 49 | 11 | 4 | 0 | 3 | 0 | 0 |
| Outdoor | 43 | 13 | 11 | 5 | 4 | 8 | 1 | 1 | |
| Undetermined | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| p-value > 0.05 | |||||||||
| Health status | Healthy | 18 | 13 | 1 | 1 | 0 | 2 | 0 | 1 |
| Sick | 94 | 51 | 21 | 8 | 4 | 9 | 1 | 0 | |
| p-value < 0.05 | |||||||||
| Vaccination * | Yes | 42 | 29 | 10 | 3 | 0 | 0 | 0 | 0 |
| No | 20 | 12 | 2 | 1 | 4 | 1 | 0 | 0 | |
| Undetermined | 50 | 23 | 10 | 5 | 0 | 10 | 1 | 1 | |
| p-value > 0.05 | |||||||||
| 112 | 64 | 22 | 9 | 4 | 11 | 1 | 1 | ||
| Percentages (%) | 100 | 57.1 | 19.6 | 8 | 3.6 | 9.8 | 0.9 | 0.9 |
| Health Status | Total | ||||
|---|---|---|---|---|---|
| Sick | Healthy | ||||
| Sex p < 0.01 | Female | Count | 39 | 14 | 53 |
| % within sex | 73.6% | 26.4% | 100.0% | ||
| Male | Count | 55 | 4 | 59 | |
| % within sex | 93.2% | 6.8% | 100.0% | ||
| Total | Count | 94 | 18 | 112 | |
| % within sex | 83.9% | 16.1% | 100.0% | ||
| Lifestyle p < 0.001 | Outdoor | Count | 42 | 1 | 43 |
| % within lifestyle | 97.7% | 2.3% | 100.0% | ||
| Indoor | Count | 50 | 17 | 67 | |
| % within lifestyle | 74.6% | 25.4% | 100.0% | ||
| Total | Count | 92 | 18 | 110 | |
| % within lifestyle | 83.6% | 16.4% | 100.0% | ||
| Positivity | Total | ||||
|---|---|---|---|---|---|
| FeLV | FeLV and Others | ||||
| Vaccine p > 0.05 | No | Count | 12 | 8 | 20 |
| % within vaccine | 60.0% | 40.0% | 100.0% | ||
| Yes | Count | 29 | 13 | 42 | |
| % within vaccine | 69.0% | 31.0% | 100.0% | ||
| Total | Count | 41 | 21 | 62 | |
| % within vaccine | 66.1% | 33.9% | 100.0% | ||
| Total | Only FeLV | FeLV FCoV | FeLV FPV | FeLV FCoV FPV | FeLV DCH | FeLV DCH FCoV | FeLV DCH FPV | |
|---|---|---|---|---|---|---|---|---|
| Signs and symptoms | 94 | |||||||
| Leukopenia | 22 | 10 | 4 | 3 | 4 | 1 | 0 | 0 |
| Icterus | 13 | 7 | 2 | 1 | 1 | 2 | 0 | 0 |
| Anaemia | 10 | 2 | 2 | 1 | 4 | 1 | 0 | 0 |
| Ascites/pleural effusion | 17 | 7 | 7 | 1 | 1 | 0 | 1 | 0 |
| Uveitis | 12 | 7 | 2 | 0 | 1 | 2 | 0 | 0 |
| Stomatitis/Gingivitis | 24 | 15 | 3 | 3 | 2 | 1 | 0 | 0 |
| Rhinitis | 11 | 5 | 5 | 1 | 0 | 0 | 0 | 0 |
| Lymphadenopathy | 16 | 6 | 5 | 1 | 3 | 1 | 0 | 0 |
| Fever | 11 | 5 | 1 | 2 | 2 | 1 | 0 | 0 |
| Anorexia | 50 | 23 | 16 | 5 | 4 | 2 | 0 | 0 |
| Lethargy | 34 | 14 | 9 | 5 | 4 | 2 | 0 | 0 |
| Incoordination | 17 | 11 | 2 | 2 | 0 | 2 | 0 | 0 |
| Skin diseases | 4 | 1 | 1 | 2 | 0 | 0 | 0 | 0 |
| Respiratory diseases | 9 | 4 | 1 | 2 | 1 | 1 | 0 | 0 |
| Urogenital diseases | 6 | 5 | 1 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal diseases | 13 | 4 | 2 | 3 | 3 | 1 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korkulu, E.; Şenlik, E.İ.; Adıgüzel, E.; Artut, F.G.; Çetinaslan, H.D.; Erdem-Şahinkesen, E.; Oğuzoğlu, T.Ç. Status Quo of Feline Leukaemia Virus Infection in Turkish Cats and Their Antigenic Prevalence. Animals 2024, 14, 385. https://doi.org/10.3390/ani14030385
Korkulu E, Şenlik Eİ, Adıgüzel E, Artut FG, Çetinaslan HD, Erdem-Şahinkesen E, Oğuzoğlu TÇ. Status Quo of Feline Leukaemia Virus Infection in Turkish Cats and Their Antigenic Prevalence. Animals. 2024; 14(3):385. https://doi.org/10.3390/ani14030385
Chicago/Turabian StyleKorkulu, Emrah, Elif İrem Şenlik, Ece Adıgüzel, Fatma Gökçe Artut, Hüseyin Doğukan Çetinaslan, Eda Erdem-Şahinkesen, and Tuba Çiğdem Oğuzoğlu. 2024. "Status Quo of Feline Leukaemia Virus Infection in Turkish Cats and Their Antigenic Prevalence" Animals 14, no. 3: 385. https://doi.org/10.3390/ani14030385
APA StyleKorkulu, E., Şenlik, E. İ., Adıgüzel, E., Artut, F. G., Çetinaslan, H. D., Erdem-Şahinkesen, E., & Oğuzoğlu, T. Ç. (2024). Status Quo of Feline Leukaemia Virus Infection in Turkish Cats and Their Antigenic Prevalence. Animals, 14(3), 385. https://doi.org/10.3390/ani14030385





