Factors Associated with Reproductive Success in Captive Vancouver Island Marmots (Marmota vancouverensis)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Studbook Data Analyses
2.2. Fecal Sample Collection and Hormone Extraction for Endocrine Analyses
2.3. Enzyme-Immunoassay (EIA)
2.4. Assay Validation
2.5. Calculating ‘Species Baseline’ of FGM in Vancouver Island Marmots
2.6. Statistical Analyses
3. Results
3.1. Studbook Data Analyses
3.1.1. Univariable Analysis
3.1.2. Multivariable Analysis
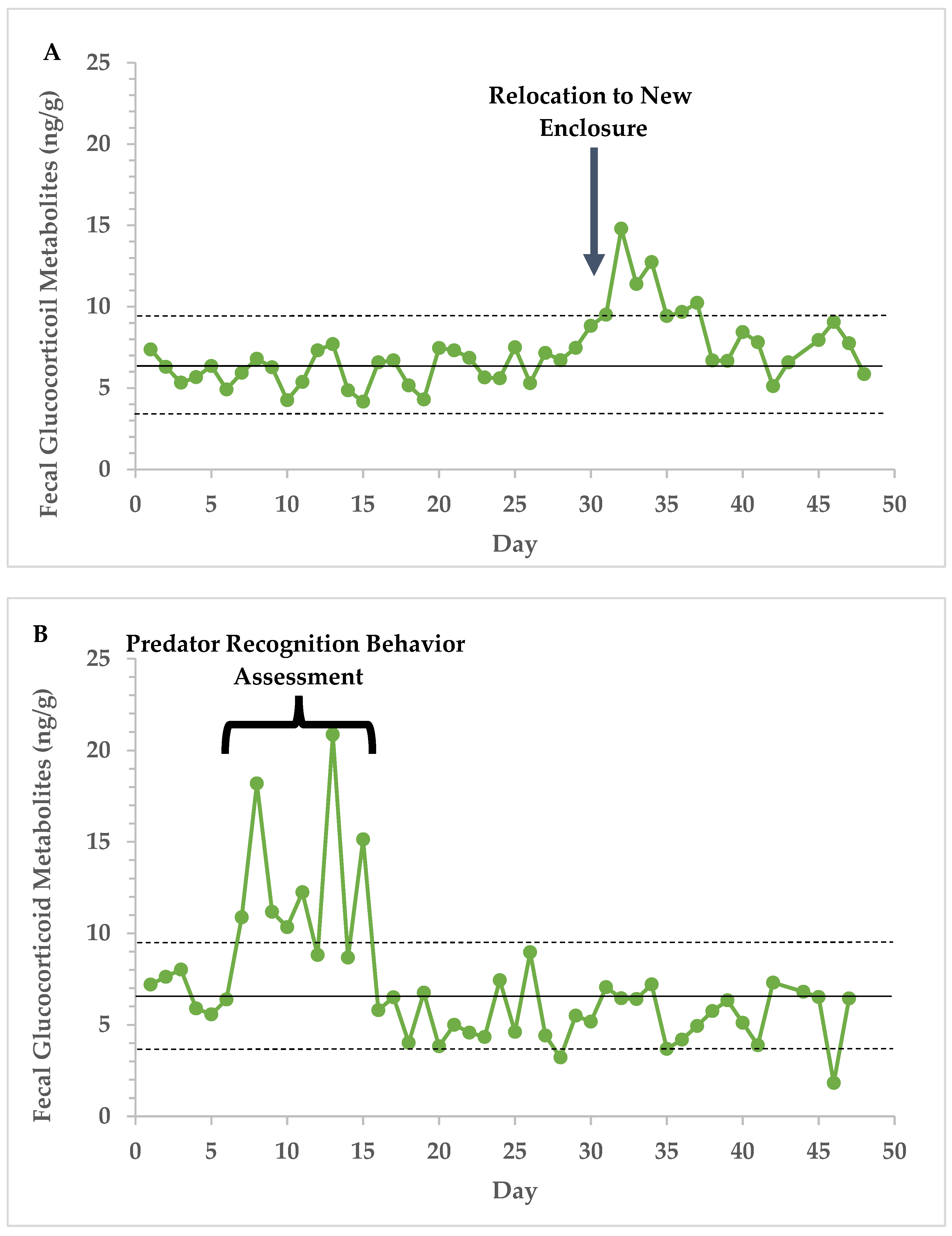
3.2. Endocrine Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COSEWIC. Assessment and Update Status Report on the Vancouver Island Marmot Marmota vancouverensis in Canada; Committee on the Status of Endangered Wildlife in Canada: Ottawa, ON, Canada, 2019; vii + 29p, Available online: http://publications.gc.ca/pub?id=9.878603&sl=0 (accessed on 20 January 2024).
- Roach, N. Marmota vancouverensis. In The IUCN Red List of Threatened Species 2017; IUCN: Gland, Switzerland, 2017. [Google Scholar] [CrossRef]
- Bryant, A.A.; Janz, D.W. Distribution and abundance of Vancouver Island marmots (Marmota vancouverensis). Can. J. Zool. 1996, 74, 667–677. [Google Scholar] [CrossRef]
- Brashares, J.S.; Werner, J.R.; Sinclair, A.R.E. Social ‘meltdown’ in the demise of an island endemic: Allee effects and the Vancouver Island marmot. J. Anim. Ecol. 2010, 79, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.A. Reproduction and persistence of Vancouver Island marmots (Marmota vancouverensis) in natural and logged habitats. Can. J. Zool. 1996, 74, 678–687. [Google Scholar] [CrossRef]
- Vancouver Island Marmot Recovery Team. Recovery Plan for the Vancouver Island Marmot (Marmota vancouverensis) in British Columbia; B.C. Ministry of Environment: Victoria, BC, Canada, 2017; 41p. [Google Scholar]
- McAdie, M. History and Current Status of the Vancouver Island marmot (Marmota vancouverensis) Captive-breeding Program. In Proceedings of the Species at Risk 2004 Pathways to Recovery Conference, Victoria, BC, Canada, 2–6 March 2004; Hooper, T.D., Ed.; Available online: https://www.arlis.org/docs/vol1/69415913/mcadie_edited_final_feb_21.pdf (accessed on 29 December 2023).
- Marmot Recovery Foundation. Available online: http://marmots.org (accessed on 29 December 2023).
- McAdie, M. Vancouver Island Marmot Captive Management Meeting Minutes 2023. Personal Communication, 2023. [Google Scholar]
- Jackson, C.; Baker, A.; Doyle, D.; Franke, M.; Jackson, V.; Lloyd, N.; McAdie, M.; Stephens, T.; Traylor-Holzer, K. (Eds.) Vancouver Island Marmot Population and Habitat Viability Assessment Workshop Final Report; IUCN SSC Conservation Breeding Specialist Group: Apple Valley, MN, USA, 2015. [Google Scholar]
- Kaumanns, W.; Begum, N.; Hofer, H. Animals are designed for breeding: Captive population management needs a new perspective. J. Zoo Aquar. Res. 2020, 8, 76–85. [Google Scholar] [CrossRef]
- Carnio, J. Vancouver Island Marmot Studbook 2023. Personal Communication, 2023. [Google Scholar]
- Bauman, K.; Sahrmann, J.; Franklin, A.; Asa, C.; Agnew, M.; Traylor-Holzer, K.; Powell, D. Reproductive Viability Analysis (RVA) as a New Tool for Ex Situ Population Management. Zoo Biol. 2018, 38, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Aymen, J.; Freedman, M.; Delnatte, P.; McAdie, M.; Beaufrère, H. Retrospective Analysis of Hibernation Parameters and Breeding Success in Captive Vancouver Island Marmots (Marmota vancouverensis): 1997–2018. Zoo Biol. 2021, 40, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.; Schwrzenberger, F.; Mostl, E.; Galama, W.; Savage, A. A versatile enzyme immunoassay for the determination of progestogens in feces and serum. Zoo Biol. 2001, 20, 227–236. [Google Scholar] [CrossRef]
- Leishman, E.M.; Franke, M.; Marvin, J.; McCart, D.; Bradford, C.; Gyimesi, Z.S.; Nichols, A.; Lessard, M.-P.; Page, D.; Breiter, C.-J.; et al. The adrenal cortisol response to increasing ambient temperature in polar bears (Ursus maritimus). Animals 2022, 12, 672. [Google Scholar] [CrossRef]
- Graham, L.H.; Reid, K.; Webster, T.; Richards, M.; Joseph, S. Endocrine patterns associated with reproduction in the Nile hippopotamus (Hippopotamus amphibius) as assessed by fecal progestagen analysis. Gen. Comp. Endocrinol. 2002, 128, 74–81. [Google Scholar] [CrossRef]
- Casimir, D.L.; Moehrenschlager, A.; Barclay, R.M.R. Factors influencing reproduction in captive Vancouver Island marmots: Implications for captive breeding and reintroduction programs. J. Mammal. 2007, 88, 1412–1419. [Google Scholar] [CrossRef]
- Bryant, A.A. Reproductive rates of wild and captive Vancouver Island marmots (Marmota vancouverensis). Can. J. Zool. 2005, 83, 664–673. [Google Scholar] [CrossRef]
- St Lawrence, S.; Dumas, M.N.; Petelle, M.; Blumstein, D.T.; Martin, J.G. Sex-specific reproductive strategies in wild yellow-bellied marmots (Marmota flaviventer): Senescence and genetic variance in annual reproductive success differ between the sexes. Behav. Ecol. Sociobiol. 2022, 76, 84. [Google Scholar] [CrossRef]
- Hackländer, K.; Arnold, W. Male-caused failure of female reproduction and its adaptive value in alpine marmots (Marmota marmota). Behav. Ecol. 1999, 10, 592–597. [Google Scholar] [CrossRef]
- Arnold, W. Energetics of Social Hibernation. In Life in the Cold: Ecological, Physiological and Molecular Mechanisms; Carey, C., et al., Eds.; Westview Press: Boulder, CO, USA, 1993; pp. 65–80. [Google Scholar]
- Armitage, K.B. Hibernation as a major determinant of life-history traits in marmots. J. Mammal. 2017, 98, 321–331. [Google Scholar] [CrossRef]
- Wingfield, J.C.; Sapolsky, R.M. Reproduction and resistance to stress: When and how. J. Neuroendocrinol. 2003, 15, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Toufexis, D.; Rivarola, M.A.; Lara, H.; Viau, V. Stress and the reproductive axis. J. Neuroendocrinol. 2014, 26, 573–586. [Google Scholar] [CrossRef]
- Romero, L.M.; Dickens, M.J.; Cyr, N.E. The Reactive scope model—A new model integrating homeostasis, allostasis, and stress. Horm. Behav. 2009, 55, 375–389. [Google Scholar] [CrossRef]
- Wright, J.; Buch, K.; Beattie, U.K.; Gormally, B.M.G.; Romero, L.M.; Fefferman, N. A mathematical representation of the reactive scope model. J. Math. Biol. 2023, 87, 3. [Google Scholar] [CrossRef]
- Armitage, K.B. Factors affecting corticosteroid concentrations in yellow-bellied marmots. Comp. Biochem. Physiol. A Comp. Physiol. 1991, 98, 47–54. [Google Scholar] [CrossRef]
- Smith, J.E.; Monclús, R.; Wantuck, D.; Florant, G.L.; Blumstein, D.T. Fecal glucocorticoid metabolites in wild yellow-bellied marmots: Experimental validation, individual differences and ecological correlates. Gen. Comp. Endocrinol. 2012, 178, 417–426. [Google Scholar] [CrossRef]
- Falconer, S.; McAdie, M.; Mastromonaco, G.; Schulte-Hostedde, A.I. Assessing stress physiology within a conservation breeding program for an endangered species. Cons. Physiol. 2023, 11, coad041. [Google Scholar] [CrossRef]
- Armitage, K.B. Marmot Biology: Sociality, Individual Fitness, and Population Dynamics; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Arnold, W.; Dittami, J. Reproductive Suppression in male alpine Marmots. Anim. Behav. 1997, 53, 53–66. [Google Scholar] [CrossRef]
- MacLeod, K.J.; English, S.; Ruuskanen, S.K.; Taborsky, B. Stress in the social context: A behavioural and eco-evolutionary perspective. J. Exp. Biol. 2023, 226, 245829. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, M.B.; Kaiser, S.; Sachser, N. Social buffering of the stress response: Diversity, mechanisms, and functions. Front. Neuroendocrinol. 2009, 30, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Wu, A. Social buffering of stress—Physiological and ethological perspectives. Appl. Anim. Behav. Sci. 2021, 239, 105325. [Google Scholar] [CrossRef]
- Smith, A.S.; Wang, Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol. Psych. 2014, 76, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Bales, K.L.; Ardekani, C.S.; Baxter, A.; Karaskiewicz, C.L.; Kuske, J.X.; Lau, A.R.; Savidge, L.E.; Sayler, K.R.; Witczak, L.R. What is a pair bond? Horm. Behav. 2021, 136, 105062. [Google Scholar] [CrossRef] [PubMed]
- Walum, H.; Young, L.J. The neural mechanisms and circuitry of the pair bond. Nat. Rev. Neurosci. 2018, 19, 643–654. [Google Scholar] [CrossRef]
- Young, K.A.; Gobrogge, K.L.; Liu, Y.; Wang, Z. The neurobiology of pair bonding: Insights from a socially monogamous rodent. Front. Neuroendocrinol. 2011, 32, 53–69. [Google Scholar] [CrossRef]
- Barrett, K.G.; Amaral, G.; Elphinstone, M.; McAdie, M.L.; Davis, C.S.; Janes, J.K.; Carnio, J.; Moehrenschlager, A.; Gorrell, J.C. Genetic management on the brink of extinction: Sequencing microsatellites does not improve estimates of inbreeding in wild and captive Vancouver Island marmots (Marmota vancouverensis). Conserv. Genet. 2022, 23, 417–428. [Google Scholar] [CrossRef]
- Martin-Wintle, M.S.; Wintle, N.J.P.; Díez-León, M.; Swaisgood, R.R.; Asa, C.S. Improving the sustainability of ex situ populations with mate choice. Zoo Biol. 2019, 38, 119–132. [Google Scholar] [CrossRef] [PubMed]
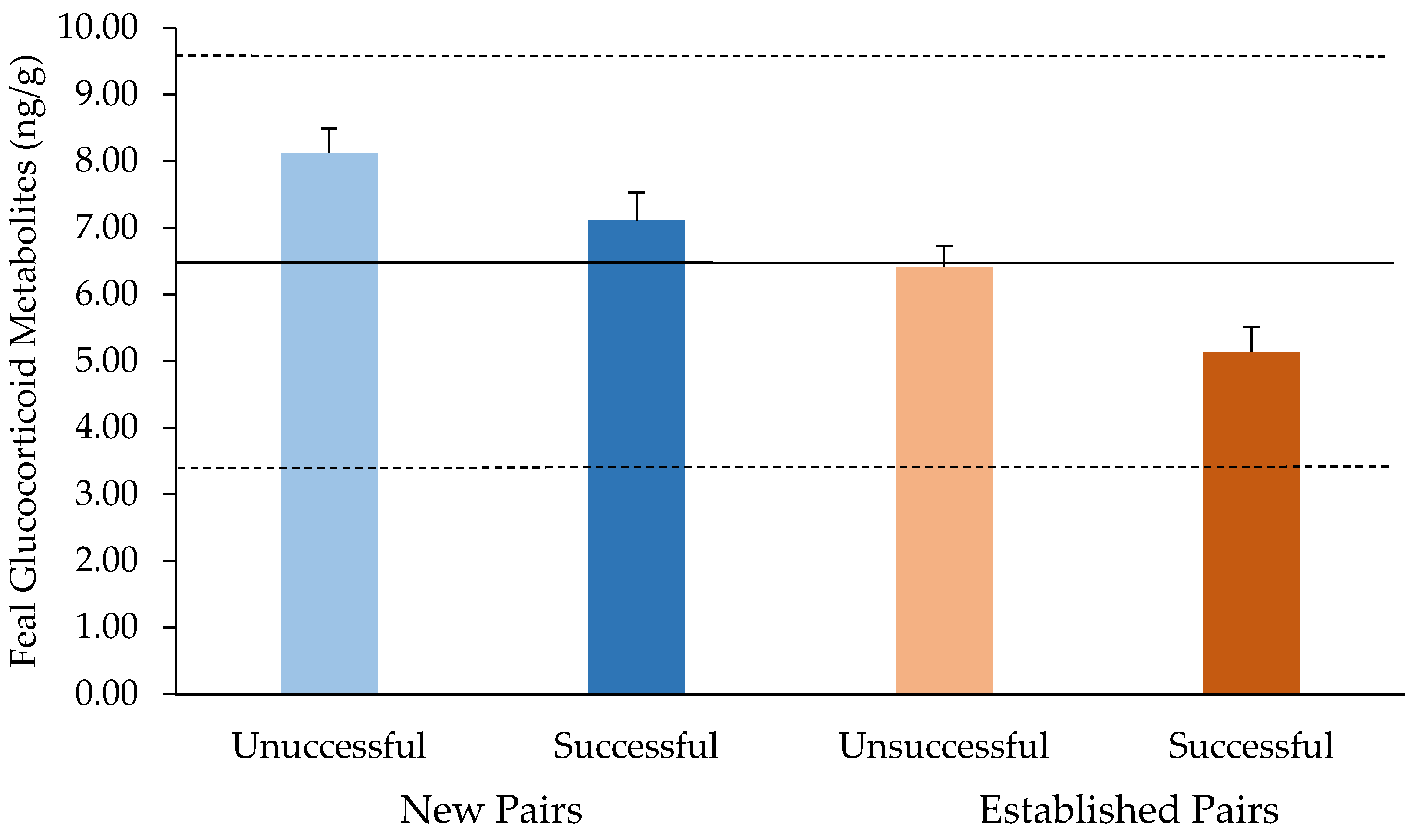
| Variable | N (%) | OR (95%CI) | p-Value |
|---|---|---|---|
| Sire age | 0.0059 | ||
| A (2–4 y) | 162 (42.7) | 2.122 (1.203–3.743) | |
| B (5–7 y) | 143 (37.7) | 2.551 (1.427–4.561) | |
| C (8–14 y) | 74 (19.5) | Reference | |
| Dam age | 0.0114 | ||
| A (2–4 y) | 158 (41.7) | 1.400 (0.855–2.294) | |
| B (5–7 y) | 113 (29.8) | 2.279 (1.326–3.918) | |
| C (8–14 y) | 108 (28.5) | Reference | |
| Any previous success–Dam | 0.1229 | ||
| No previous success | 163 (43.0) | 0.725 (0.481–1.091) | |
| Previous success | 216 (57.0) | Reference | |
| Success in previous yr–Dam | 0.0034 | ||
| Successful in previous yr | 158 (41.7) | 1.871 (1.233–2.840) | |
| Not successful in previous yr | 221 (58.3) | Reference | |
| Any previous success–Sire | 0.9939 | ||
| No previous success | 166 (43.8) | 0.998 (0.664–1.501) | |
| Previous success | 213 (56.2) | Reference | |
| Dam transferred | 0.1702 | ||
| Not transferred | 297 (78.4) | 0.706 (0.429–1.162) | |
| Transferred | 82 (21.6) | Reference | |
| Sire transferred | 0.5579 | ||
| Not transferred | 280 (73.9) | 1.147 (0.724–1.818) | |
| Transferred | 99 (26.1) | Reference | |
| Dam origin | 0.2211 | ||
| Captive | 239 (63.1) | 0.769 (0.505–1.172) | |
| Wild | 140 (36.9) | Reference | |
| Sire origin | 0.8747 | ||
| Captive | 274 (72.3) | 1.037 (0.660–1.629) | |
| Wild | 105 (27.7) | Reference | |
| Both parents wild | 0.8853 | ||
| Yes | 80 (21.1) | 1.037 (0.631–1.704) | |
| No | 299 (78.9) | Reference | |
| Both parents captive | |||
| Yes | 214 (56.5) | 0.824 (0.547–1.240) | 0.3517 |
| No | 165 (43.5) | Reference | |
| Age class difference | 0.0702 | ||
| Parents same age class | 204 (53.8) | 1.457 (0.969–2.190) | |
| Parents different age class | 175 (46.2) | Reference | |
| Sire older than dam | 0.1765 | ||
| No | 311 (82.1) | 1.439 (0.848–2.441) | |
| Yes | 68 (17.9) | Reference | |
| New pair | 0.1509 | ||
| No | 198 (52.2) | 1.346 (0.897–2.021) | |
| Yes | 181 (47.8) | Reference |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graham, L.H.; Leishman, E.M.; Demers, K.; Whiteside, D.P.; McAdie, M. Factors Associated with Reproductive Success in Captive Vancouver Island Marmots (Marmota vancouverensis). Animals 2024, 14, 387. https://doi.org/10.3390/ani14030387
Graham LH, Leishman EM, Demers K, Whiteside DP, McAdie M. Factors Associated with Reproductive Success in Captive Vancouver Island Marmots (Marmota vancouverensis). Animals. 2024; 14(3):387. https://doi.org/10.3390/ani14030387
Chicago/Turabian StyleGraham, Laura H., Emily M. Leishman, Kahlee Demers, Douglas P. Whiteside, and Malcolm McAdie. 2024. "Factors Associated with Reproductive Success in Captive Vancouver Island Marmots (Marmota vancouverensis)" Animals 14, no. 3: 387. https://doi.org/10.3390/ani14030387
APA StyleGraham, L. H., Leishman, E. M., Demers, K., Whiteside, D. P., & McAdie, M. (2024). Factors Associated with Reproductive Success in Captive Vancouver Island Marmots (Marmota vancouverensis). Animals, 14(3), 387. https://doi.org/10.3390/ani14030387





