Molecular Identification of Babesia and Theileria Infections in Livestock in the Qinghai–Tibetan Plateau Area, China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Molecular Detection of Theileria and Babesia Species
2.3. Sequencing Reaction
2.4. GenBank Accession Numbers
2.5. Statistical Analyses
3. Results
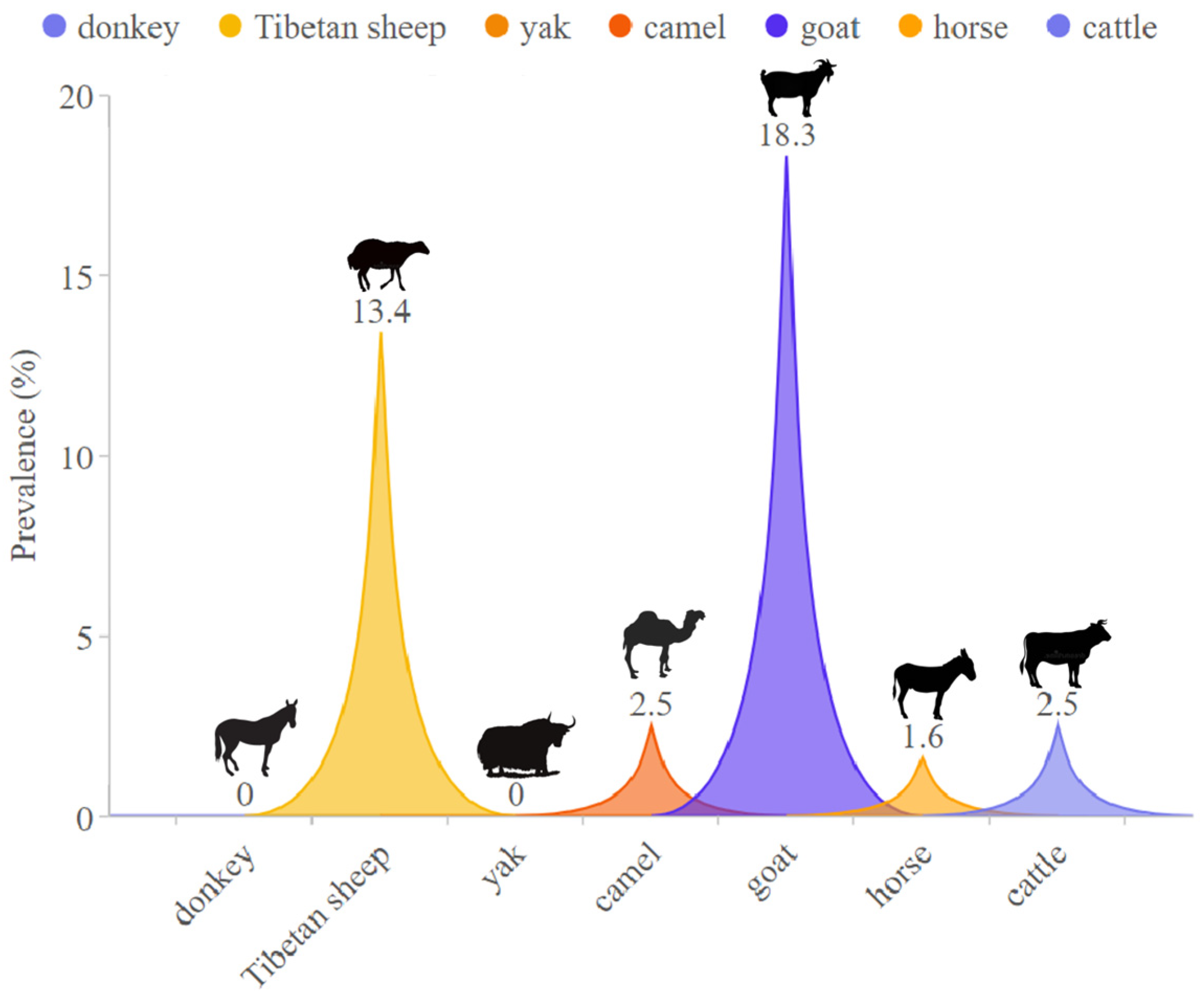
3.1. Overall Infection Rates
3.2. Sequencing Analyses
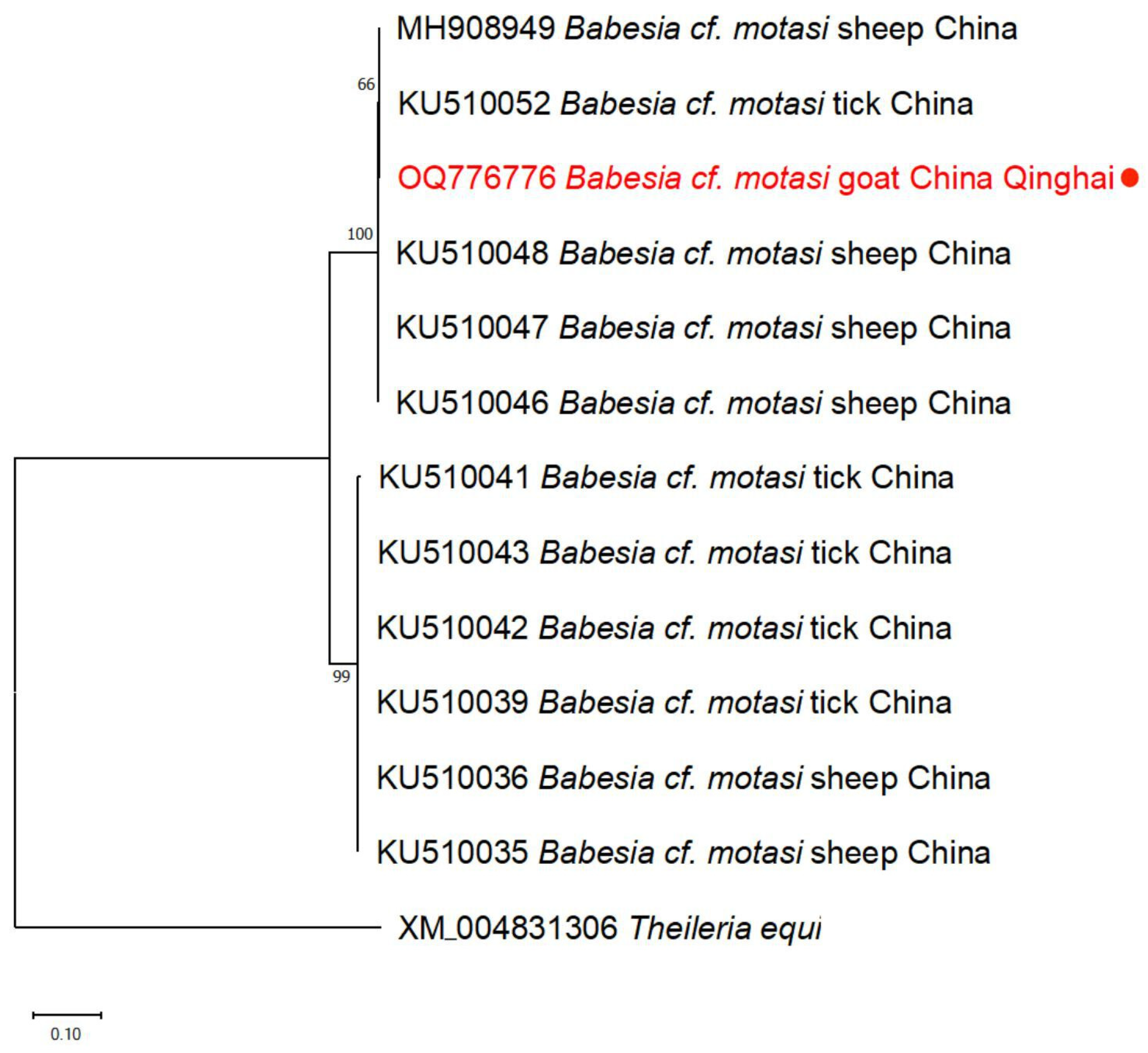
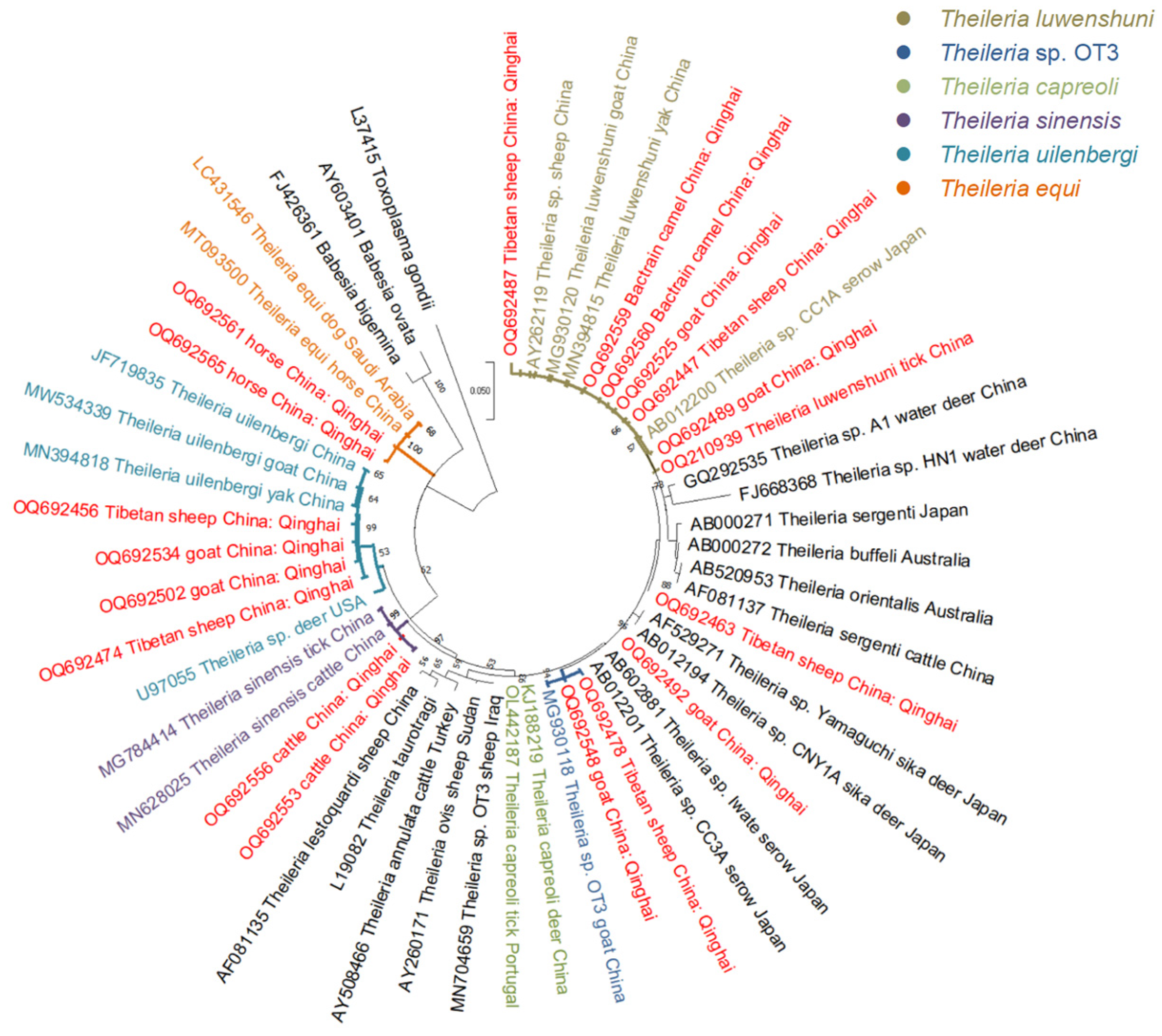
3.3. Phylogenetic Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, W.; Zhou, T.; Sun, J.; Li, Y.; Li, W. Accelerated urban expansion in Lhasa city and the implications for sustainable development in a plateau city. Sustainability 2017, 9, 1499. [Google Scholar] [CrossRef]
- Long, R.J.; Ding, L.M.; Shang, Z.H.; Guo, X.H. The yak grazing system on the Qinghai-Tibetan plateau and its status. Rangeland J. 2008, 30, 241. [Google Scholar] [CrossRef]
- Spicer, R.A.; Su, T.; Valdes, P.J.; Farnsworth, A.; Wu, F.-X.; Shi, G.; Spicer, T.E.V.; Zhou, Z. Why ‘the uplift of the Tibetan plateau’ is a myth? Natl. Sci. Rev. 2020, 8, nwaa091. [Google Scholar] [CrossRef]
- Qi, T.; Ai, J.; Yang, J.; Zhu, H.; Zhou, Y.; Zhu, Y.; Zhang, H.; Qin, Q.; Kang, M.; Sun, Y.; et al. Seroepidemiology of neosporosis in various animals in the Qinghai-Tibetan plateau. Front. Vet. Sci. 2022, 9, 953380. [Google Scholar] [CrossRef]
- Prosser, D.J.; Cui, P.; Takekawa, J.Y.; Tang, M.; Hou, Y.; Collins, B.M.; Yan, B.; Hill, N.J.; Li, T.; Li, Y.; et al. Wild bird migration across the Qinghai-Tibetan Plateau: A transmission route for highly pathogenic H5N1. PLoS ONE 2011, 6, e17622. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, S.; He, H. Research status of tick distribution and tick-borne diseases in Qinghai Province. Prog. Vet. Med. 2022, 43, 115–119. [Google Scholar]
- Hasle, G.; Bjune, G.; Edvardsen, E.; Jakobsen, C.; Linnehol, B.; Røer, J.E.; Mehl, R.; Røed, K.H.; Pedersen, J.; Leinaas, H.P. Transport of ticks by migratorypasserine birds to Norway. J. Parasitol. 2009, 95, 1342–1351. [Google Scholar] [CrossRef]
- Hasle, G.; Leinaas, H.P.; Røed, K.H.; Øines, Ø. Transport of Babesia venatorum-infected Ixodes ricinus to Norway by northward migrating passerine birds. Acta Vet. Scand. 2011, 53, 41. [Google Scholar] [CrossRef]
- He, J.; Chen, J.; Xiao, J.; Zhao, T.; Cao, P. Defining Important Areas for Ecosystem Conservation in Qinghai Province under the Policy of Ecological Red Line. Sustainability 2023, 15, 5524. [Google Scholar] [CrossRef]
- Yue, J.; Shi, Y. Epidemiological investigation of Lyme disease in parts of forest areas in Qinghai province. Chin. J. Vector Biol. Control 2009, 20, 358–359. [Google Scholar]
- Yin, H.; Schnittger, L.; Luo, J.; Seitzer, U.; Ahmed, J.S. Ovine theileriosis in China: A new look at an old story. Parasitol. Res. 2007, 101, 191–195. [Google Scholar] [CrossRef]
- Jian, Y.; Zhang, X.; Li, X.; Wang, G.; Wang, G.; Ma, L. Identification of ticks and tick-borne pathogens in different areas of Haibei of Qinghai province. Chin. Qinghai J. Anim. Vet. Sci. 2020, 50, 35. [Google Scholar]
- Li, J.; Jian, Y.; Jia, L.; Galon, E.M.; Benedicto, B.; Wang, G.; Cai, Q.; Liu, M.; Li, Y.; Ji, S.; et al. Molecular characterization of tick-borne bacteria and protozoans in yaks (Bos grunniens), Tibetan sheep (Ovis aries) and bactrian camels (Camelus bactrianus) in the Qinghai-Tibetan plateau area, China. Ticks Tick Borne Dis. 2020, 11, 101466. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Zhang, Q.; Li, Y.; Yang, Z.; Han, S.; Yuan, G.; Wang, S.; He, H. The common occurrence of Theileria ovis in Tibetan sheep and the first report of Theileria sinensis in yaks from southern Qinghai, China. Acta Parasit. 2021, 66, 1177–1185. [Google Scholar] [CrossRef]
- Li, J.; Ma, L.; Moumouni, P.F.A.; Jian, Y.; Wang, G.; Zhang, X.; Li, X.; Wang, G.; Lee, S.H.; Galon, E.M.; et al. Molecular survey and characterization of tick-borne pathogens in sheep from Qinghai, China. Small Rumin. Res. 2019, 175, 23–30. [Google Scholar] [CrossRef]
- Clark, I.A.; Jacobson, L.S. Do babesiosis and malaria share a common disease process? Ann. Trop. Med. Parasitol. 1998, 92, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Liu, J.; Liu, A.; Li, Y.; Niu, Q.; Gao, J.; Luo, J.; Chauvin, A.; Yin, H.; Moreau, E. A Member of the HSP90 family from ovine Babesia in China: Molecular characterization, phylogenetic analysis and antigenicity. Parasitology 2015, 142, 1387–1397. [Google Scholar] [CrossRef]
- Niu, Q.; Liu, Z.; Yu, P.; Yang, J.; Abdallah, M.O.; Guan, G.; Liu, G.; Luo, J.; Yin, H. Genetic characterization and molecular survey of Babesia bovis, Babesia bigemina and Babesia ovata in cattle, dairy cattle and yaks in China. Parasites Vectors 2015, 8, 518. [Google Scholar] [CrossRef]
- Sun, M.; Guan, G.; Liu, Z.; Wang, J.; Wang, D.; Wang, S.; Ma, C.; Cheng, S.; Yin, H.; Luo, J. Molecular survey and genetic diversity of Babesia spp. and Theileria spp. in cattle in Gansu province, China. Acta Parasit. 2020, 65, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.Y.; Wang, J.L.; Ning, H.R.; Tan, Q.D.; Yin, M.Y.; Zhang, X.X.; Zhou, D.H.; Zhu, X.Q. First report of Babesia bigemina infection in white yaks in China. Acta Trop. 2015, 145, 52–54. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Ayllón, N.; de la Fuente, J. Impact of climate trends on tick-borne pathogen transmission. Front. Physiol. 2012, 3, 64. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, W.; Meng, Q. Research progress of ticks and tick-borne disease. J. Anhui Agric. 2013, 41, 1107–1109. [Google Scholar]
- Yu, Z.; Wang, H.; Wang, T.; Sun, W.; Yang, X.; Liu, J. Tick-borne pathogens and the vector potential of ticks in China. Parasites Vectors 2015, 8, 24. [Google Scholar] [CrossRef]
- Gao, Y. Distribution of Tick Species and Epidemiological Investigation of 3 Species of Bacterial and Protozoal in Qinghai Province. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2018. [Google Scholar]
- Li, Y.; Liu, P.; Wang, C.; Chen, G.; Kang, M.; Liu, D.; Li, Z.; He, H.; Dong, Y.; Zhang, Y. Serologic evidence for Babesia bigemina infection in wild yak (Bos mutus) in Qinghai province, China. J. Wildl. Dis. 2015, 51, 872–875. [Google Scholar] [CrossRef]
- Qinghai Province. Available online: https://www.gov.cn/test/2013-03/26/content_2362781.htm (accessed on 22 August 2023).
- Terkawi, M.A.; Huyen, N.X.; Shinuo, C.; Inpankaew, T.; Maklon, K.; Aboulaila, M.; Ueno, A.; Goo, Y.K.; Yokoyama, N.; Jittapalapong, S.; et al. Molecular and serological prevalence of Babesia bovis and Babesia bigemina in water buffaloes in the northeast region of Thailand. Vet. Parasitol. 2011, 178, 201–207. [Google Scholar] [CrossRef]
- Alhassan, A.; Pumidonming, W.; Okamura, M.; Hirata, H.; Battsetseg, B.; Fujisaki, K.; Yokoyama, N.; Igarashi, I. Development of a single-round and multiplex PCR method for the simultaneous detection of Babesia caballi and Babesia equi in horse blood. Vet. Parasitol. 2005, 129, 43–49. [Google Scholar] [CrossRef]
- Niu, Q.; Liu, Z.; Yang, J.; Yu, P.; Pan, Y.; Zhai, B.; Luo, J.; Yin, H. Genetic diversity and molecular characterization of Babesia motasi-like in small uuminants and Ixodid ticks from China. Infect. Genet. Evol. 2016, 41, 8–15. [Google Scholar] [CrossRef]
- Aktaş, M.; Altay, K.; Dumanlı, N. Development of a polymerase chain reaction method for diagnosis of Babesia ovis infection in sheep and goats. Vet. Parasitol. 2005, 133, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhang, S.; Jia, L.; Xue, S.; Yu, L.; Kamyingkird, K.; Moumouni, P.F.A.; Moussa, A.A.E.M.; Zhou, M.; Zhang, Y.; et al. Molecular Detection of Theileria Species in Sheep from Northern China. J. Vet. Med. Sci. 2013, 75, 1227–1230. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Han, R.; Yang, J.F.; Mukhtar, M.U.; Chen, Z.; Niu, Q.L.; Lin, Y.Q.; Liu, G.Y.; Luo, J.X.; Yin, H.; Liu, Z.J. Molecular detection of Anaplasma Infections in Ixodid ticks from the Qinghai-Tibet plateau. Infect. Dis. Poverty 2019, 8, 12. [Google Scholar] [CrossRef]
- VanderWaal, K.L.; Ezenwa, V.O. Heterogeneity in Pathogen Transmission: Mechanisms and Methodology. Funct. Ecol. 2016, 30, 1606–1622. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, H.; Tang, X.; Zhao, W.; Yi, X.; Li, Q.; Sun, X. Organization and complexity of the yak (Bos grunniens) immunoglobulin loci. Front. Immunol. 2022, 13, 876509. [Google Scholar] [CrossRef]
- Guan, J.; Long, K.; Ma, J.; Zhang, J.; He, D.; Jin, L.; Tang, Q.; Jiang, A.; Wang, X.; Hu, Y.; et al. Comparative Analysis of the MicroRNA Transcriptome between Yak and Cattle Provides Insight into High-Altitude Adaptation. PeerJ 2017, 5, e3959. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, A.; Zhang, Y.; Yang, F. The Quantitative Attribution of Climate Change to Runoff Increase over the Qinghai-Tibetan Plateau. Sci. Total Environ. 2023, 897, 165326. [Google Scholar] [CrossRef]
- Faber, J.; Fonseca, L.M. How sample size influences research outcomes. Dental Press. J. Orthod. 2014, 19, 27–29. [Google Scholar] [CrossRef]
| Pathogen | Target Gene | Method | Primer Sequence (5′→3′) | Annealing Temperature (°C) | Amplicon Size (bp) | Reference | |
|---|---|---|---|---|---|---|---|
| B. bovis | sbp-4 | PCR | F | AGTTGTTGGAGGAGGCTAAT | 55 | 907 | [27] |
| R | TCCTTCTCGGCGTCCTTTTC | ||||||
| nPCR | nF | GAAATCCCTGTTCCAGAG | 55 | 503 | |||
| nR | TCGTTGATAACACTGCAA | ||||||
| B. bigemina | rap-1a | PCR | F | GAGTCTGCCAAATCCTTAC | 55 | 879 | [27] |
| R | TCCTCTACAGCTGCTTCG | ||||||
| nPCR | nF | AGCTTGCTTTCACAACTCGCC | 55 | 412 | |||
| nR | TTGGTGCTTTGACCGACGACAT | ||||||
| B. caballi | bc48 | PCR | F | GGCTCCCAGCGACTCTGTGG | 63 | 570 | [28] |
| R | CTTAAGTGCCCTCTTGATGC | ||||||
| B. motasi-like (Babesia sp. L/N/T) | rap-1b | PCR | F | TGCGCCTTCGAGTTGTACAAGAG | 58 | 765 | [29] |
| R | GACGGGTTGCRTAGGCTGAC | ||||||
| nPCR | nF | TGCGTGGAAGATAGAAAGTTAGCC | 60 | 536 | |||
| nR | ATGACTGATCTCGACTCTCCATTAGCTGG | ||||||
| B. ovis | ssu rRNA | PCR | F | TGGGCAGGACCTTGGTTCTTCT | 62 | 549 | [30] |
| R | CCGCGTAGCGCCGGCTAAATA | ||||||
| Theileria spp. | 18S rRNA | PCR | F | GAAACGGCTACCACATCT | 55 | 778 | [31] |
| R | AGTTTCCCCGTGTTGAGT | ||||||
| nPCR | nF | TTAAACCTCTTCCAGAGT | 581 | ||||
| nR | TCAGCCTTGCGACCATAC | ||||||
| Obtained Sequences | The Closest BLASTn Match | ||||||
|---|---|---|---|---|---|---|---|
| Pathogen | Isolation Source | Target Gene | GenBank Number | Length (bp) | Identity (%) | Pathogen | GenBank Number (Host, Country) |
| Theileria spp. | Sheep | 18s rRNA | OQ692447 | 584 | 99.14% | T. luwenshuni | MN394815 (yak, China) |
| Sheep | 18s rRNA | OQ692473 | 586 | 98.64% | T. capreoli | KJ188219 (deer, China) | |
| Sheep | 18s rRNA | OQ692474 | 580 | 99.66% | T. uilenbergi | MN394818 (yak, China) | |
| Sheep | 18s rRNA | OQ692478 | 589 | 100% | Theileria sp. OT3 | MG930118 (goat, China) | |
| Goat | 18s rRNA | OQ692489 | 582 | 99.83% | T. luwenshuni | MN394815 (yak, China) | |
| Goat | 18s rRNA | OQ692492 | 593 | 97.98% | Theileria sp. Iwate | AB602881 (serow, Japan) | |
| Goat | 18s rRNA | OQ692502 | 579 | 99.66% | T. uilenbergi | MN394818 (yak, China) | |
| Goat | 18s rRNA | OQ692548 | 589 | 100% | Theileria sp. OT3 | MG930118 (goat, China) | |
| Cattle | 18s rRNA | OQ692553 | 579 | 100% | T. sinensis | MN628025 (cattle, China) | |
| Horse | 18s rRNA | OQ692565 | 583 | 99.83% | T. equi | MT093500 (horse, China) | |
| Camel | 18s rRNA | OQ692560 | 582 | 100% | T. luwenshuni | MN394815 (yak, China) | |
| Babesia spp. | Goat | rap-1b | OQ776776 | 535 | 99.63% | Babesia cf. motasi | MH908949 (sheep, China) |
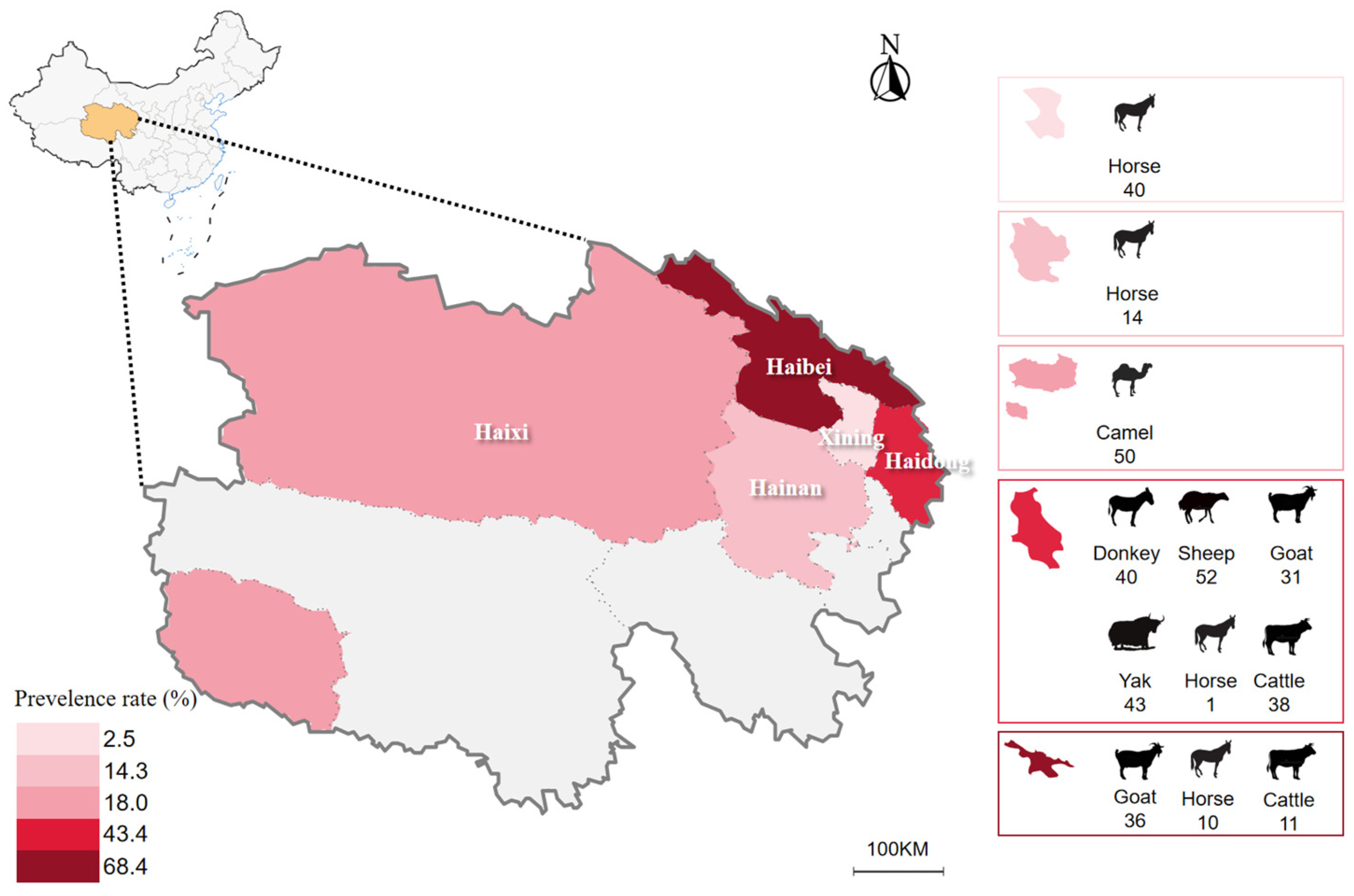
| Area | Animal Species | TBP 1 | Babesia spp. | Theileria spp. | ||||
|---|---|---|---|---|---|---|---|---|
| n | B. bigemina | B. bovis | B. caballi | Babesia cf. motasi | B. ovis | |||
| Haidong | Donkey | 40 | - | - | - | - | - | - |
| Tibetan sheep | 52 | - | - | - | - | - | 49 (94.2) | |
| Goat | 31 | - | - | - | 1 (3.2) | - | 31 (100.0) | |
| Yak | 43 | - | - | - | - | - | - | |
| Horse | 1 | - | - | - | - | - | - | |
| Cattle | 38 | - | - | - | - | - | 9 (23.7) | |
| Subtotal | 205 | - | - | - | 1 (0.5) | - | 89 (43.4) | |
| Haibei | Goat | 36 | - | - | - | - | - | 36 (100.0) |
| Horse | 10 | - | - | - | - | - | 3 (30.0) | |
| Cattle | 11 | - | - | - | - | - | - | |
| Subtotal | 57 | - | - | - | - | - | 39 (68.4) | |
| Hainan | Horse | 14 | - | - | - | - | - | 2 (14.3) |
| Subtotal | 14 | - | - | - | - | - | 2 (14.3) | |
| Haixi | Bactrian camels | 50 | - | - | - | - | - | 9 (18.0) |
| Subtotal | 50 | - | - | - | - | - | 9 (18.0) | |
| Xining | Horse | 40 | - | - | - | - | - | 1 (2.5) |
| Subtotal | 40 | - | - | - | - | - | 1 (2.5) | |
| Total | 366 | 1 (0.3) | 140 (38.2) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Jian, Y.; Wang, G.; Li, X.; Wang, G.; Hu, Y.; Yokoyama, N.; Ma, L.; Xuan, X. Molecular Identification of Babesia and Theileria Infections in Livestock in the Qinghai–Tibetan Plateau Area, China. Animals 2024, 14, 476. https://doi.org/10.3390/ani14030476
Ma Y, Jian Y, Wang G, Li X, Wang G, Hu Y, Yokoyama N, Ma L, Xuan X. Molecular Identification of Babesia and Theileria Infections in Livestock in the Qinghai–Tibetan Plateau Area, China. Animals. 2024; 14(3):476. https://doi.org/10.3390/ani14030476
Chicago/Turabian StyleMa, Yihong, Yingna Jian, Geping Wang, Xiuping Li, Guanghua Wang, Yong Hu, Naoaki Yokoyama, Liqing Ma, and Xuenan Xuan. 2024. "Molecular Identification of Babesia and Theileria Infections in Livestock in the Qinghai–Tibetan Plateau Area, China" Animals 14, no. 3: 476. https://doi.org/10.3390/ani14030476







