A Multidimensional Evaluation of the Factors in the Animal Welfare Assessment Grid (AWAG) That Are Associated with, and Predictive of, Behaviour Disorders in Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
Data Analysis
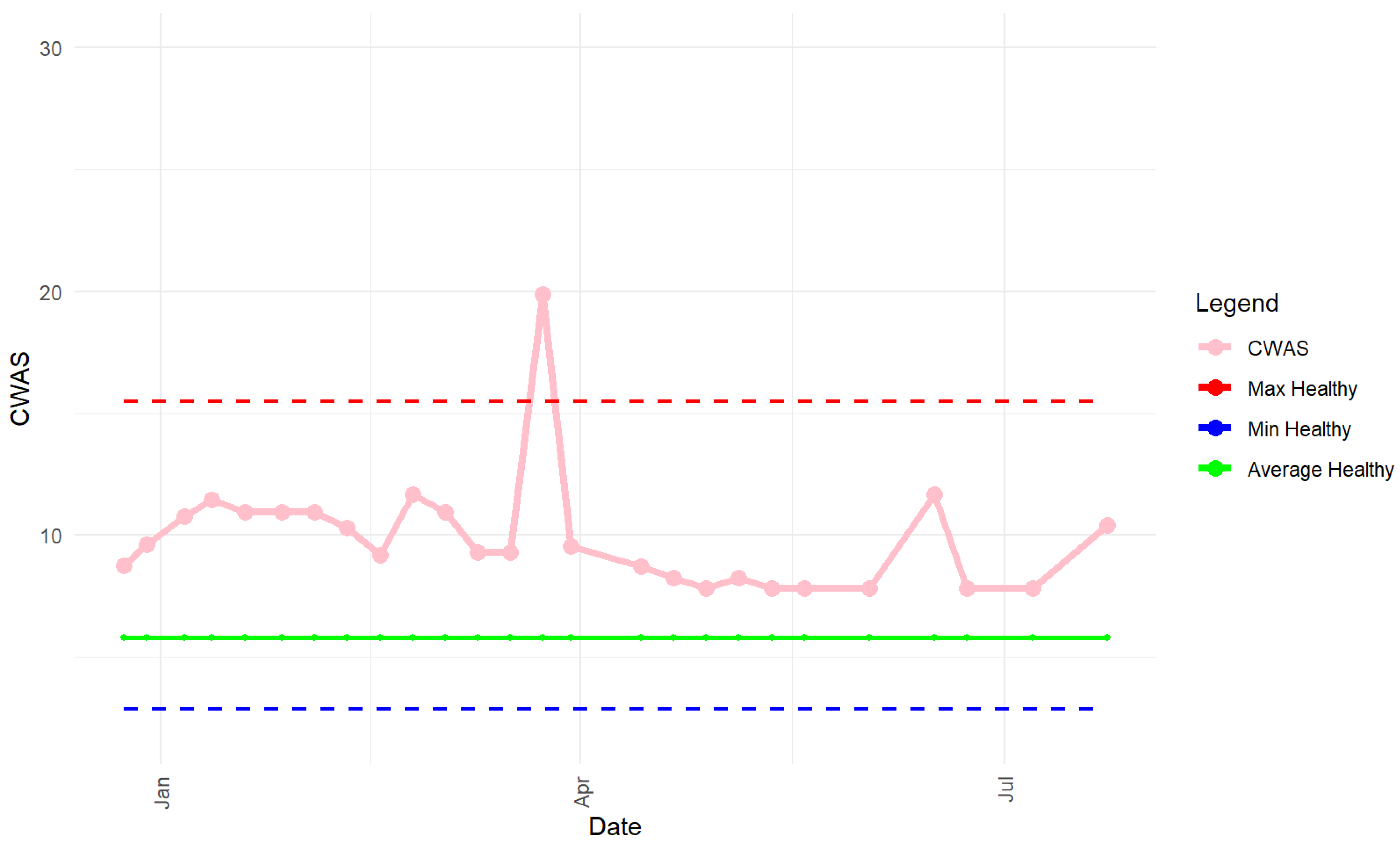
3. Results
3.1. Healthy vs. Behaviour Disorders
3.2. Factor Correlation
3.3. Factors That Are Predictors of Behaviour Disorders
4. Discussion
5. Limitations
Future Research
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Storengen, L.M.; Lingaas, F. Noise sensitivity in 17 dog breeds: Prevalence, breed risk and correlation with fear in other situations. Appl. Anim. Behav. Sci. 2015, 171, 152–160. [Google Scholar] [CrossRef]
- Dinwoodie, I.R.; Dwyer, B.; Zottola, V.; Gleason, D.; Dodman, N.H. Demographics and comorbidity of behavior problems in dogs. J. Vet. Behav. 2019, 32, 62–71. [Google Scholar] [CrossRef]
- Tiira, K.; Sulkama, S.; Lohi, H. Prevalence, comorbidity, and behavioral variation in canine anxiety. J. Vet. Behav. 2016, 16, 36–44. [Google Scholar] [CrossRef]
- Salonen, M.; Sulkama, S.; Mikkola, S.; Puurunen, J.; Hakanen, E.; Tiira, K.; Araujo, C.; Lohi, H. Prevalence, comorbidity, and breed differences in canine anxiety in 13,700 Finnish pet dogs. Sci. Rep. 2020, 10, 2962. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, E.J.; Bradshaw, J.W.S.; Casey, R.A. Fear responses to noises in domestic dogs: Prevalence, risk factors and co-occurrence with other fear related behaviour. Appl. Anim. Behav. Sci. 2013, 145, 15–25. [Google Scholar] [CrossRef]
- PDSA. PDSA Animal Wellbeing Report; PDSA: Telford, UK, 2023. [Google Scholar]
- Fatjó, J.; Bowen, J. Making the case for multi-axis assessment of behavioural problems. Animals 2020, 10, 383. [Google Scholar] [CrossRef]
- Sonntag, Q.; Overall, K. Key determinants of dog and cat welfare: Behaviour, breeding and household lifestyle. Rev. Sci. Tech. 2014, 33, 213–220. [Google Scholar] [CrossRef]
- Hall, N.J.; Wynne, C.D.L. The canid genome: Behavioral geneticists’ best friend? Genes Brain Behav. 2012, 11, 889–902. [Google Scholar] [CrossRef]
- Bray, E.E.; MacLean, E.L.; Hare, B.A. Increasing arousal enhances inhibitory control in calm but not excitable dogs. Anim. Cogn. 2015, 18, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Overall, K.L.; Dunham, A.E.; Scheifele, P.; Malowski, K.S. Fear of noises affects canine problem solving behavior and locomotion in standardized cognitive tests. Appl. Anim. Behav. Sci. 2019, 221, 104863. [Google Scholar] [CrossRef]
- Salehi, B.; Cordero, M.I.; Sandi, C. Learning under stress: The inverted-U-shape function revisited. Learn. Mem. 2010, 17, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Sümegi, Z.; Oláh, K.; Topál, J. Emotional contagion in dogs as measured by change in cognitive task performance. Appl. Anim. Behav. Sci. 2014, 160, 106–115. [Google Scholar] [CrossRef]
- Jakovcevic, A.; Elgier, A.M.; Mustaca, A.E.; Bentosela, M. Frustration Behaviors in Domestic Dogs. J. Appl. Anim. Welf. Sci. 2013, 16, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Engel, O.; Müller, H.W.; Klee, R.; Francke, B.; Mills, D.S. Effectiveness of imepitoin for the control of anxiety and fear associated with noise phobia in dogs. J. Vet. Intern. Med. 2019, 33, 2675–2684. [Google Scholar] [CrossRef] [PubMed]
- Mills, D.S. Perspectives on assessing the emotional behavior of animals with behavior problems. Curr. Opin. Behav. Sci. 2017, 16, 66–72. [Google Scholar] [CrossRef]
- McPeake, K.J.; Mills, D.S. The use of imepitoin (Pexion™) on fear and anxiety related problems in dogs—A case series. BMC Vet. Res. 2017, 13, 173. [Google Scholar] [CrossRef]
- Tooley, C.; Heath, S.E. Emotional Arousal Impacts Physical Health in Dogs: A Review of Factors Influencing Arousal, with Exemplary Case and Framework. Animals 2023, 13, 465. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Malkani, R.; Paramasivam, S.; Wolfensohn, S. Preliminary validation of a novel tool to assess dog welfare: The Animal Welfare Assessment Grid. Front. Vet. Sci. 2022, 9, 940017. [Google Scholar] [CrossRef]
- Czycholl, I.; Kniese, C.; Büttner, K.; Beilage, E.G.; Schrader, L.; Krieter, J. Interobserver reliability of the ‘Welfare Quality® Animal Welfare Assessment Protocol for Growing Pigs’. SpringerPlus 2016, 5, 1114. [Google Scholar] [CrossRef]
- Bateson, M.; Martin, P. Measuring Behaviour: An Introductory Guide, 4th ed.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Friedman, J.H.; Hastie, T.; Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Salt, C.; Morris, P.J.; Wilson, D.; Lund, E.M.; German, A.J. Association between life span and body condition in neutered client-owned dogs. J. Vet. Intern. Med. 2019, 33, 89–99. [Google Scholar] [CrossRef] [PubMed]
- German, A.J.; Woods, G.R.T.; Holden, S.L.; Brennan, L.; Burke, C. Dangerous trends in pet obesity. Vet. Rec. 2018, 182, 25. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, A.-M.; Mills, D.S.; Zulch, H. Clinical indicators of occult musculoskeletal pain in aggressive dogs. Vet. Rec. 2015, 176, 465. [Google Scholar] [CrossRef]
- Fagundes, A.L.L.; Hewison, L.; McPeake, K.J.; Zulch, H.; Mills, D.S. Noise sensitivities in dogs: An exploration of signs in dogs with and without musculoskeletal pain using qualitative content analysis. Front. Vet. Sci. 2018, 5, 17. [Google Scholar] [CrossRef]
- Camps, T.; Amat, M.; Manteca, X. A review of medical conditions and behavioral problems in dogs and cats. Animals 2019, 9, 1133. [Google Scholar] [CrossRef] [PubMed]
- Luño, I.; Muniesa, A.; Palacio, J.; García-Belenguer, S.; Rosado, B. Detection of owner-perceived emotional eating in companion dogs: A regression modelling approach. Vet. Rec. 2021, 189, e63. [Google Scholar] [CrossRef]
- Rosado, B.; García-Belenguer, S.; León, M.; Chacón, G.; Villegas, A.; Palacio, J. Blood concentrations of serotonin, cortisol and dehydroepiandrosterone in aggressive dogs. Appl. Anim. Behav. Sci. 2010, 123, 124–130. [Google Scholar] [CrossRef]
- Risbon, R.E.; Clifford, C.A.; Skorupski, K. An overview of canine histiocytic disorders. Vet. Med. 2007, 102, 464–480. [Google Scholar]
- Appleby, D.L.; Bradshaw, J.W.S.; Casey, R.A. Relationship between aggressive and avoidance behaviour by dogs and their experience in the first six months of life. Vet. Rec. 2002, 150, 434–438. [Google Scholar] [CrossRef]
- Tiira, K. Resilience in Dogs? Lessons from Other Species. Vet. Med. Res. Rep. 2019, 10, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Amat, M.; Camps, T.; Le Brech, S.; Manteca, X. Separation anxiety in dogs: The implications of predictability and contextual fear for behavioural treatment. Anim. Welf. 2014, 23, 263–266. [Google Scholar] [CrossRef]
- Rooney, N.J.; Clark, C.C.A.; Casey, R.A. Minimizing fear and anxiety in working dogs: A review. J. Vet. Behav. 2016, 16, 53–64. [Google Scholar] [CrossRef]
- Cobb, M.L.; Otto, C.M.; Fine, A.H. The Animal Welfare Science of Working Dogs: Current Perspectives on Recent Advances and Future Directions. Front. Vet. Sci. 2021, 8, 666898. [Google Scholar] [CrossRef]
- Puurunen, J.; Hakanen, E.; Salonen, M.K.; Mikkola, S.; Sulkama, S.; Araujo, C.; Lohi, H. Inadequate socialisation, inactivity, and urban living environment are associated with social fearfulness in pet dogs. Sci. Rep. 2020, 10, 3527. [Google Scholar] [CrossRef]
- Taylor, P.S.; Schrobback, P.; Verdon, M.; Lee, C. An effective environmental enrichment framework for the continual improvement of production animal welfare. Anim. Welf. 2023, 32, e14. [Google Scholar] [CrossRef]
- Fernandez, E.J. Training as enrichment: A critical review. Anim. Welf. 2022, 31, 1–12. [Google Scholar] [CrossRef]
- Houser, B.; Vitale, K.R. Increasing shelter cat welfare through enrichment: A review. Appl. Anim. Behav. Sci. 2022, 248, 105585. [Google Scholar] [CrossRef]
- Cunningham-Smith, P.; Emery, K. Dogs and People: Exploring the Human-Dog Connection. J. Ethnobiol. 2020, 40, 409–413. [Google Scholar] [CrossRef]
- Prato Previde, E.; Valsecchi, P. The Immaterial Cord: The Dog-Human Attachment Bond. In The Social Dog: Behavior and Cognition; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar] [CrossRef]
- Payne, E.; DeAraugo, J.; Bennett, P.; McGreevy, P. Exploring the existence and potential underpinnings of dog–human and horse–human attachment bonds. Behav. Process. 2016, 125, 114–121. [Google Scholar] [CrossRef]
- Smuts, B. Social Behaviour among Companion Dogs with an Emphasis on Play. In The Social Dog: Behavior and Cognition; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar] [CrossRef]
- Udell, M.A.R.; Brubaker, L. Are Dogs Social Generalists? Canine Social Cognition, Attachment, and the Dog-Human Bond. Curr. Dir. Psychol. Sci. 2016, 25, 327–333. [Google Scholar] [CrossRef]
- Morrill, K.; Hekman, J.; Li, X.; McClure, J.; Logan, B.; Goodman, L.; Gao, M.; Dong, Y.; Alonso, M.; Carmichael, E.; et al. Ancestry-inclusive dog genomics challenges popular breed stereotypes. Science 2022, 376, eabk0639. [Google Scholar] [CrossRef] [PubMed]
- Beerda, B.; Schilder, M.B.H.; Van Hooff, J.A.R.A.M.; De Vries, H.W.; Mol, J.A. Chronic stress in dogs subjected to social and spatial restriction. I. Behavioral responses. Physiol. Behav. 1999, 66, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Stellato, A.C.; Flint, H.E.; Dewey, C.E.; Widowski, T.M.; Niel, L. Risk-factors associated with veterinary-related fear and aggression in owned domestic dogs. Appl. Anim. Behav. Sci. 2021, 241, 105374. [Google Scholar] [CrossRef]
- Edwards, P.T.; Smith, B.P.; McArthur, M.L.; Hazel, S.J. Fearful Fido: Investigating dog experience in the veterinary context in an effort to reduce distress. Appl. Anim. Behav. Sci. 2019, 213, 14–25. [Google Scholar] [CrossRef]
- Riemer, S.; Heritier, C.; Windschnurer, I.; Pratsch, L.; Arhant, C.; Affenzeller, N. A review on mitigating fear and aggression in dogs and cats in a veterinary setting. Animals 2021, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Forster, B.; Engel, O.; Erhard, M.; Bartels, A. Short-term imepitoin treatment reduces stress level in dogs with generalized anxiety disorder. J. Vet. Behav. 2020, 38, 67–73. [Google Scholar] [CrossRef]
- Crowell-Davis, S.L. Understanding behavior: Generalized anxiety disorder. Compend. Contin. Educ. Vet. 2009, 31, 427–430. [Google Scholar]
- Hekman, J.P.; Karas, A.Z.; Sharp, C.R. Psychogenic stress in hospitalized dogs: Cross species comparisons, implications for health care, and the challenges of evaluation. Animals 2014, 4, 331–347. [Google Scholar] [CrossRef]
- Oshio, A.; Taku, K.; Hirano, M.; Saeed, G. Resilience and Big Five personality traits: A meta-analysis. Pers. Individ. Differ. 2018, 127, 54–60. [Google Scholar] [CrossRef]
- Sipple, N.; Thielke, L.; Smith, A.; Vitale, K.R.; Udell, M.A.R. Intraspecific and Interspecific Attachment between Cohabitant Dogs and Human Caregivers. Integr. Comp. Biol. 2021, 61, 132–139. [Google Scholar] [CrossRef]
- Mills, D.S.; Demontigny-Bédard, I.; Gruen, M.; Klinck, M.P.; McPeake, K.J.; Barcelos, A.M.; Hewison, L.; Van Haevermaet, H.; Denenberg, S.; Hauser, H.; et al. Pain and problem behavior in cats and dogs. Animals 2020, 10, 318. [Google Scholar] [CrossRef]
- Watson, F.; Packer, R.M.A.; Rusbridge, C.; Volk, H.A. Behavioural changes in dogs with idiopathic epilepsy. Vet. Rec. 2020, 186, 93. [Google Scholar] [CrossRef]
- Watson, F.; Rusbridge, C.; Packer, R.M.A.; Casey, R.A.; Heath, S.; Volk, H.A. A review of treatment options for behavioural manifestations of clinical anxiety as a comorbidity in dogs with idiopathic epilepsy. Vet. J. 2018, 238, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Camps, T.; Amat, M.; Mariotti, V.M.; Le Brech, S.; Manteca, X. Pain-related aggression in dogs: 12 clinical cases. J. Vet. Behav. 2012, 7, 99–102. [Google Scholar] [CrossRef]
- Landsberg, G.M.; Hunthausen, W.L.; Ackerman, L.J. Behavioural Problems of the Dog and Cat; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Bleuer-Elsner, S.; Muller, G.; Beata, C.; Zamansky, A.; Marlois, N. Effect of fluoxetine at a dosage of 2–4 mg/kg daily in dogs exhibiting hypersensitivity-hyperactivity syndrome, a retrospective study. J. Vet. Behav. 2021, 44, 25–31. [Google Scholar] [CrossRef]
- van Haaften, K.A.; Grigg, E.K.; Kolus, C.; Hart, L.; Kogan, L.R. A survey of dog owners’ perceptions on the use of psychoactive medications and alternatives for the treatment of canine behavior problems. J. Vet. Behav. 2020, 35, 27–33. [Google Scholar] [CrossRef]
- Warnes, C.E. Using psychopharmacology in the treatment of problem behaviours in dogs and cats. Companion Anim. 2021, 26, 1–10. [Google Scholar] [CrossRef]
- Murray, J.K.; Kinsman, R.H.; Lord, M.S.; Da Costa, R.E.P.; Woodward, J.L.; Owczarczak-Garstecka, S.C.; Tasker, S.; Knowles, T.G.; Casey, R.A. ‘Generation Pup’—Protocol for a longitudinal study of dog behaviour and health. BMC Vet. Res. 2021, 17, 1. [Google Scholar] [CrossRef]
- Pegram, C.; Gray, C.; Packer, R.M.A.; Richards, Y.; Church, D.B.; Brodbelt, D.C.; O’neill, D.G. Proportion and risk factors for death by euthanasia in dogs in the UK. Sci. Rep. 2021, 11, 9145. [Google Scholar] [CrossRef]
- Tulloch, J.S.P.; Owczarczak-Garstecka, S.C.; Fleming, K.M.; Vivancos, R.; Westgarth, C. English hospital episode data analysis (1998–2018) reveal that the rise in dog bite hospital admissions is driven by adult cases. Sci. Rep. 2021, 11, 1767. [Google Scholar] [CrossRef]
- Tayari, H.; Bell, A. Dexmedetomidine infusion as perioperative adjuvant in a dog undergoing craniotomy. Vet. Rec. Case Rep. 2019, 7, e000727. [Google Scholar] [CrossRef]
- Fernández-Gallego, A.; Del-Pozo, J.; Boag, A.; Maxwell, S.; Pérez-Acino, J. Xanthogranulomatous Pituitary Adenoma in a Dog with Typical Hyperadrenocorticism. J. Comp. Pathol. 2020, 180, 115–121. [Google Scholar] [CrossRef]
- Zulch, H. Considering Medical Influences on Behaviour. In Companion Animal Behaviour Problems; CABI: Wallingford, UK, 2022. [Google Scholar] [CrossRef]
- Bennaim, M.; Shiel, R.E.; Mooney, C.T. Diagnosis of spontaneous hyperadrenocorticism in dogs. Part 1: Pathophysiology, aetiology, clinical and clinicopathological features. Vet. J. 2019, 252, 105342. [Google Scholar] [CrossRef]
- Rutherford, K.M.D. Assessing pain in animals. Anim. Welf. 2002, 11, 31–53. [Google Scholar] [CrossRef]
- Reyes-Sotelo, B.; Mota-Rojas, D.; Martínez-Burnes, J.; Gómez, J.; Lezama, K.; González-Lozano, M.; Hernández-Ávalos, I.; Casas, A.; Herrera, Y.; Mora-Medina, P. Tail docking in dogs: Behavioural, physiological and ethical aspects. CABI Rev. 2020, 15, 039. [Google Scholar] [CrossRef]
- Lane, D.M.; Hill, S.A. Pressure algometry measurement of canine muscular pain near the thoracolumbar junction: Evaluation of a modified technique. Vet. Anaesth. Analg. 2016, 43, 227–234. [Google Scholar] [CrossRef]
- Amanieh, H.; Dorey, N.R. How Animals Learn. In Animal Behavior for Shelter Veterinarians and Staff; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Ooi, Y.P.; Ang, R.P.; Fung, D.S.S.; Wong, G.; Cai, Y. The impact of parent–child attachment on aggression, social stress and self-esteem. Sch. Psychol. Int. 2006, 27, 552–566. [Google Scholar] [CrossRef]
- Beckmann, L.; Bergmann, M.C.; Fischer, F.; Mößle, T. Risk and Protective Factors of Child-to-Parent Violence: A Comparison Between Physical and Verbal Aggression. J. Interpers. Violence 2021, 36, NP1309-1334NP. [Google Scholar] [CrossRef]
- Riggio, G.; Gazzano, A.; Zsilák, B.; Carlone, B.; Mariti, C. Quantitative behavioral analysis and qualitative classification of attachment styles in domestic dogs: Are dogs with a secure and an insecure-avoidant attachment different? Animals 2021, 11, 14. [Google Scholar] [CrossRef]
- Masson, S.; de la Vega, S.; Gazzano, A.; Mariti, C.; Pereira, G.D.G.; Halsberghe, C.; Leyvraz, A.M.; McPeake, K.; Schoening, B. Electronic training devices: Discussion on the pros and cons of their use in dogs as a basis for the position statement of the European Society of Veterinary Clinical Ethology. J. Vet. Behav. 2018, 25, 71–75. [Google Scholar] [CrossRef]
- Guilherme Fernandes, J.; Olsson, I.A.S.; Vieira de Castro, A.C. Do aversive-based training methods actually compromise dog welfare?: A literature review. Appl. Anim. Behav. Sci. 2017, 196, 1–12. [Google Scholar] [CrossRef]
- Casey, R.A.; Naj-Oleari, M.; Campbell, S.; Mendl, M.; Blackwell, E.J. Dogs are more pessimistic if their owners use two or more aversive training methods. Sci. Rep. 2021, 11, 19023. [Google Scholar] [CrossRef]
- Coccaro, E.F.; Fanning, J.R.; Phan, K.L.; Lee, R. Serotonin and impulsive aggression. CNS Spectr. 2015, 20, 295–302. [Google Scholar] [CrossRef]
- da Cunha-Bang, S.; Knudsen, G.M. The Modulatory Role of Serotonin on Human Impulsive Aggression. Biol. Psychiatry 2021, 90, 447–457. [Google Scholar] [CrossRef]
- Denenberg, S. Small Animal Veterinary Psychiatry; CABI Publishing: Oxon, UK, 2021; ISBN 9781786394552. [Google Scholar]
- Çakiroǧlu, D.; Meral, Y.; Sancak, A.A.; Çifti, G. Relationship between the serum concentrations of serotonin and lipids and aggression in dogs. Vet. Rec. 2007, 161, 59–61. [Google Scholar] [CrossRef]
- León, M.; Rosado, B.; García-Belenguer, S.; Chacón, G.; Villegas, A.; Palacio, J. Assessment of serotonin in serum, plasma, and platelets of aggressive dogs. J. Vet. Behav. 2012, 7, 348–352. [Google Scholar] [CrossRef]
- Hersey, M.; Reneaux, M.; Berger, S.N.; Mena, S.; Buchanan, A.M.; Ou, Y.; Tavakoli, N.; Reagan, L.P.; Clopath, C.; Hashemi, P. A tale of two transmitters: Serotonin and histamine as in vivo biomarkers of chronic stress in mice. J. Neuroinflammation 2022, 19, 167. [Google Scholar] [CrossRef]
- Boissy, A. Fear and fearfulness in animals. Q. Rev. Biol. 1995, 70, 165–191. [Google Scholar] [CrossRef]
- Ohl, F.; Arndt, S.S.; van der Staay, F.J. Pathological anxiety in animals. Vet. J. 2008, 175, 18–26. [Google Scholar] [CrossRef]
- Bubier, J.L.; Drabick, D.A.G. Co-occurring anxiety and disruptive behavior disorders: The roles of anxious symptoms, reactive aggression, and shared risk processes. Clin. Psychol. Rev. 2009, 29, 658–669. [Google Scholar] [CrossRef]



| Physical | Psychological | Environmental | Procedural |
|---|---|---|---|
| Mobility | Aggression towards caregiver | Choice, control, and predictability | Behaviour during assessment |
| Body condition | Aggression towards unfamiliar people | Enrichment | Change in daily routine |
| Clinical assessment | Fears and anxieties frequency | Social interactions | Handling during assessment |
| Eating and drinking | Reaction to stressors | Procedure pain |
| Factor | W Value | p Value |
|---|---|---|
| Aggression towards caregiver | 8034 | <0.001 |
| Aggression towards unfamiliar people | 8071 | <0.001 |
| Behaviour during assessment | 6516 | <0.001 |
| Body condition score | 8330.5 | 0.02019 |
| Change in daily routine | 2124.5 | <0.001 |
| Choice, control, and predictability | 12,438 | <0.001 |
| Clinical assessment | 337 | <0.001 |
| Eating and drinking | 9182.5 | <0.001 |
| Enrichment | 10,876 | <0.001 |
| Fears and anxieties frequency | 936.5 | <0.001 |
| Handing during assessment | 12,956 | <0.001 |
| Mobility/activity | 450 | <0.001 |
| Procedure pain | 7754.7 | 0.08476 |
| Reaction to stressors | 12,698 | <0.001 |
| Social interactions | 9827.5 | <0.001 |
| Aggression towards Caregiver | Aggression towards Unfamiliar People | Behaviour during Assessment | Body Condition | Change in Daily Routine | Choice Control and Predictability | Clinical Assessment | Eating and Drinking | Enrichment | Fears and Anxieties Frequency | Handling during Assessment | Procedure Pain | Mobility Activity | Reaction to Stressors | Social Interactions | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Social interactions | 0.707 | 0.011 | −0.275 | 0.461 | 0 | 0.014 | 0.379 | 0.186 | 0.568 | −0.689 | 0.2 | 0.386 | 0.114 | 0.711 | 1 |
| Reaction to stressors | 0.418 | −0.175 | 0.246 | 0.043 | −0.293 | 0.561 | 0.104 | −0.175 | 0.821 | −0.168 | 0.396 | 0.075 | −0.071 | 1 | 0.711 |
| Procedure pain | 0.707 | 0.6 | −0.518 | 0.493 | 0.768 | −0.321 | 0.789 | 0.696 | 0.096 | −0.654 | 0.193 | 1 | 0.646 | 0.075 | 0.386 |
| Mobility Activity | 0.257 | 0.354 | −0.204 | 0.657 | 0.421 | −0.4 | 0.8 | 0.429 | −0.139 | −0.325 | 0.179 | 0.646 | 1 | −0.071 | 0.114 |
| Handling during assessment | 0.482 | 0.511 | −0.075 | 0.407 | 0.243 | 0.139 | 0.289 | 0.286 | 0.161 | −0.146 | 1 | 0.193 | 0.179 | 0.396 | 0.2 |
| Fears and anxieties frequency | −0.789 | −0.293 | 0.729 | −0.657 | −0.457 | 0.464 | −0.557 | −0.625 | −0.114 | 1 | −0.146 | −0.654 | −0.325 | −0.168 | −0.689 |
| Enrichment | 0.282 | −0.329 | 0.293 | −0.232 | −0.246 | 0.618 | −0.129 | −0.136 | 1 | −0.114 | 0.161 | 0.096 | −0.139 | 0.821 | 0.568 |
| Eating and drinking | 0.586 | 0.764 | −0.757 | 0.411 | 0.789 | −0.546 | 0.496 | 1 | −0.136 | −0.625 | 0.286 | 0.696 | 0.429 | −0.175 | 0.186 |
| Clinical assessment | 0.568 | 0.575 | −0.468 | 0.754 | 0.514 | −0.489 | 1 | 0.496 | −0.129 | −0.557 | 0.289 | 0.789 | 0.8 | 0.104 | 0.379 |
| Choice control and predictability | −0.086 | −0.368 | 0.786 | −0.561 | −0.436 | 1 | −0.489 | −0.546 | 0.618 | 0.464 | 0.139 | −0.321 | −0.4 | 0.561 | 0.014 |
| Change in daily routine | 0.493 | 0.782 | −0.571 | 0.364 | 1 | −0.436 | 0.514 | 0.789 | −0.246 | −0.457 | 0.243 | 0.768 | 0.421 | −0.293 | 0 |
| Body condition | 0.479 | 0.4 | −0.429 | 1 | 0.364 | −0.561 | 0.754 | 0.411 | −0.232 | −0.657 | 0.407 | 0.493 | 0.657 | 0.043 | 0.461 |
| Behaviour during assessment | −0.564 | −0.5 | 1 | −0.429 | −0.571 | 0.786 | −0.468 | −0.757 | 0.293 | 0.729 | −0.075 | −0.518 | −0.204 | 0.246 | −0.275 |
| Aggression towards unfamiliar people | 0.514 | 1 | −0.5 | 0.4 | 0.782 | −0.368 | 0.575 | 0.764 | −0.329 | −0.293 | 0.511 | 0.6 | 0.354 | −0.175 | 0.011 |
| Aggression towards caregiver | 1 | 0.514 | −0.564 | 0.479 | 0.493 | −0.086 | 0.568 | 0.586 | 0.282 | −0.789 | 0.482 | 0.707 | 0.257 | 0.418 | 0.707 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malkani, R.; Paramasivam, S.; Wolfensohn, S. A Multidimensional Evaluation of the Factors in the Animal Welfare Assessment Grid (AWAG) That Are Associated with, and Predictive of, Behaviour Disorders in Dogs. Animals 2024, 14, 528. https://doi.org/10.3390/ani14040528
Malkani R, Paramasivam S, Wolfensohn S. A Multidimensional Evaluation of the Factors in the Animal Welfare Assessment Grid (AWAG) That Are Associated with, and Predictive of, Behaviour Disorders in Dogs. Animals. 2024; 14(4):528. https://doi.org/10.3390/ani14040528
Chicago/Turabian StyleMalkani, Rachel, Sharmini Paramasivam, and Sarah Wolfensohn. 2024. "A Multidimensional Evaluation of the Factors in the Animal Welfare Assessment Grid (AWAG) That Are Associated with, and Predictive of, Behaviour Disorders in Dogs" Animals 14, no. 4: 528. https://doi.org/10.3390/ani14040528
APA StyleMalkani, R., Paramasivam, S., & Wolfensohn, S. (2024). A Multidimensional Evaluation of the Factors in the Animal Welfare Assessment Grid (AWAG) That Are Associated with, and Predictive of, Behaviour Disorders in Dogs. Animals, 14(4), 528. https://doi.org/10.3390/ani14040528





