Simple Summary
The systematic status of Fuscozetes is not clear in the literature. Therefore, the morphological ontogeny of F. fuscipes, the type species of this genus, was investigated and compared with its congeners in this study, and a new diagnosis of Fuscozetes is given. The juveniles of F. fuscipes are light brown, with a brown prodorsum, sclerites, epimeres, and legs. In all juveniles, a humeral organ and a humeral macrosclerite are present. The gastronotum of the larva has 12 pairs of setae (h3 is present), while the nymphs have 15 pairs. In the larva, the gastronotal shield is weakly developed, most of the gastronotal setae are short and inserted on the microsclerites, and several other macrosclerites and many microsclerites are present on the hysterosoma. In the nymphs, the gastronotal shield is well developed, with 10 pairs of setae (d-, l-, and h-series, and p1), and setae p2 and p3 are located on a large posteroventral macrosclerite. In all the instars, femora I and II are oval in cross-section, without a large ventral carina. Mitochondrial COI sequence data revealed a deep split between the Nearctic and Palearctic populations of F. fuscipes, and a less, but significant, divergence within each continent. These strong geographical barriers were contrasted with multiple cases of shared haplotypes over long distances in the Palearctic, indicating high migration rates in modern times.
Abstract
The systematic status of Fuscozetes Sellnick, 1928, is not clear in the literature. Therefore, the morphological ontogeny of F. fuscipes (C.L. Koch, 1844), the type species of this genus, was investigated and compared with its congeners in this study, and a new diagnosis of Fuscozetes is given. The juveniles of F. fuscipes are light brown, with a brown prodorsum, sclerites, epimeres, and legs. In all juveniles, a humeral organ and a humeral macrosclerite are present. The gastronotum of the larva has 12 pairs of setae (h3 is present), whereas the nymphs have 15 pairs. In the larva, the gastronotal shield is weakly developed, and most gastronotal setae are short except for a slightly longer h2. Most of the gastronotal setae are inserted on the microsclerites except for h3, and several other macrosclerites and many microsclerites are present on the hysterosoma. In the nymphs, the gastronotal shield is well developed, with 10 pairs of setae (d-, l-, and h-series, and p1), and setae p2 and p3 are located on a large posteroventral macrosclerite. In all the instars, femora I and II are oval in cross-section, without a large ventral carina. Mitochondrial COI sequence data revealed a deep split between the Nearctic and Palearctic populations of F. fuscipes, and a less, but significant, divergence within each continent. These strong geographical barriers were contrasted with multiple cases of shared haplotypes over long distances in the Palearctic, indicating high migration rates in modern times.
1. Introduction
Fuscozetes Sellnick, 1928, with the type species F. fuscipes (C.L Koch, 1844), is an average genus in terms of its number of species. It includes 15 species according to Subías [1], and two of them are considered species inquirendae. Fuscozetes belongs to the subfamily Spherozetinae sensu Shaldybina [2], which also contains the genera Edwardzetes Berlese, 1913; Ghilarovizetes Shaldybina, 1969; Melanozetes Hull, 1916; and Sphaerozetes Berlese, 1885.
The systematics of Fuscozetes are not clear in the literature, and its diagnosis has varied over time. Sellnick [3] paid particular attention to the skeletal characters of adults, whereas Shaldybina [2] added the number of notogastral setae, which can provide information on the phylogeny of moss mites [4,5,6]. Based on the morphology of the juvenile stages and adults of the Fuscozetes species, Shaldybina [2,7,8] limited the number of setae on the notogaster of adults to 10 or 11 pairs. Behan-Pelletier [9,10] expanded the diagnosis of Fuscozetes to 10–14 pairs, including c3, but this diagnosis was problematic because it also included Melanozetes Hull, 1916, which has 14 pairs of notogastral setae. Based on the morphology of the juvenile stages and adults of the Fuscozetes species, Seniczak et al. [11] restricted the diagnosis of Fuscozetes to 10–13 pairs of notogastral setae, including seta c2, and some or all the setae of the d-series. Next, Seniczak et al. [12] and Seniczak and Seniczak [13,14] added the length and position of solenidion ω2 on tarsus I, which clearly separated Fuscozetes from Melanozetes, both in the nymphs and the adults. In Fuscozetes, solenidion ω2 is shorter than ω1 and is placed posterolaterally to ω1, whereas in Melanozetes, solenidion ω2 is as long as or longer than ω1 and is placed anteriorly to ω1.
Shaldybina [2] included Fuscozetes in the subfamily Sphearozetinae, which clearly differs from Ceratozetinae and Trichoribatinae in both the adult and juvenile stages. Weigmann [15] omitted this proposal and included Fuscozetes and related genera in Ceratozetidae, indicating that the systematics of this family need further investigation.
The juvenile instars of the Fuscozetes species are relatively well studied. According to the catalogue by Norton and Ermilov [16], Seniczak et al. [12], and Seniczak and Seniczak [13,14], the morphological ontogeny of seven species of Fuscozetes is known: F. coulsoni A. and S. Seniczak, 2020; F. fuscipes; F. kamchatkicus Seniczak et al., 2016; F. pseudosetosus Shaldybina, 1969; F. setiger (Trägårdh, 1910); F. setosus (C.L. Koch, 1839); and F. tatricus Seniczak, 1993. The morphological ontogeny of F. fuscipes has already been investigated by Seniczak [17], but this study was general and omitted the lateral aspect of the larvae, tritonymphs, and adults, as well as the leg setation, which is important for the morphological comparison of species within the genus. Here, we investigated specimens of F. fuscipes from Norway, which differed slightly from those studied by Seniczak [17] from Poland, illustrating some regional variability in this species. We also illustrated the morphological structures of F. fuscipes with SEM figures to clarify the miniscule characters of this species. In addition, we compared the molecular data (COI) of F. fuscipes from different locations, based on our own and public data.
2. Materials and Methods
2.1. Morphological and Biological Studies
The specimens of F. fuscipes used in this study were collected on 15 June 2018 by A. Seniczak from patches of Sphagnum mosses on the shore of lake Skomakerdiket (Bergen, Vestland, Norway, 60°23′39.7″ N, 5°21′04.7″ E, 320 m a. s. l.). The samples were extracted in Berlese funnels in the laboratory of the Department of Natural History, University Museum of Bergen (Norway), over ten days. The juveniles of F. fuscipes were identified using the specific characters given by Seniczak [17]. In 30 adults selected at random, the sex ratio and the number of gravid females were determined, as well as the body length and width. We also measured the morphological characters of all the instars of F. fuscipes, namely the total body length (in the lateral aspect, from the tip of the rostrum to the posterior edge of the notogaster), the width (in the dorsal aspect, at the widest part of the notogaster), and the length of the anal and genital openings and the setae, perpendicularly to their length. All measurements are given in µm. All light microscopy was performed using a Nicon Eclipse Ni.
We illustrated the dorsal and lateral aspects of the larvae, tritonymphs, and adults; some leg segments of these stages; the ventral regions of all instars; and the palps and chelicerae of the adults. The illustrations were prepared from individuals mounted temporarily on slides in lactic acid. In the text and figures, the following abbreviations are used: rostral (ro), lamellar (le), interlamellar (in) and exobothridial (ex) setae, lamella (La), translamella (Tr), bothridium (bo), bothridial seta (bs), notogastral or gastronotal setae (c-, d-, l-, h-, and p-series), porose areas (Aa, A1, A2, and A3), opisthonotal gland opening (gla), pteromorph (Ptm), cupules or lyrifissures (ia, im, ip, ih, ips, and iad), tutorium (Tut), pedotectum (Pd), circumpedal carina (cp), custodium (cus), discidium (Dis), humeral sclerite (hs), humeral organ (oh), subcapitular setae (a, m, and h), cheliceral setae (cha and chb), Trägårdh organ (Tg), palp setae (sup, inf, l, d, cm, acm, it, vt, ul, and su), solenidion ω, adanal and anal setae (ad- and an-series), epimeral setae (1a–c, 2a, 3a–c, and 4a–c), genital (g) and aggenital (ag) setae, leg solenidia (σ, φ, and ω), famulus (ε), and setae (bv, d, l, ft, tc, it, p, u, a, s, pv, pl, and v). The terminology used follows that of Grandjean [4,5,18,19,20,21], Behan-Pelletier [9,10], and Norton and Behan-Pelletier [22]. The species nomenclature follows Subías [1] and Norton and Ermilov [16].
For scanning electron microscopy (SEM), the mites were air-dried, coated with Au/Pd in a Polaron SC502 sputter-coater, and placed on Al stubs with double-sided sticky carbon tape. The observations and micrographs were made with a Quanta™ 450 FEG scanning electron microscope.
2.2. DNA Barcoding
The specimens of F. fuscipes used for molecular studies were collected in Southern, Central, and Northern Norway. We also used public sequences from the BOLD database, which originated from Canada, Finland, and Germany. The outgroup sequences represented two other Fuscozetes species, F. setosus and F. setiger, and representatives of all the other genera of the subfamily Spherozetinae (Edwardzetes Berlese, 1913; Ghilarovizetes Shaldybina, 1969; Melanozetes Hull, 1916; and Sphaerozetes Berlese, 1885) and representatives of two other related subfamilies, Trichoribatinae (Diapterobates Grandjean, 1936; Neogymnobates Ewing, 1917; Svalbardia Thor, 1930; and Trichoribates Berlese, 1910) and Ceratozetinae (Ceratozetes Berlese, 1908).
The specimens of F. fuscipes were DNA-barcoded at the Canadian Centre for DNA Barcoding (CCDB) in Guelph, Canada. Before sending the samples to the CCDB, each specimen was photographed, and these photos are vouchers available at the Barcode of Life Data System (BOLD, http://boldsystems.org, accessed on 20 November 2023). The specimens were placed in a well containing 50 mL of 90% ethanol in a 96-well microplate and submitted to the CCDB. The mites were sequenced for the barcode region of the COI gene according to standard protocols at the CCDB (www.ccdb.ca, accessed on 20 October 2020), using either the LepF1/LepR1 [23] or the LCO1490/m HCO2198 [24] primer pairs. The DNA extracts were placed in archival storage at −80 °C, mainly at the CCDB, and some (sequencing code starting with UMNFO) at the University Museum of Bergen (ZMUB). The COI sequence chromatograms were checked for double peaks and potential NUMTs, and were blasted in GenBank to detect and exclude possible contaminants. The sequences are available in GenBank (Table 1).

Table 1.
Information about sequenced specimens of Fuscozetes fuscipes and other oribatid species used in this study. Na—not available; these sequences are public in BOLD, but without some data.
The sequences were aligned by eye in MEGA11: Molecular Evolutionary Genetics Analysis, version 11 [29]. A Bayesian inference (BI) analysis was conducted in MrBayes 3.2 using a GTR + G + I model of nucleotide substitutions [30]. Posterior probabilities were generated from 10 million generations of sampling from two independent runs using one cold (temp = 0.3) and three heated chains, excluding the first 25% of generations as burn-in. The chain convergence was assessed using a standard deviation of the split frequencies approaching 0.01 and a potential scale reduction factor (PSRF) of 1.0. The consensus tree topology was visualized in FigTree 1.4.2 (available at http://tree.bio.ed.ac.uk/software/figtree, accessed on 20 November 2023) and edited in Adobe Illustrator.
3. Results
3.1. Diagnosis of Fuscozetes Sellnick, 1928
Based on the morphological characters given by Seniczak et al. [11,12] and Seniczak and Seniczak [13,14] and F. fuscipes studied herein, the diagnosis of Fuscozetes is as follows: the adults are of a medium size (423–897), brown to dark brown, and with characters of Ceratozetidae [2]. A translamella is present or absent, the lamellar cusp is rounded or with teeth, and the bothridial seta is clavate or fusiform. The notogastral setae are short to long (10–13 pairs, including c2 and all or some setae of the d-series), and the porose areas (4 pairs) are of a similar size or with a larger Aa. Femora I and II are oval in cross-section, and solenidion ω2 on tarsus I is shorter than ω1 and is located posterolaterally to solenidion ω1.
The juveniles are light brown with a brown prodorsum, sclerites, epimeres, and legs. The bothridial seta is clavate or fusiform and the gastronotal setae are short to long. In the larva, a humeral organ and a humeral macrosclerite are present or absent. The gastronotal seta c1 is inserted on the humeral macrosclerite or microsclerite, or on the unsclerotized integument, while setae c2 and c3 are inserted on the microsclerites or the unsclerotized integument. The gastronotal shield is uniform, divided in two parts, structured as a pygidium, or absent (in which case most gastronotal setae are located on microsclerites); other sclerites and microsclerites are present or absent. In the nymphs, the humeral macrosclerite is present, whereas the humeral organ is present or absent. The gastronotal shield has 10 pairs of setae (d-, l-, and h-series, and p1), where setae p2 and p3 are placed on the macrosclerite or the unsclerotized integument. In all the juveniles, the femora are oval in cross-section, without a large ventral carina. Solenidion ω2 on tarsus I is shorter than solenidion ω1, and is located posterolaterally to ω1.
3.2. Morphological Ontogeny of Fuscozetes fuscipes (C.L. Koch, 1844) (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15, Figure 16, Figure 17, Figure 18, Figure 19, Figure 20 and Figure 21)
Oribata fuscipes C.L. Koch, 1844: Michael [31].
Fuscozetes fuscipes: Sellnick [3], Willmann [32], Shaldybina [2], Mehl [33], Karppinen and Krivolutsky [34], Golosova et al. [35], Karppinen et al. [36,37], Marshall et al. [38], Olszanowski et al. [39], Niemi et al. [40], Ryabinin and Pankov [41], Subías [1], Weigmann [15], Miko [42], Murvanidze and Mumladze [43], Schatz [44], and Murvanidze et al. [45].
3.2.1. Diagnosis
The adults are brown, of a medium size (629–897), and with the characters of Fuscozetes given above. The translamella is present, the lamellar cusp is long with teeth, and the bothridial seta is fusiform. The notogastral setae are long (10 pairs, including c2), and porose area Aa is rounded and slightly larger than the other porose areas.
In the juveniles, the bothridial seta is fusiform and a humeral organ and humeral macrosclerite are present. In the larva, the gastronotal shield is absent; most of the gastronotal setae are short and inserted on the microsclerites; and other sclerites and microsclerites are also present. In the nymphs, the gastronotal shield is present, the setae of the c-series are located on the microsclerites, and setae p2 and p3 are placed on a large, posteroventral sclerite.
3.2.2. Morphology of Adults
The adults are brown to dark brown, oval in the dorsal and ventral view (Figure 1a, Figure 2 and Figure 4a,d), and of a medium size (734–897), as redescribed by Shaldybina [8] and Bayartogtokh and Weigmann [46] (but see Remarks). The mean length (and range) of females is 847.6 ± 19.7 (815–897, n = 23) and the maximum width is 566.2 ± 19.8 (538–587); the mean length (and range) of males is 791.7 ± 26.4 (734–815, n = 7) and the maximum width is 526.3 ± 20.4 (505–554). The prodorsal seta in is long, le and ro are of a medium size, and ex is short (Figure 1a, Figure 2, Figure 3a, Figure 4, Figure 5, Figure 6a and Figure 7c, Table 2). The notogastral setae (13 pairs, including c2) are long, with short barbs (Figure 1b and Figure 6a), but appear smooth under low magnification. The porose areas (four pairs) are rounded and Aa is slightly larger than the other porose areas (Figure 1a and Figure 3a). The chelicerae are chelate–dentate (Figure 3b and Figure 6c,d), with seta cha longer than chb; both setae are barbed. Most palp setae have short barbs (Figure 3c, Figure 5a and Figure 6c,d). The custodium (cus) is long and the discidium (Dis) is triangular. The epimeral setae are short and smooth, and the inner setae are slightly shorter than the other setae (Figure 2, Figure 4d, Figure 6c,d and Figure 7a,b). The genital (g), aggenital (ag), and anal (an) setae are short and smooth, and the adanal setae (ad) are longer, with short barbs on the apical part. The medium parts of femora I and II are oval in cross-section, without a large ventral carina, but femur II has an anteroventral projection (Figure 4b, Figure 5a, Figure 7c and Figure 8). Most of the leg setae have short barbs (Figure 4, Figure 5a,b, Figure 6c,d, Figure 7, Figure 8 and Figure 9a,b). The formulae of the leg setae [trochanter to tarsus (+ solenidia)] are as follows: I—1-5-3(1)-4(2)-20(2); II—1-5-3(1)-4(1)-15(2); III—2-3-1(1)-3(1)-15; and IV—1-2-2-3(1)-12.

Table 2.
Measurements of some morphological characters of juvenile stages of Fuscozetes fuscipes (mean measurements of 4–10 specimens in μm).
Remarks: The adults investigated here were larger than those studied by Willmann [32] (body size: 710 × 500), Shaldybina [8] (body size: 753–774 × 473–516), Bayartogtokh and Weigmann [46] (body size: 629–676 × 419–433), and Weigmann [15] (body length of 630–765), but the length and distribution of the notogastral setae were similar in all studies.
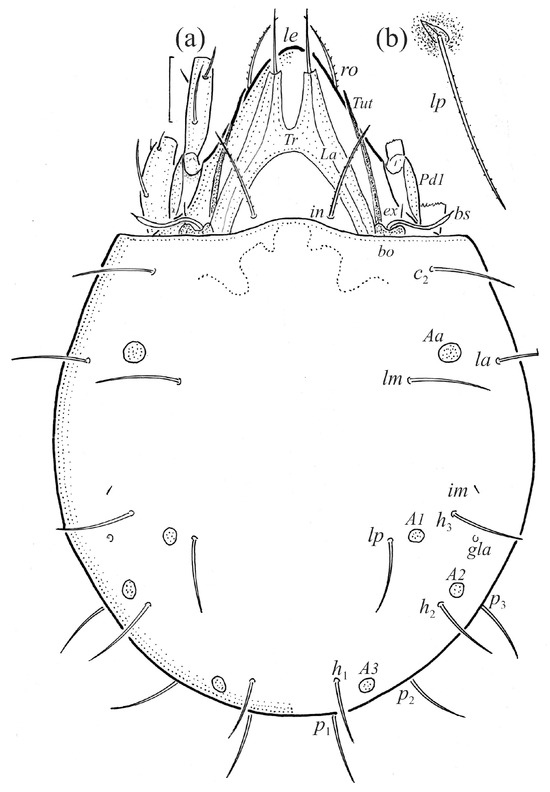
Figure 1.
Adult female Fuscozetes fuscipes. (a) Dorsal aspect, with legs partially drawn; scale bar: 50 μm. (b) Seta lp (enlarged).
Figure 1.
Adult female Fuscozetes fuscipes. (a) Dorsal aspect, with legs partially drawn; scale bar: 50 μm. (b) Seta lp (enlarged).
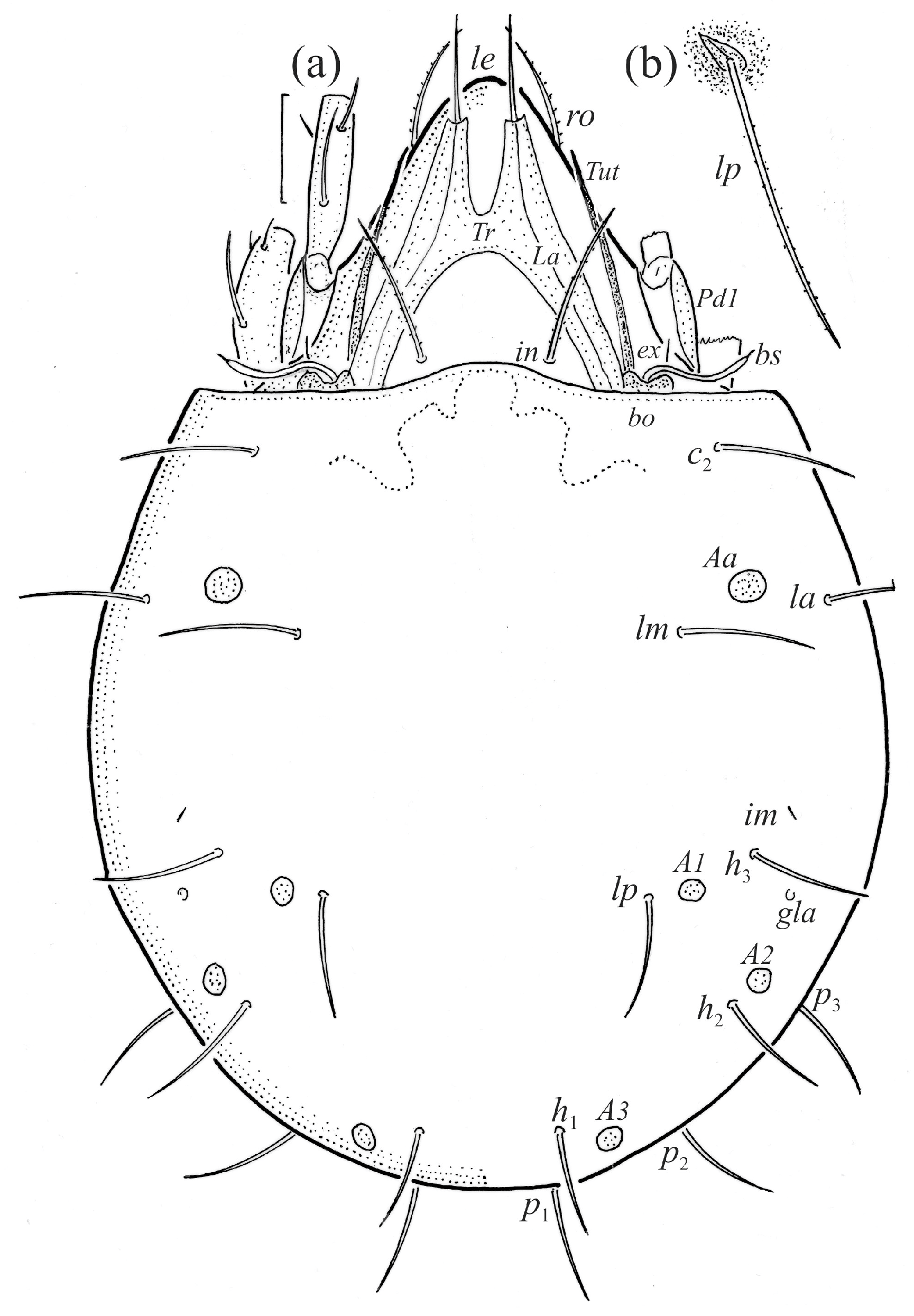
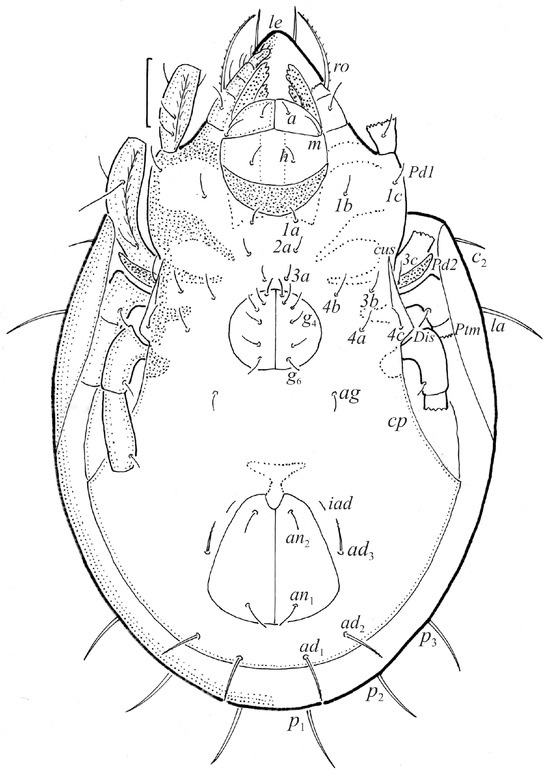
Figure 2.
Adult female Fuscozetes fuscipes, ventral aspect, with legs partially drawn; scale bar: 50 μm.
Figure 2.
Adult female Fuscozetes fuscipes, ventral aspect, with legs partially drawn; scale bar: 50 μm.
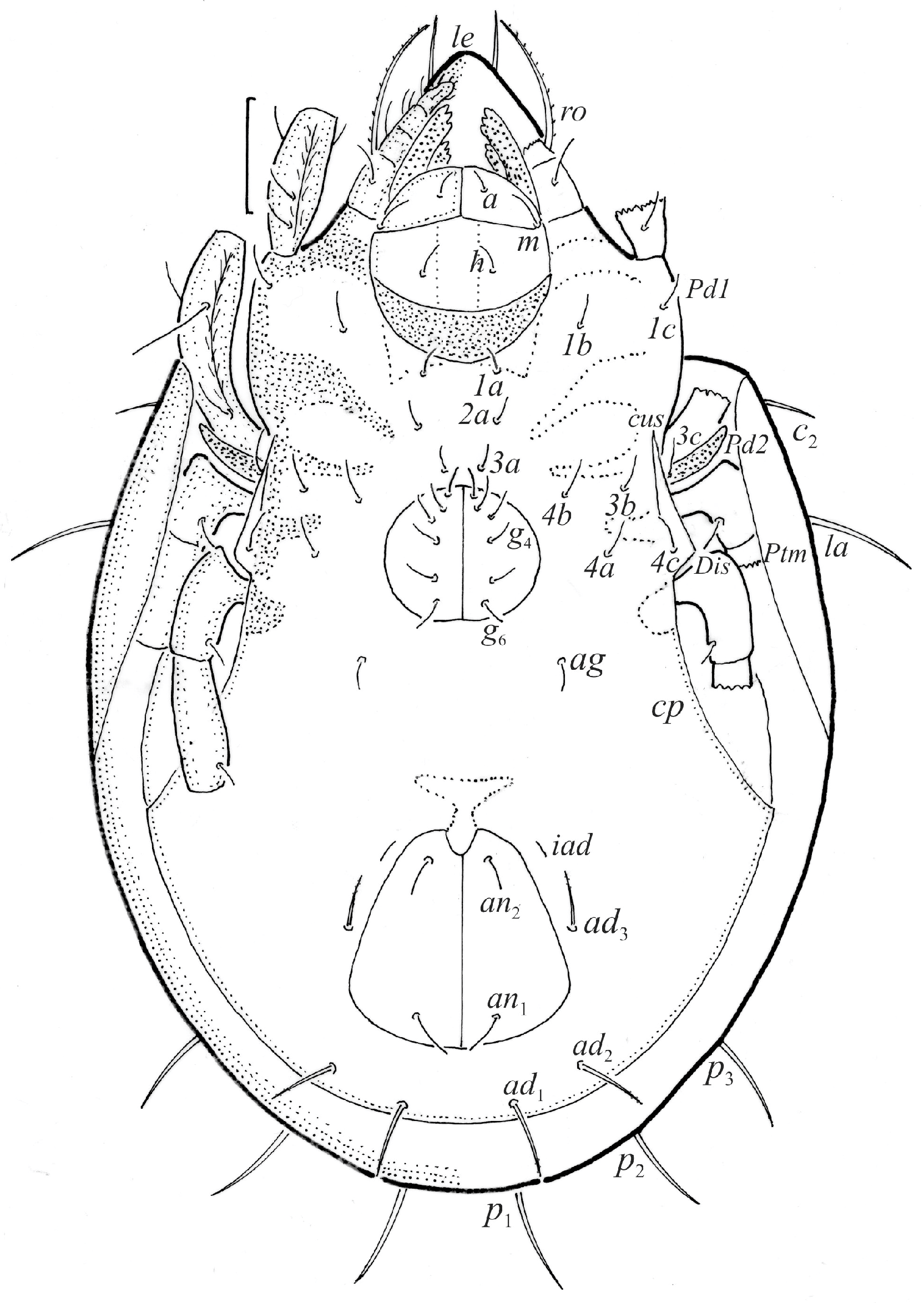
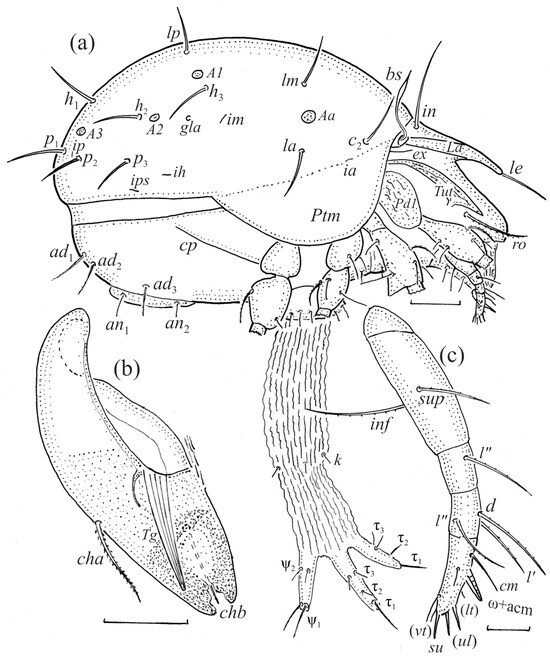
Figure 3.
Fuscozetes fuscipes. (a) Female with ejected ovipositor, lateral aspect, with legs partially drawn; scale bar: 50 μm. Mouthparts of adult, right side; scale bars: 20 μm. (b) Chelicera, paraxial aspect. (c) Palp.
Figure 3.
Fuscozetes fuscipes. (a) Female with ejected ovipositor, lateral aspect, with legs partially drawn; scale bar: 50 μm. Mouthparts of adult, right side; scale bars: 20 μm. (b) Chelicera, paraxial aspect. (c) Palp.
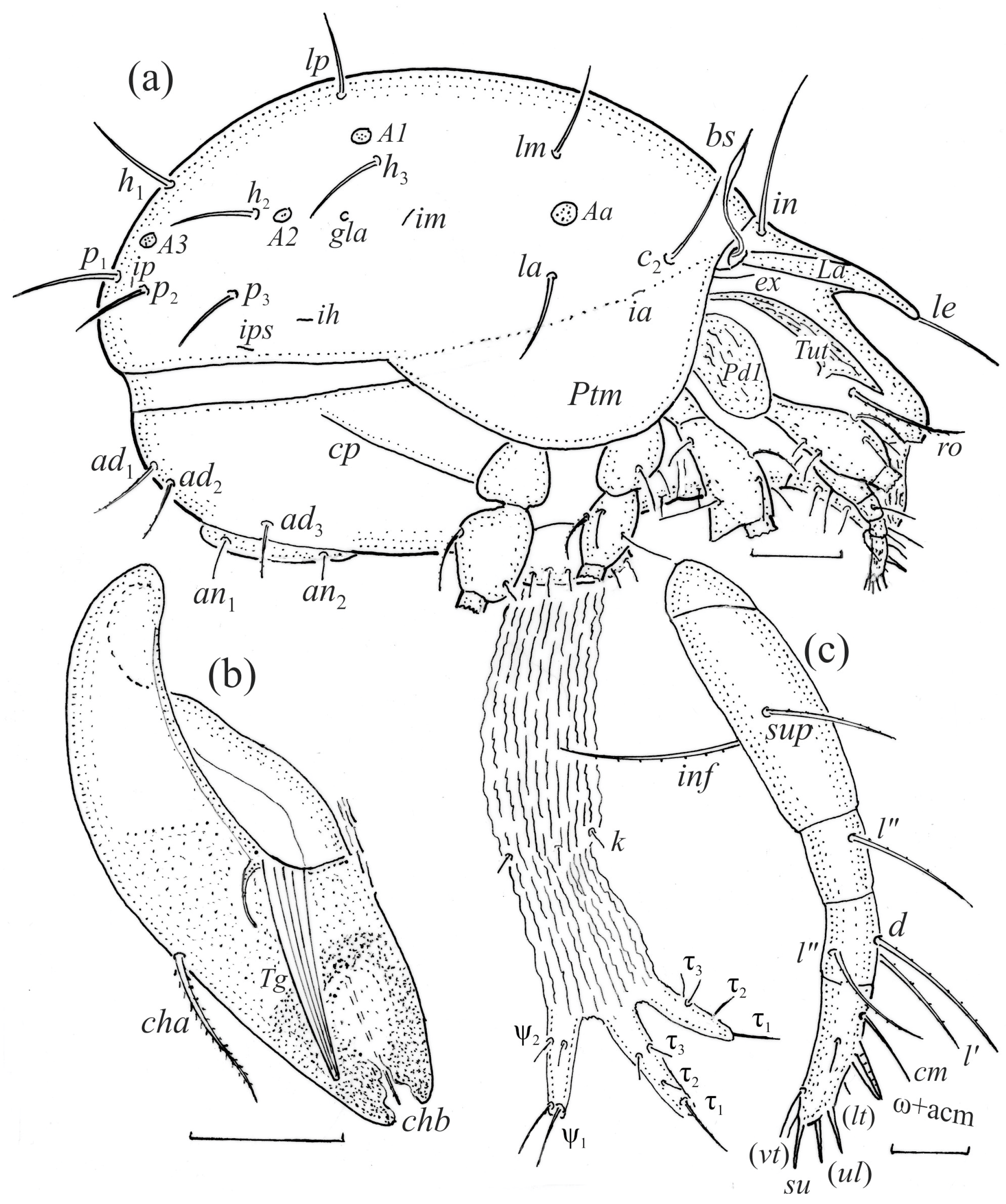

Figure 4.
SEM micrographs of adult Fuscozetes fuscipes. (a) Dorsal view, (b) lateral view, (c) dorsolateral view, and (d) ventral view.
Figure 4.
SEM micrographs of adult Fuscozetes fuscipes. (a) Dorsal view, (b) lateral view, (c) dorsolateral view, and (d) ventral view.

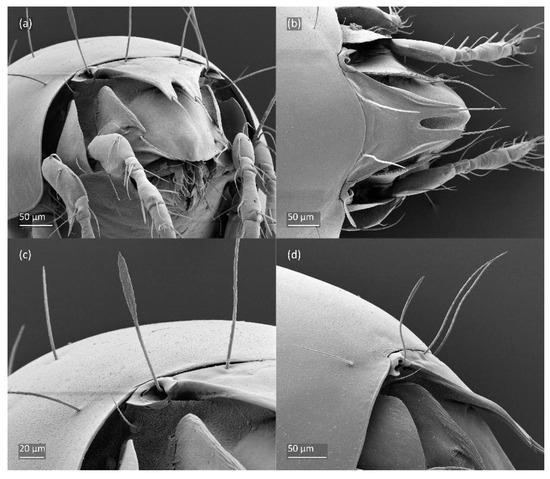
Figure 5.
SEM micrographs of adult Fuscozetes fuscipes. Anterior part of body, (a) frontal view and (b) dorsal view; bothridial seta, (c) frontal view and (d) lateral view.
Figure 5.
SEM micrographs of adult Fuscozetes fuscipes. Anterior part of body, (a) frontal view and (b) dorsal view; bothridial seta, (c) frontal view and (d) lateral view.
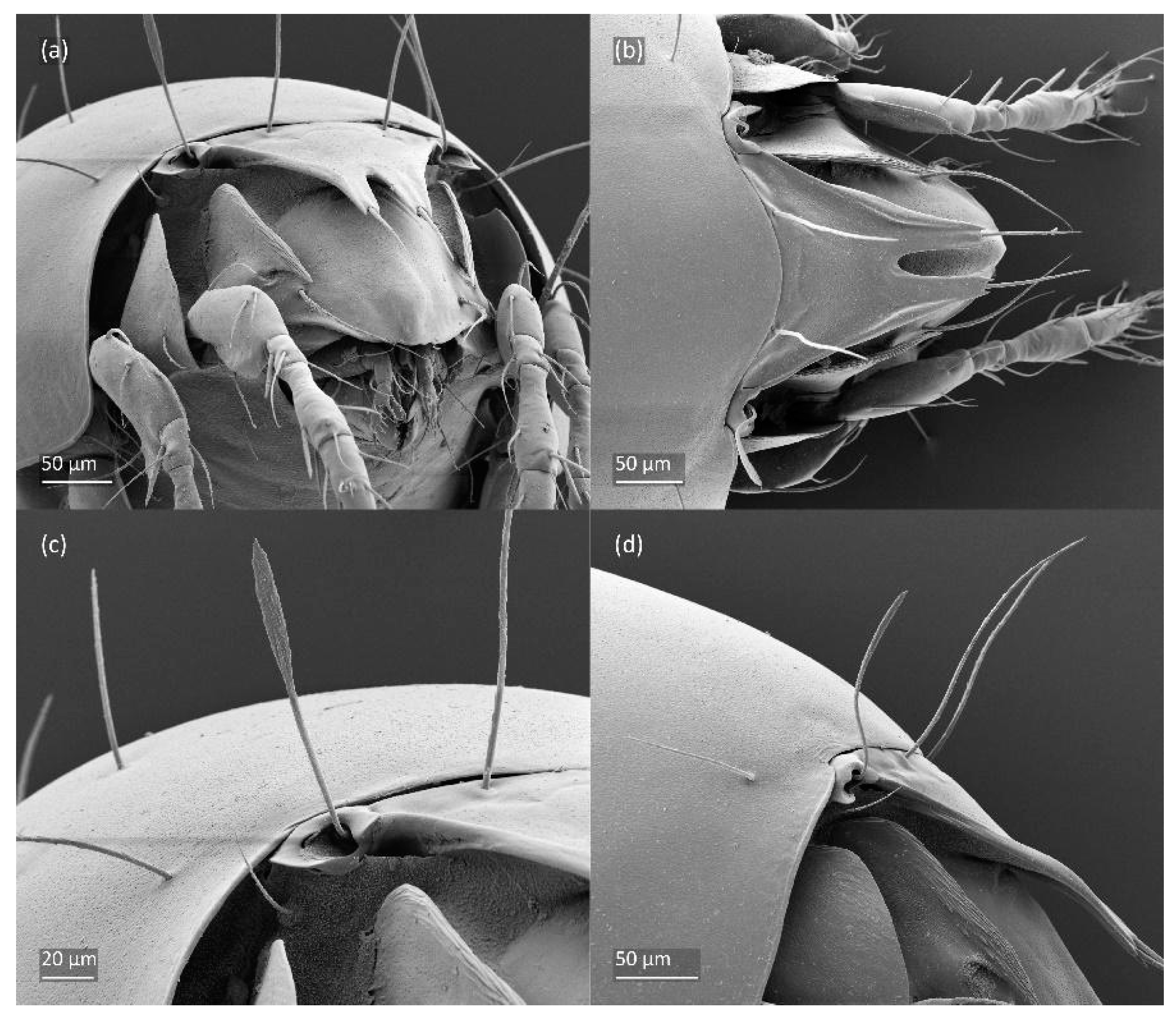
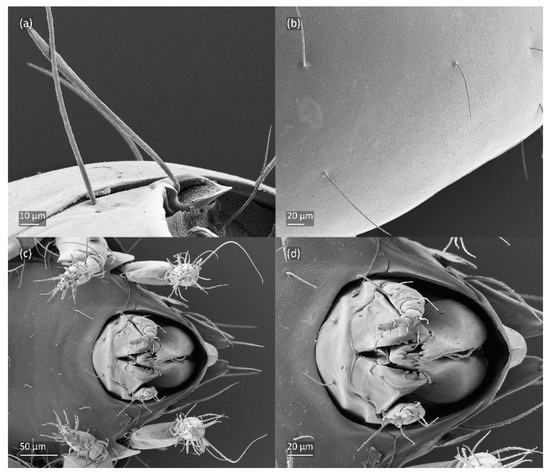
Figure 6.
SEM micrographs of adult Fuscozetes fuscipes. (a) Bothridial seta, lateral view; (b) part of notogaster, dorsal view; and (c,d) anterior part of body, ventral view.
Figure 6.
SEM micrographs of adult Fuscozetes fuscipes. (a) Bothridial seta, lateral view; (b) part of notogaster, dorsal view; and (c,d) anterior part of body, ventral view.
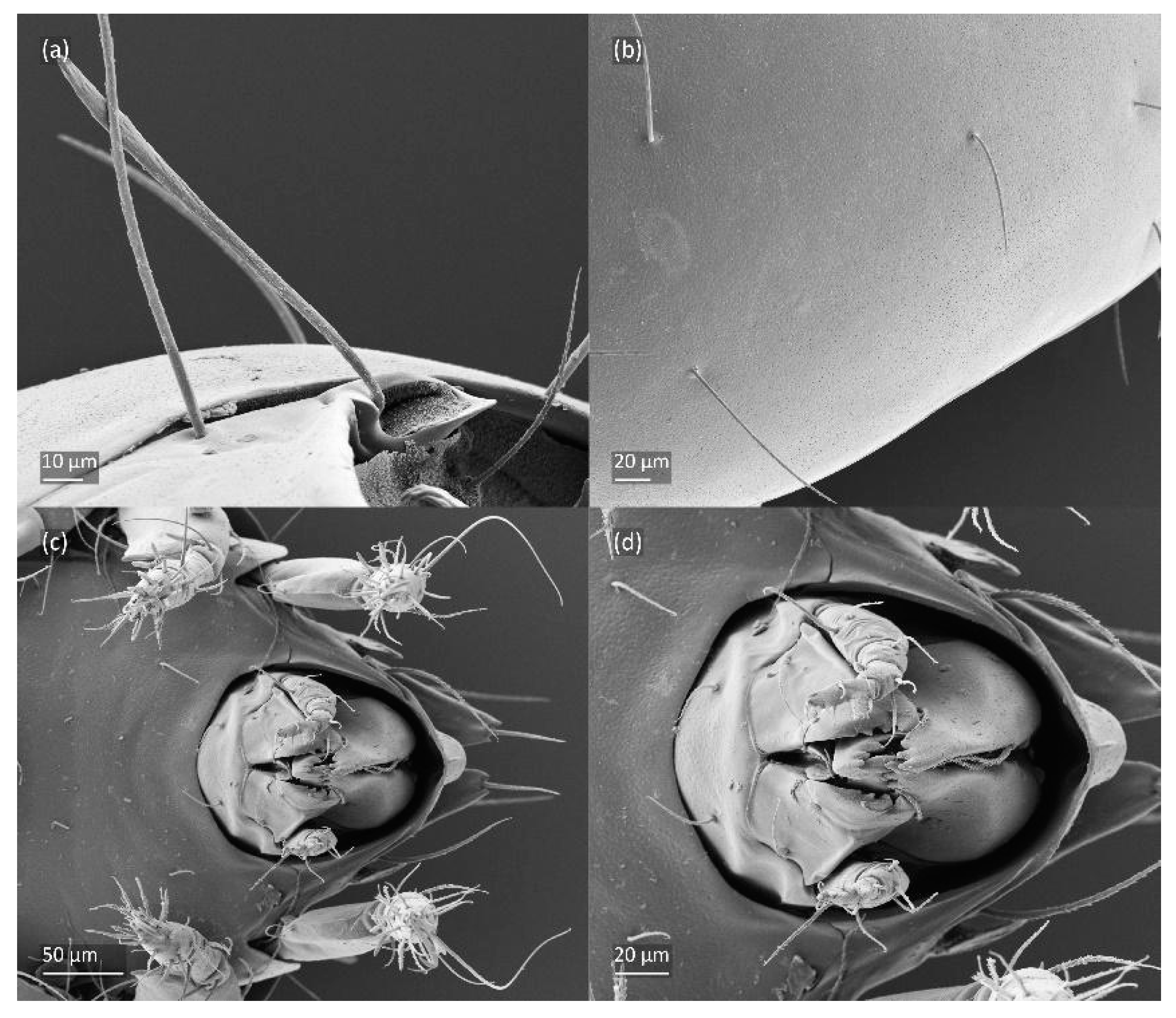
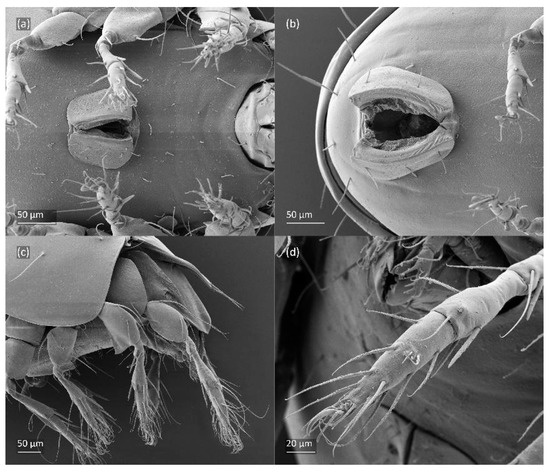
Figure 7.
SEM micrographs of adult Fuscozetes fuscipes. Ventral view of (a) medial part of body and (b) posterior part of body; (c) anterior part of body, lateral view; and (d) part of leg I, dorsal view.
Figure 7.
SEM micrographs of adult Fuscozetes fuscipes. Ventral view of (a) medial part of body and (b) posterior part of body; (c) anterior part of body, lateral view; and (d) part of leg I, dorsal view.
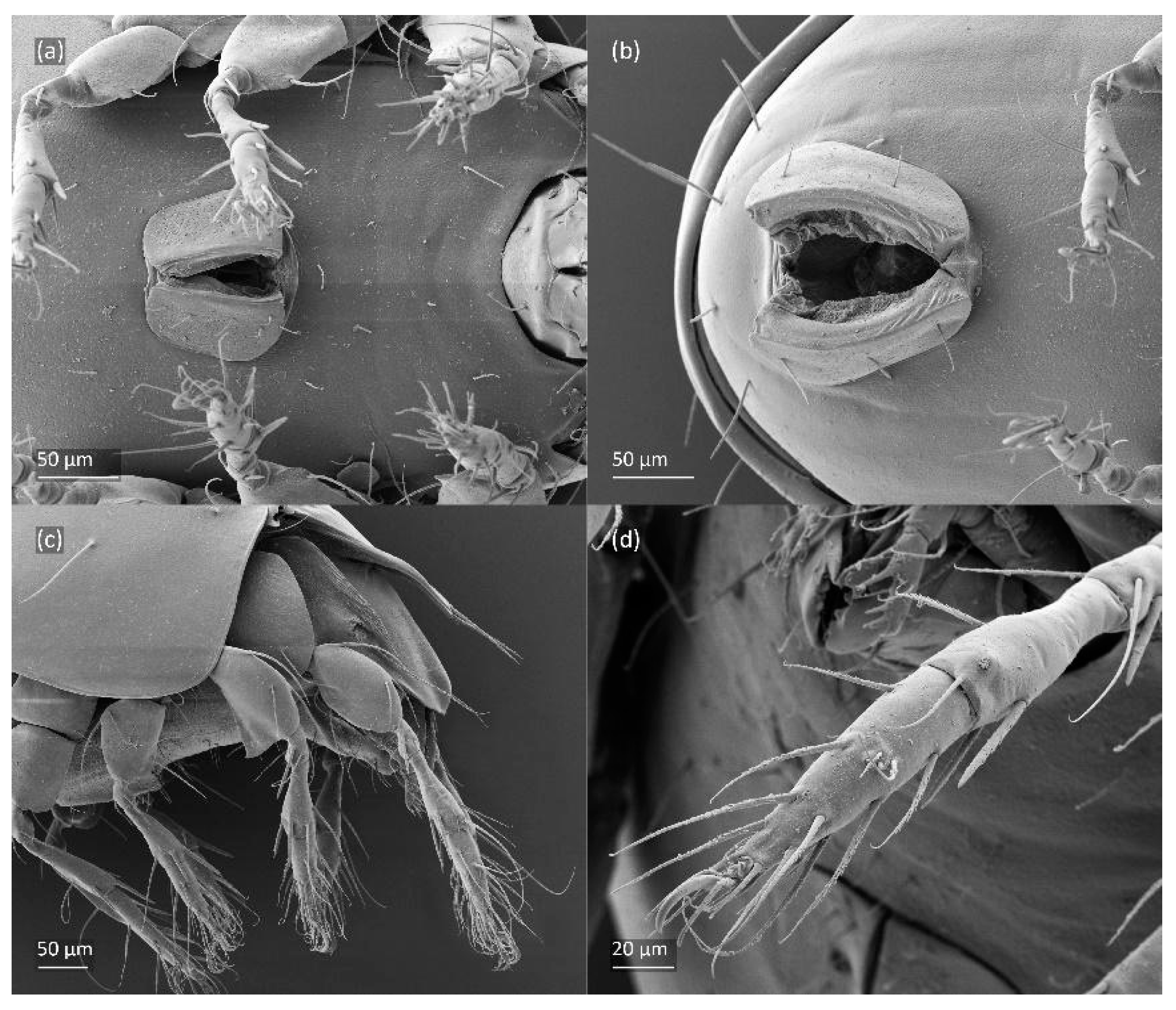
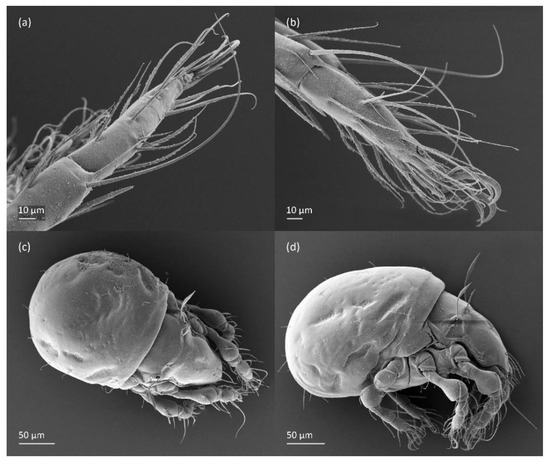
Figure 8.
SEM micrographs of Fuscozetes fuscipes. Part of leg I of adult, (a) dorsal view and (b) lateral view; larva, (c) dorsal view and (d) lateral view.
Figure 8.
SEM micrographs of Fuscozetes fuscipes. Part of leg I of adult, (a) dorsal view and (b) lateral view; larva, (c) dorsal view and (d) lateral view.
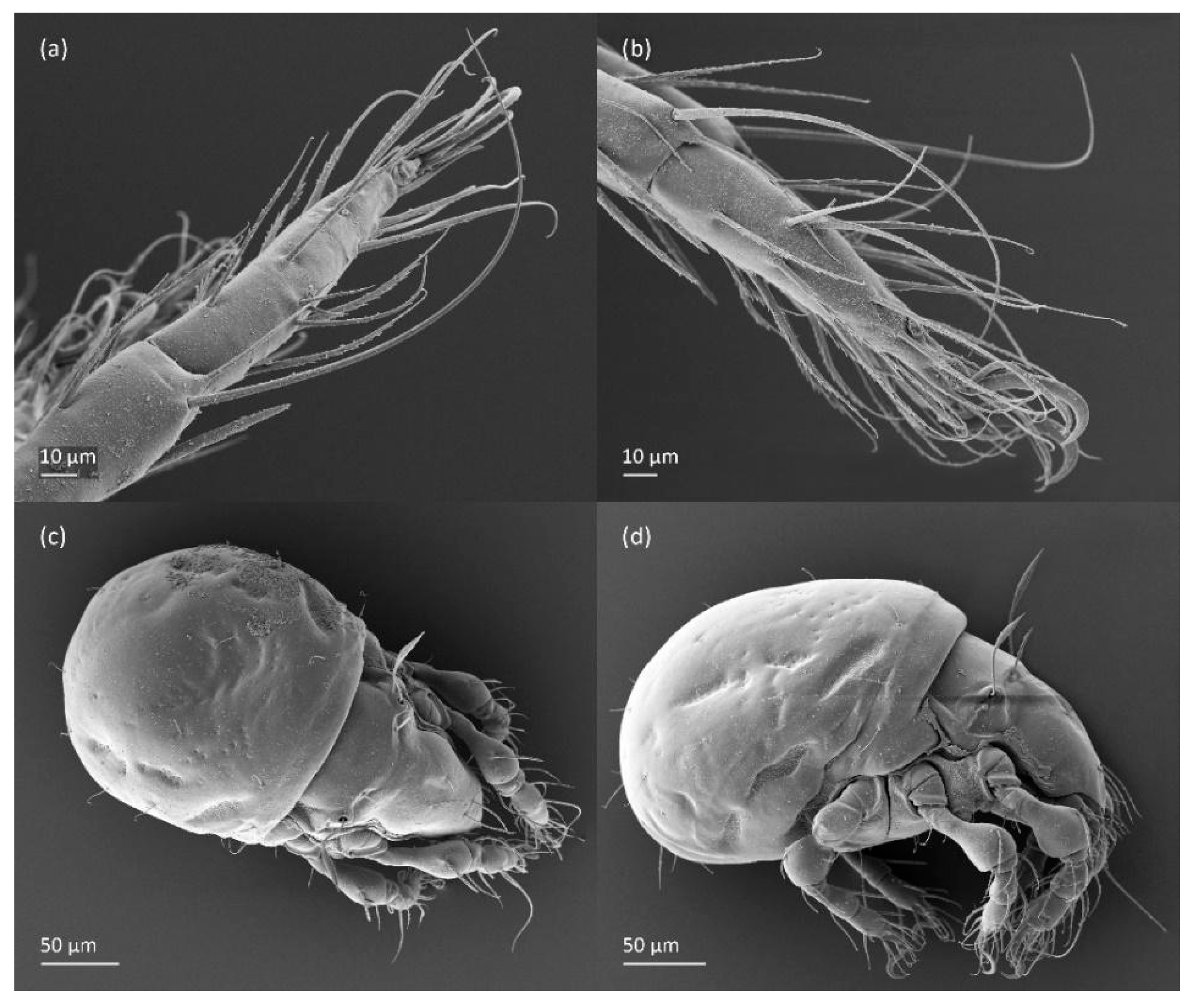
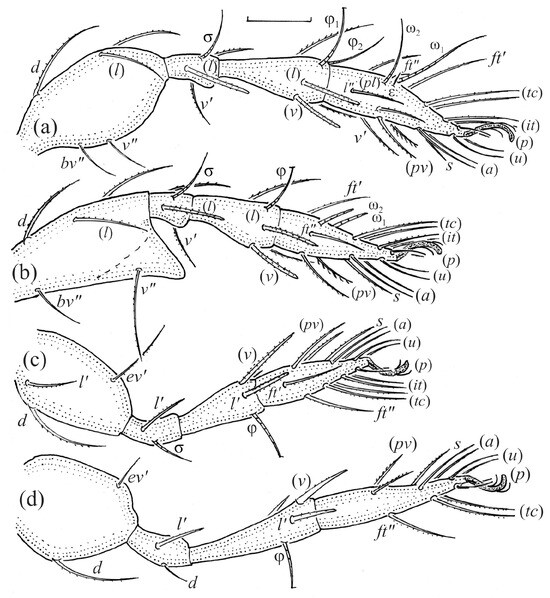
Figure 9.
Leg segments of adult Fuscozetes fuscipes (femur to tarsus), right side; scale bar: 20 μm. (a) Leg I, (b) leg II, (c) leg III, and (d) leg IV.
Figure 9.
Leg segments of adult Fuscozetes fuscipes (femur to tarsus), right side; scale bar: 20 μm. (a) Leg I, (b) leg II, (c) leg III, and (d) leg IV.
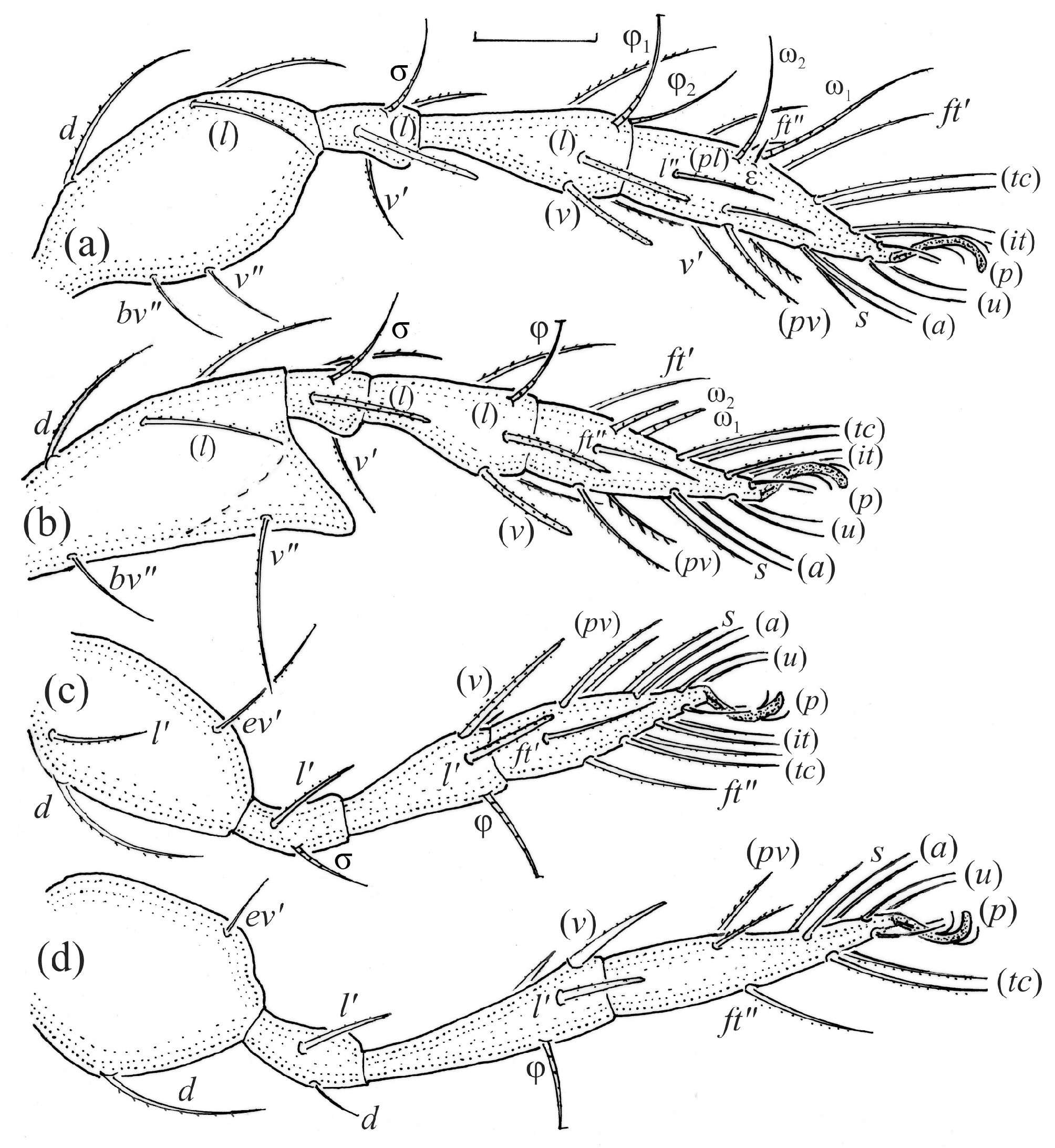
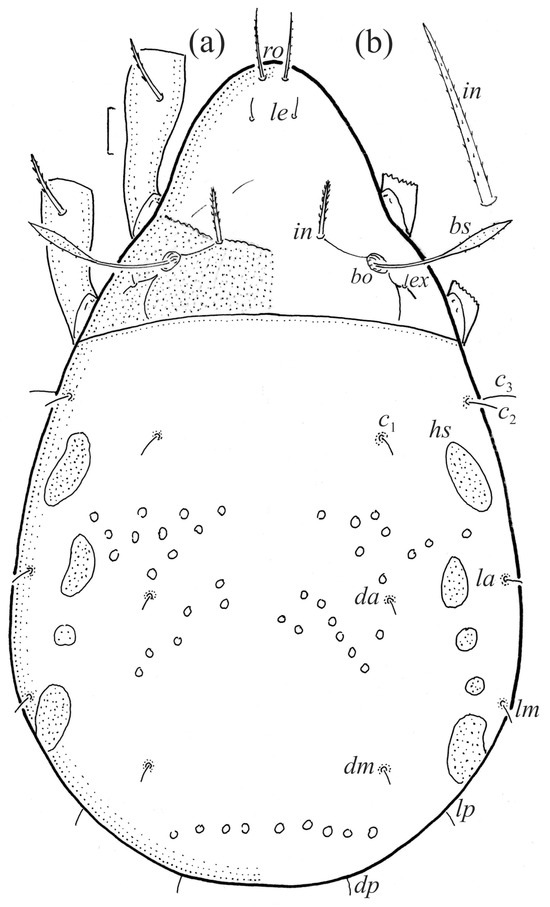
Figure 10.
Fuscozetes fuscipes larva. (a) Dorsal aspect, legs partially drawn; scale bar: 20 μm. (b) Seta in (enlarged).
Figure 10.
Fuscozetes fuscipes larva. (a) Dorsal aspect, legs partially drawn; scale bar: 20 μm. (b) Seta in (enlarged).
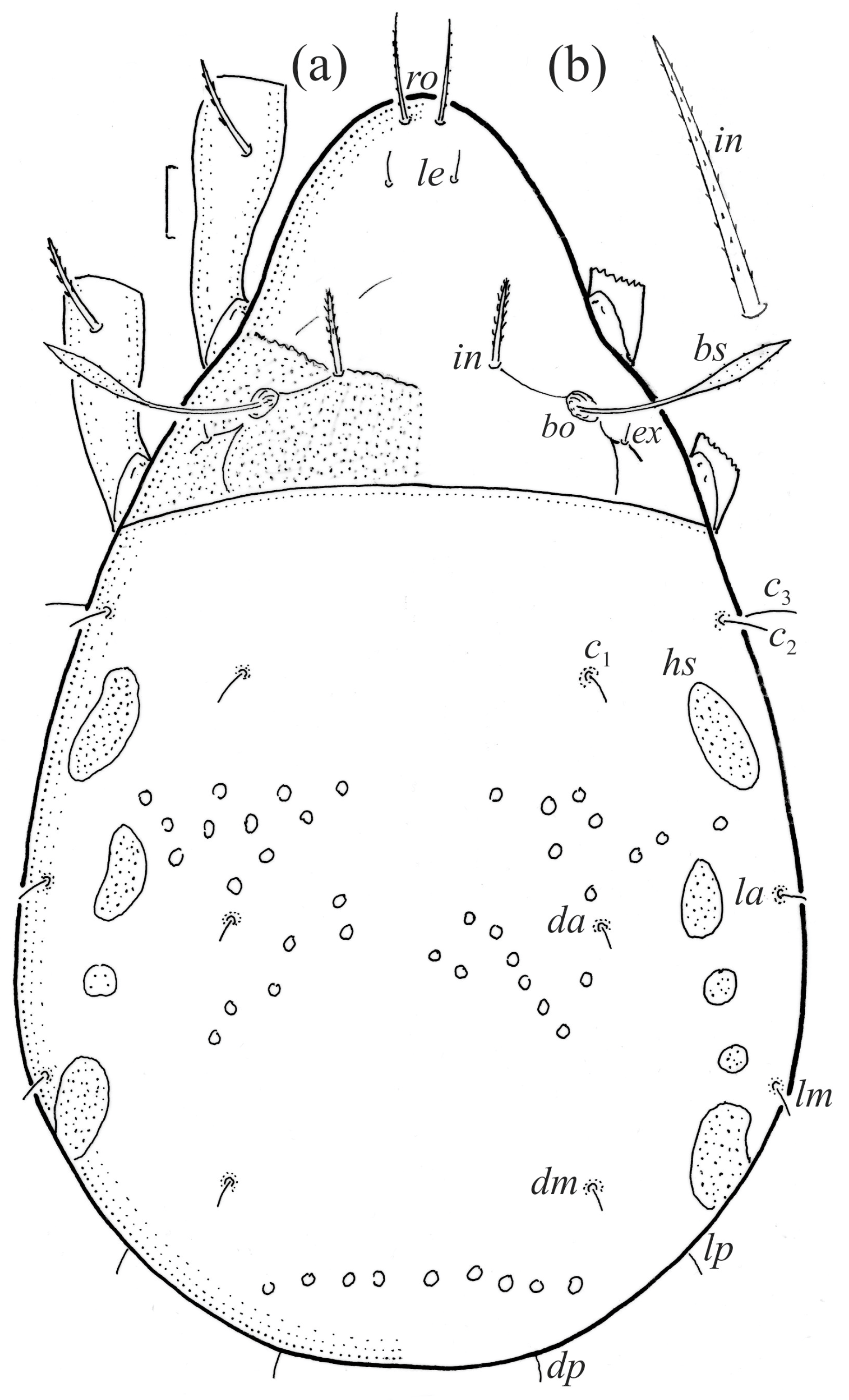
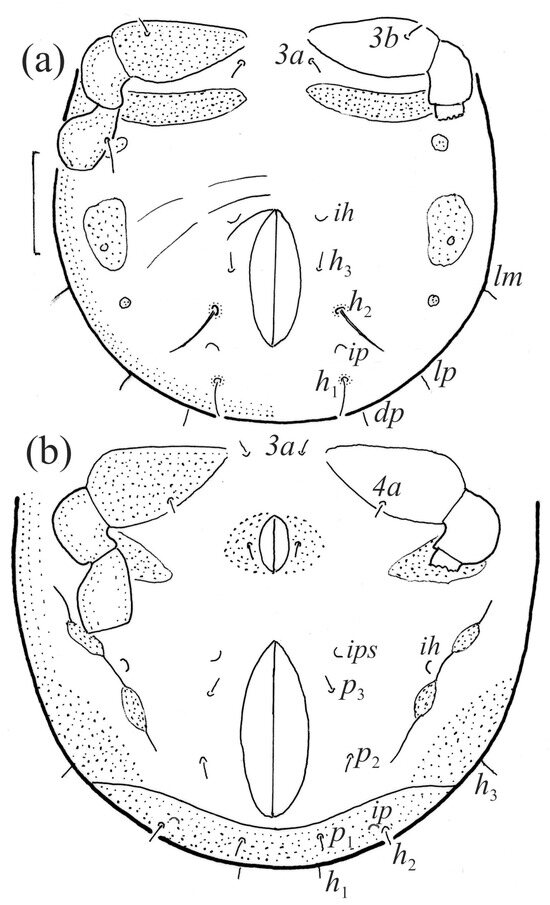
Figure 11.
Posterior part of Fuscozetes fuscipes hysterosoma, legs III and IV partially drawn; scale bar: 20 μm. (a) Larva and (b) protonymph.
Figure 11.
Posterior part of Fuscozetes fuscipes hysterosoma, legs III and IV partially drawn; scale bar: 20 μm. (a) Larva and (b) protonymph.
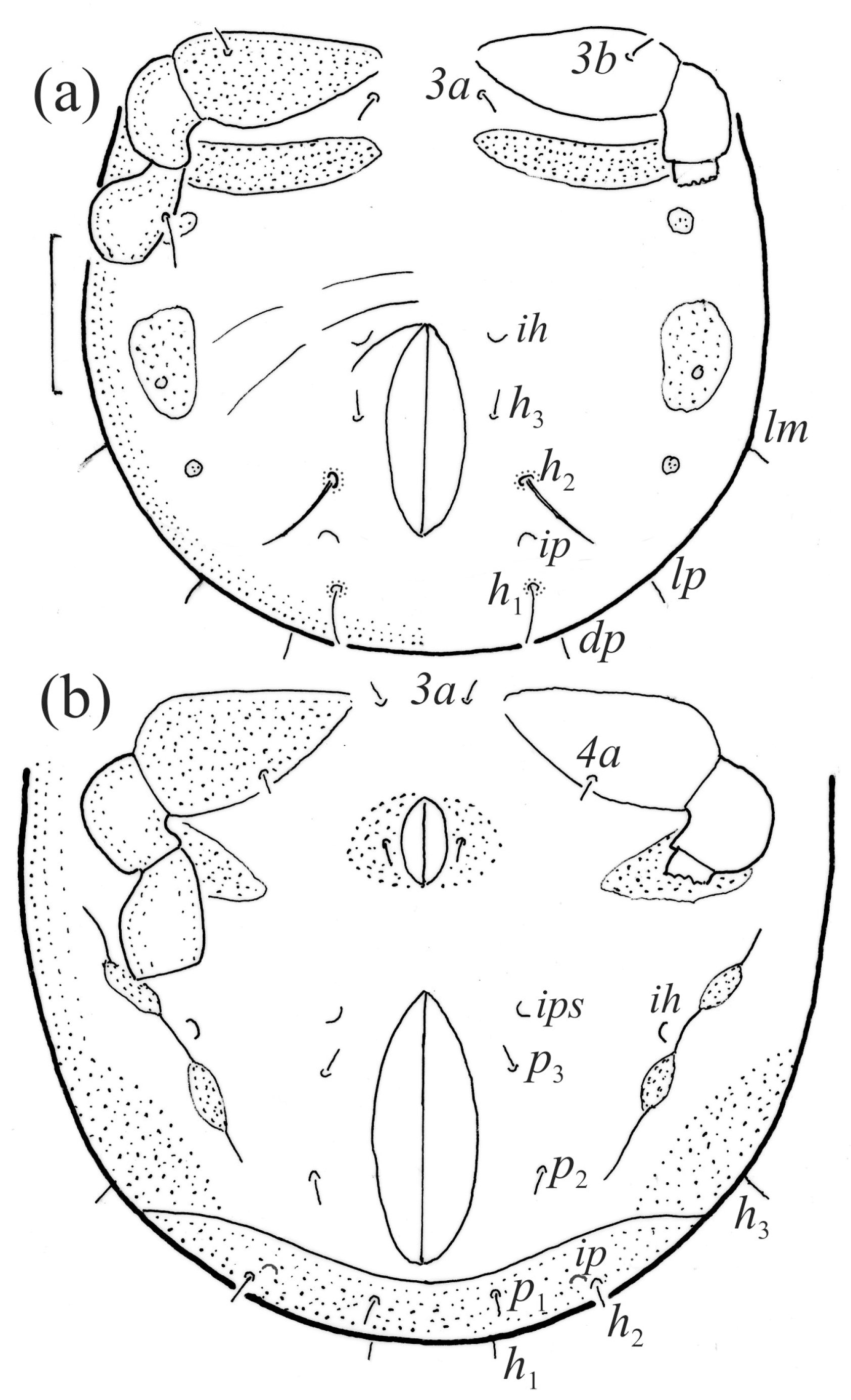
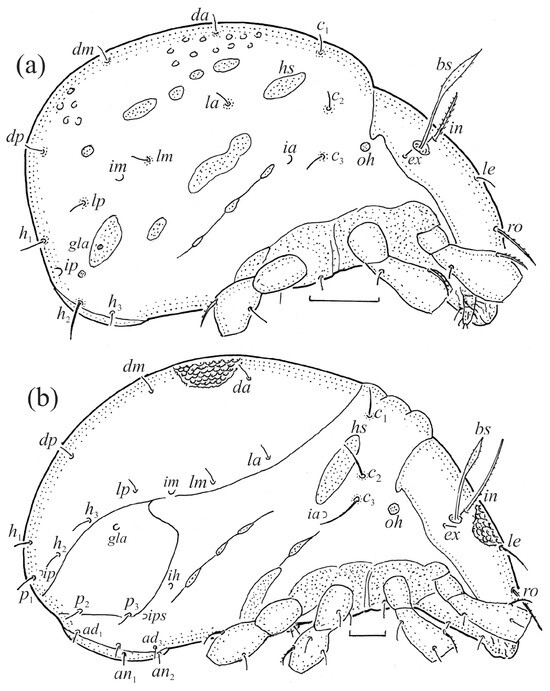
Figure 12.
Fuscozetes fuscipes, lateral aspect, legs partially drawn; scale bars: 50 μm. (a) Larva and (b) tritonymph.
Figure 12.
Fuscozetes fuscipes, lateral aspect, legs partially drawn; scale bars: 50 μm. (a) Larva and (b) tritonymph.
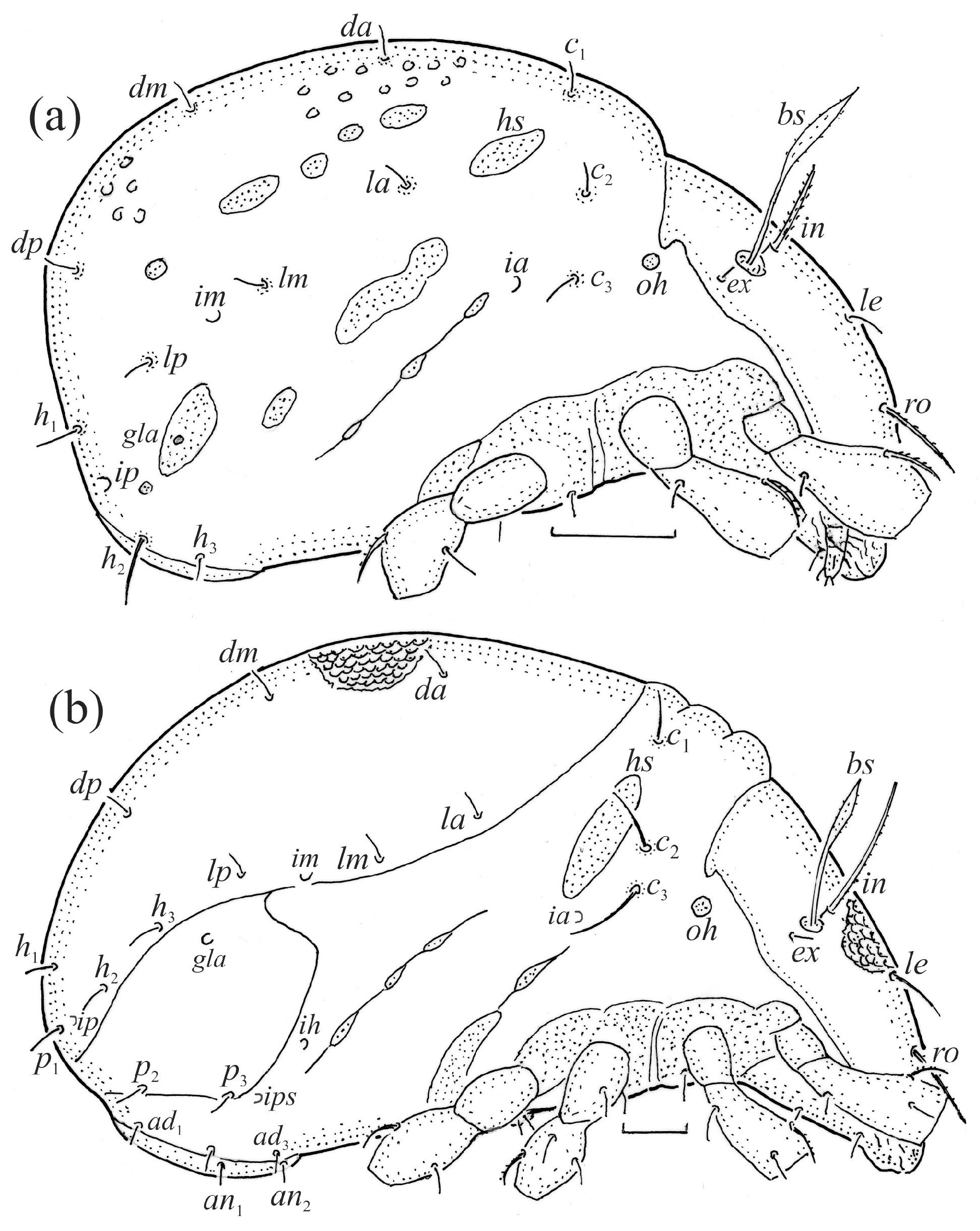

Figure 13.
SEM micrographs of Fuscozetes fuscipes larva. (a) Dorsolateral view; (b) ventral view; and dorsal view of (c) anterior and medial part of body and (d) anterior part of body.
Figure 13.
SEM micrographs of Fuscozetes fuscipes larva. (a) Dorsolateral view; (b) ventral view; and dorsal view of (c) anterior and medial part of body and (d) anterior part of body.

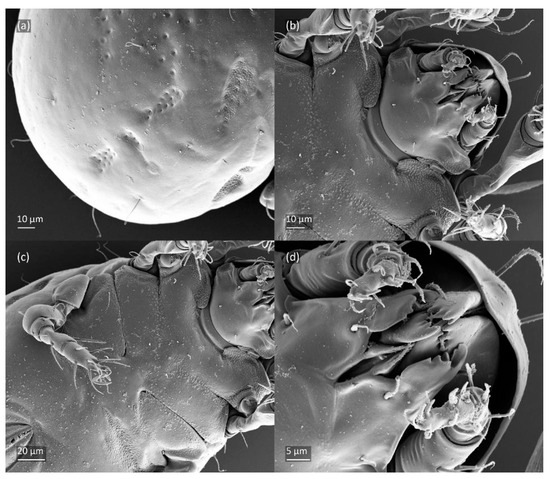
Figure 14.
SEM micrographs of Fuscozetes fuscipes larva. (a) Posterior part of body, dorsolateral view; ventral view of (b) anterior part of body, (c) medial part of body, and (d) gnathosoma.
Figure 14.
SEM micrographs of Fuscozetes fuscipes larva. (a) Posterior part of body, dorsolateral view; ventral view of (b) anterior part of body, (c) medial part of body, and (d) gnathosoma.
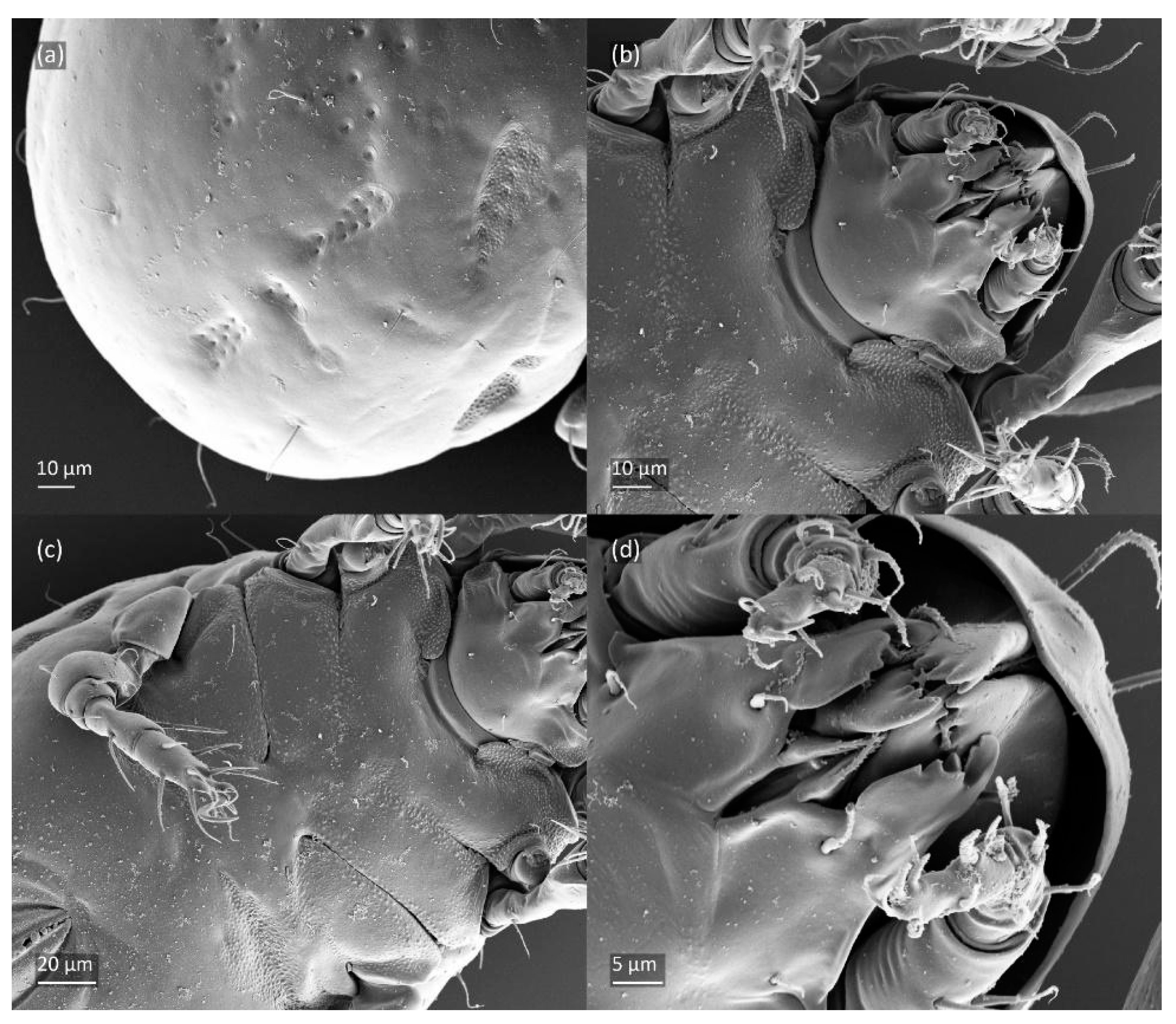
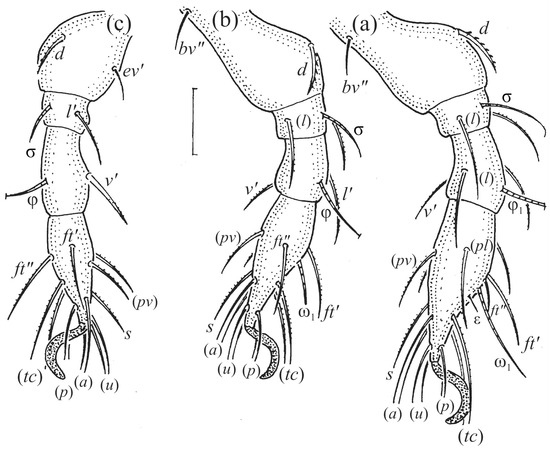
Figure 15.
Leg segments of Fuscozetes fuscipes larva (femur to tarsus), right side; scale bar: 20 μm. (a) Leg I, (b) leg II, and (c) leg III.
Figure 15.
Leg segments of Fuscozetes fuscipes larva (femur to tarsus), right side; scale bar: 20 μm. (a) Leg I, (b) leg II, and (c) leg III.
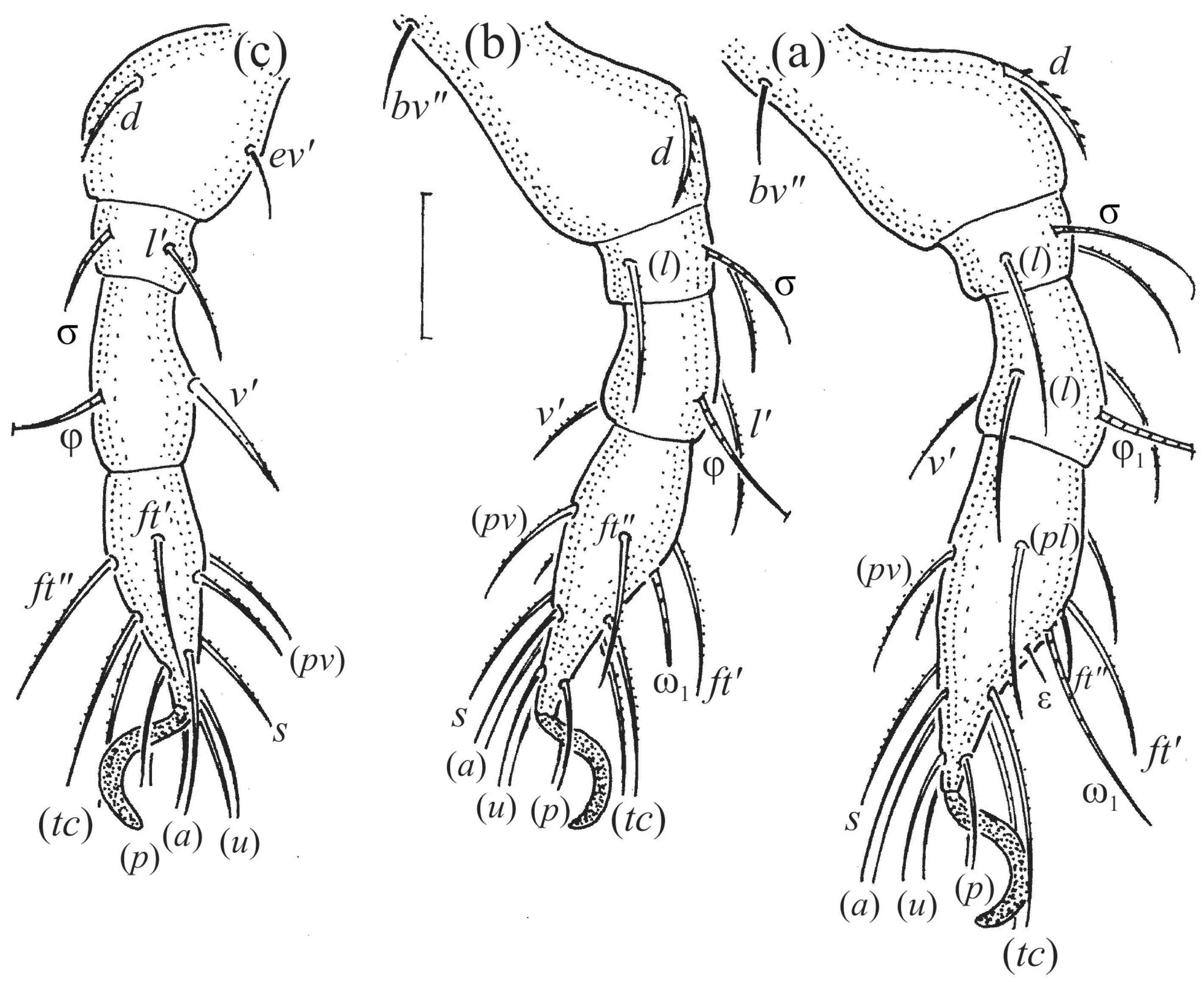
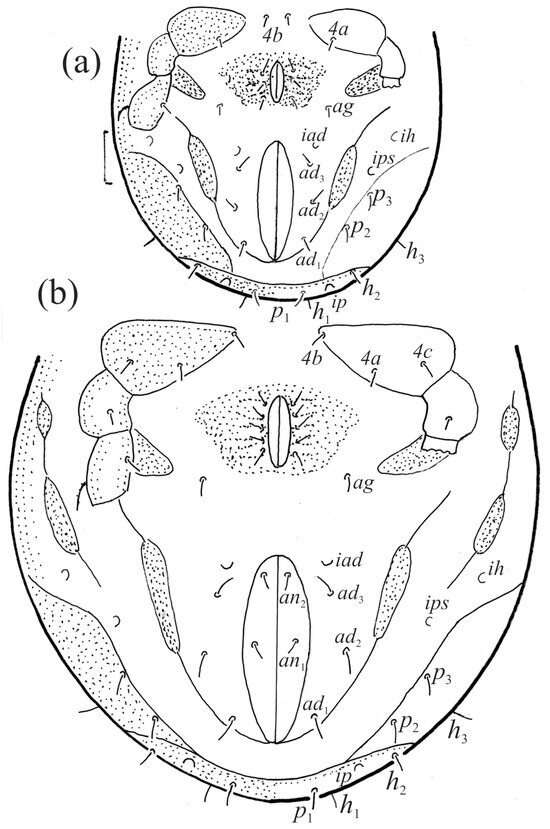
Figure 16.
Anogenital region of Fuscozetes fuscipes, legs partially drawn; scale bar: 50 μm. (a) Deutonymph and (b) tritonymph.
Figure 16.
Anogenital region of Fuscozetes fuscipes, legs partially drawn; scale bar: 50 μm. (a) Deutonymph and (b) tritonymph.
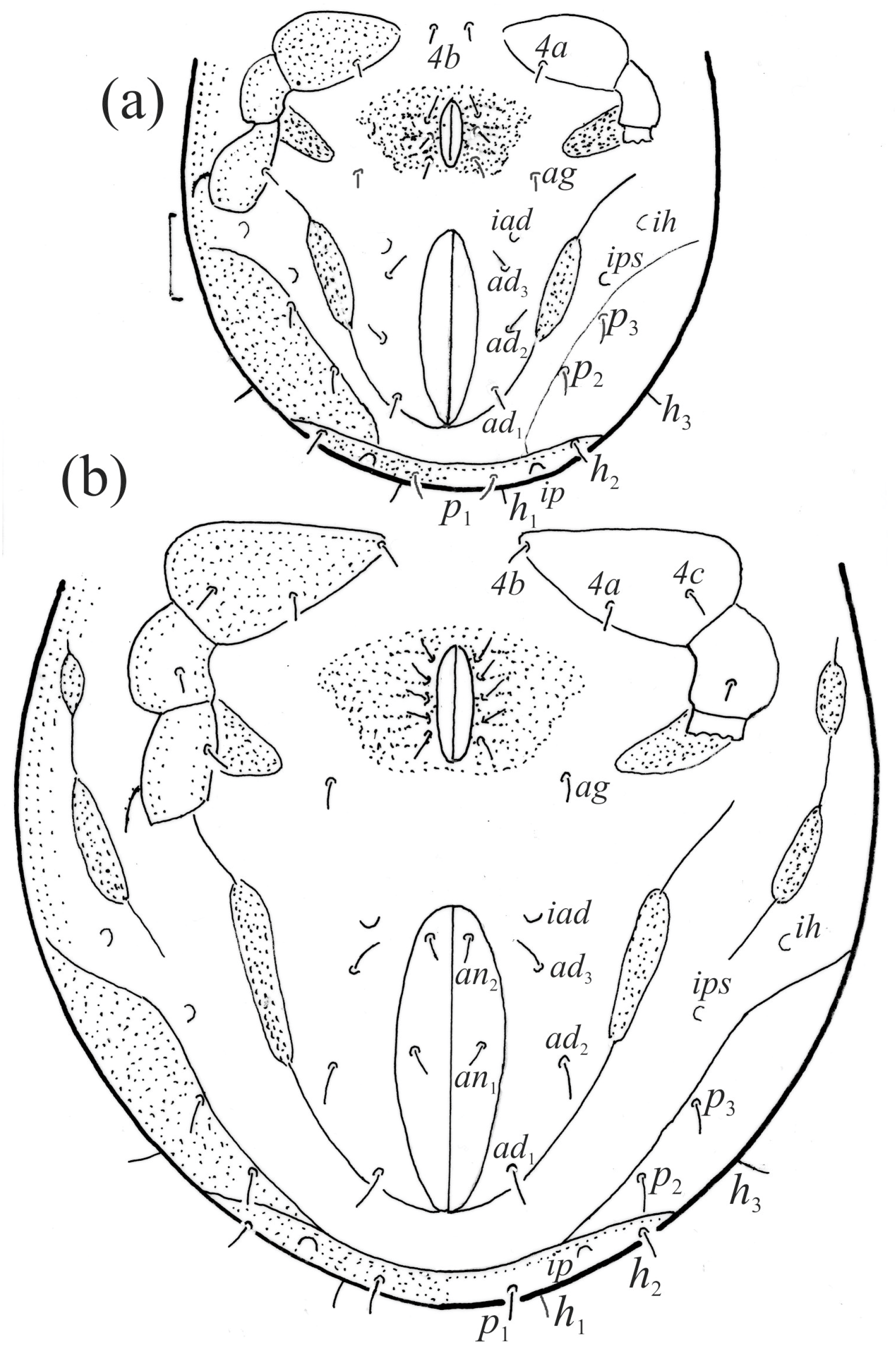
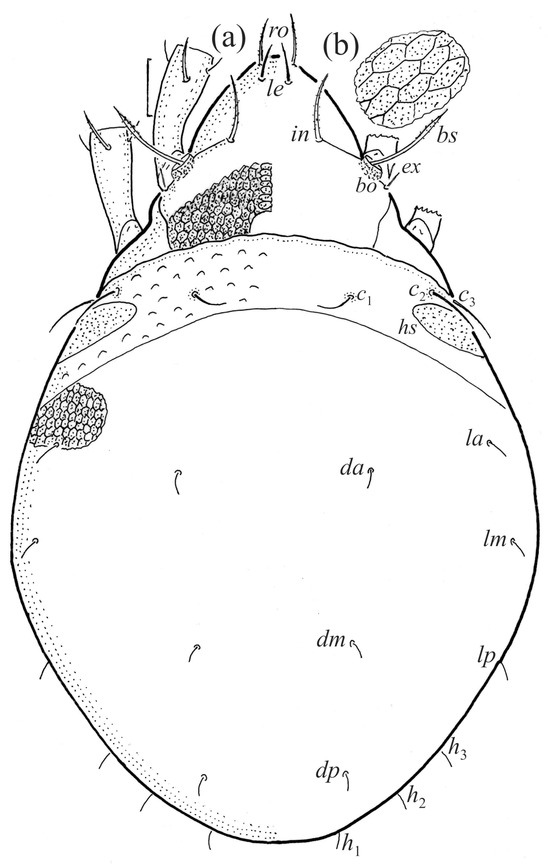
Figure 17.
Fuscozetes fuscipes tritonymph. (a) Dorsal aspect, legs partially drawn; scale bar: 50 μm. (b) Pattern of gastronotum (enlarged).
Figure 17.
Fuscozetes fuscipes tritonymph. (a) Dorsal aspect, legs partially drawn; scale bar: 50 μm. (b) Pattern of gastronotum (enlarged).
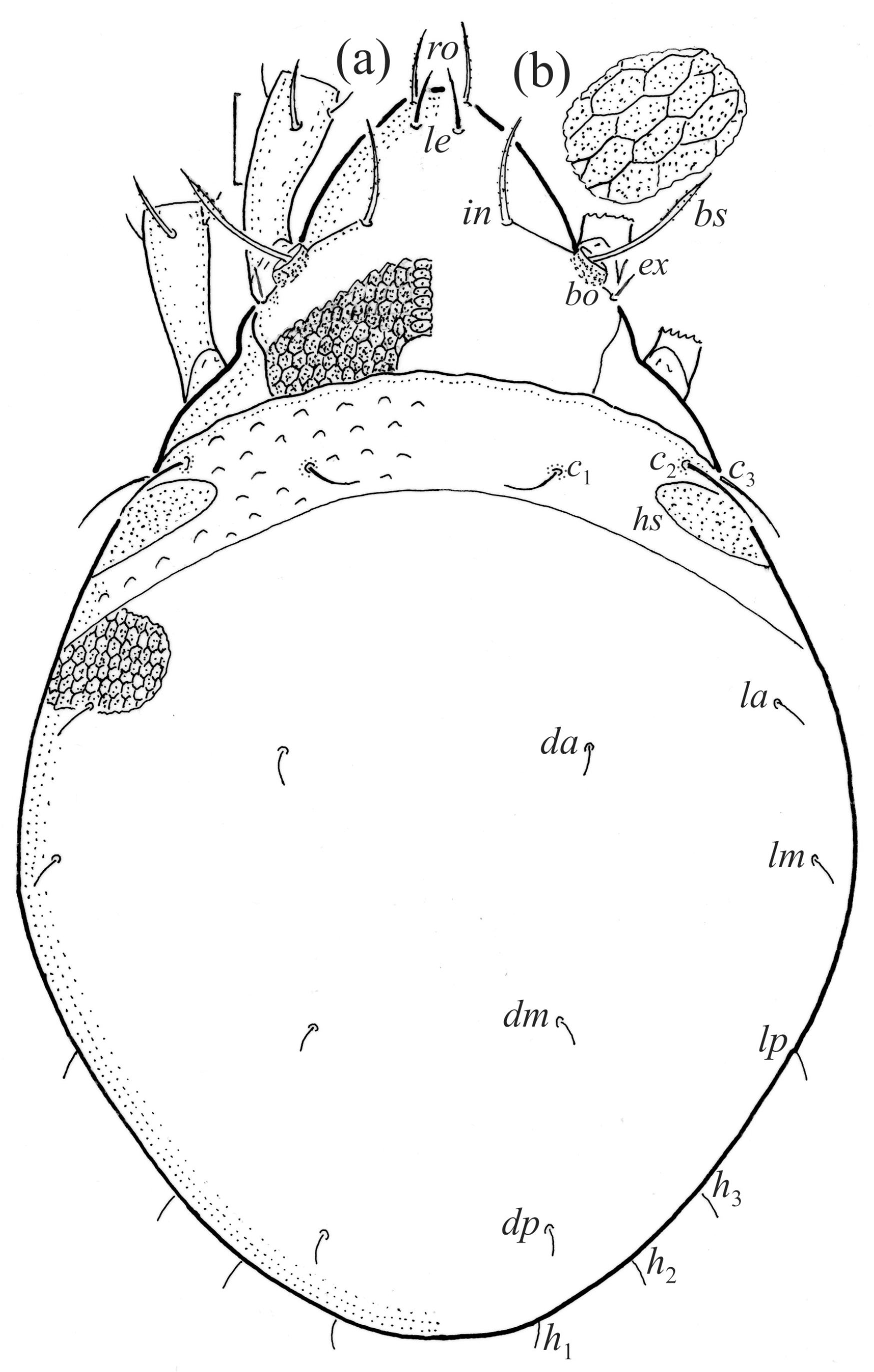
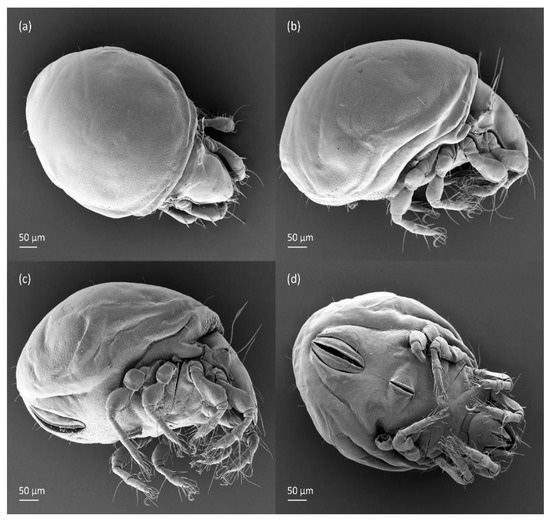
Figure 18.
SEM micrographs of Fuscozetes fuscipes tritonymph. (a) Dorsal view, (b) lateral view, (c) ventrolateral view, and (d) ventral view.
Figure 18.
SEM micrographs of Fuscozetes fuscipes tritonymph. (a) Dorsal view, (b) lateral view, (c) ventrolateral view, and (d) ventral view.
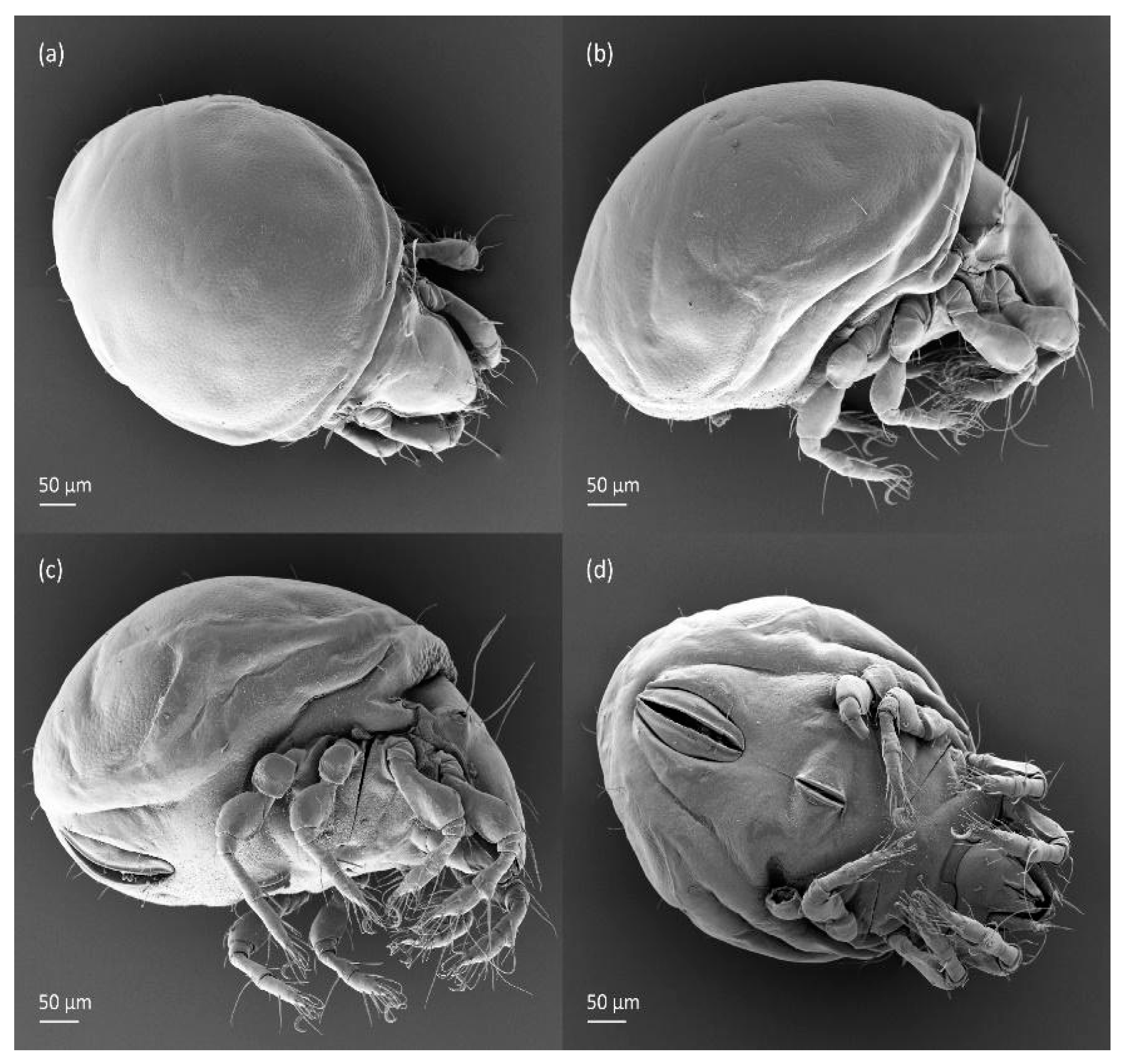
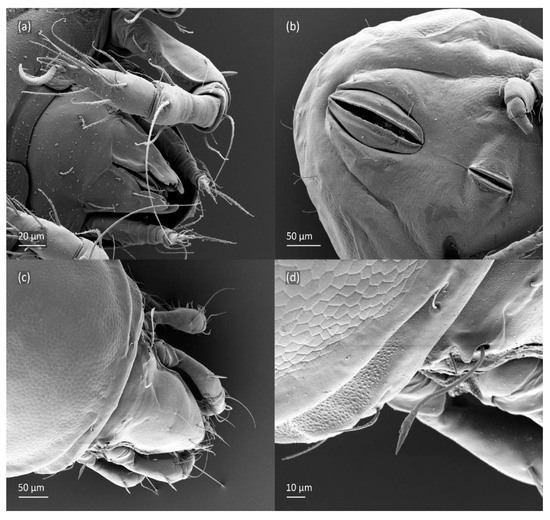
Figure 19.
SEM micrographs of Fuscozetes fuscipes tritonymph. Ventral view of (a) anterior part of body and (b) posterior part of body; dorsal view of (c) anterior and medial part of body and (d) bothridial seta.
Figure 19.
SEM micrographs of Fuscozetes fuscipes tritonymph. Ventral view of (a) anterior part of body and (b) posterior part of body; dorsal view of (c) anterior and medial part of body and (d) bothridial seta.
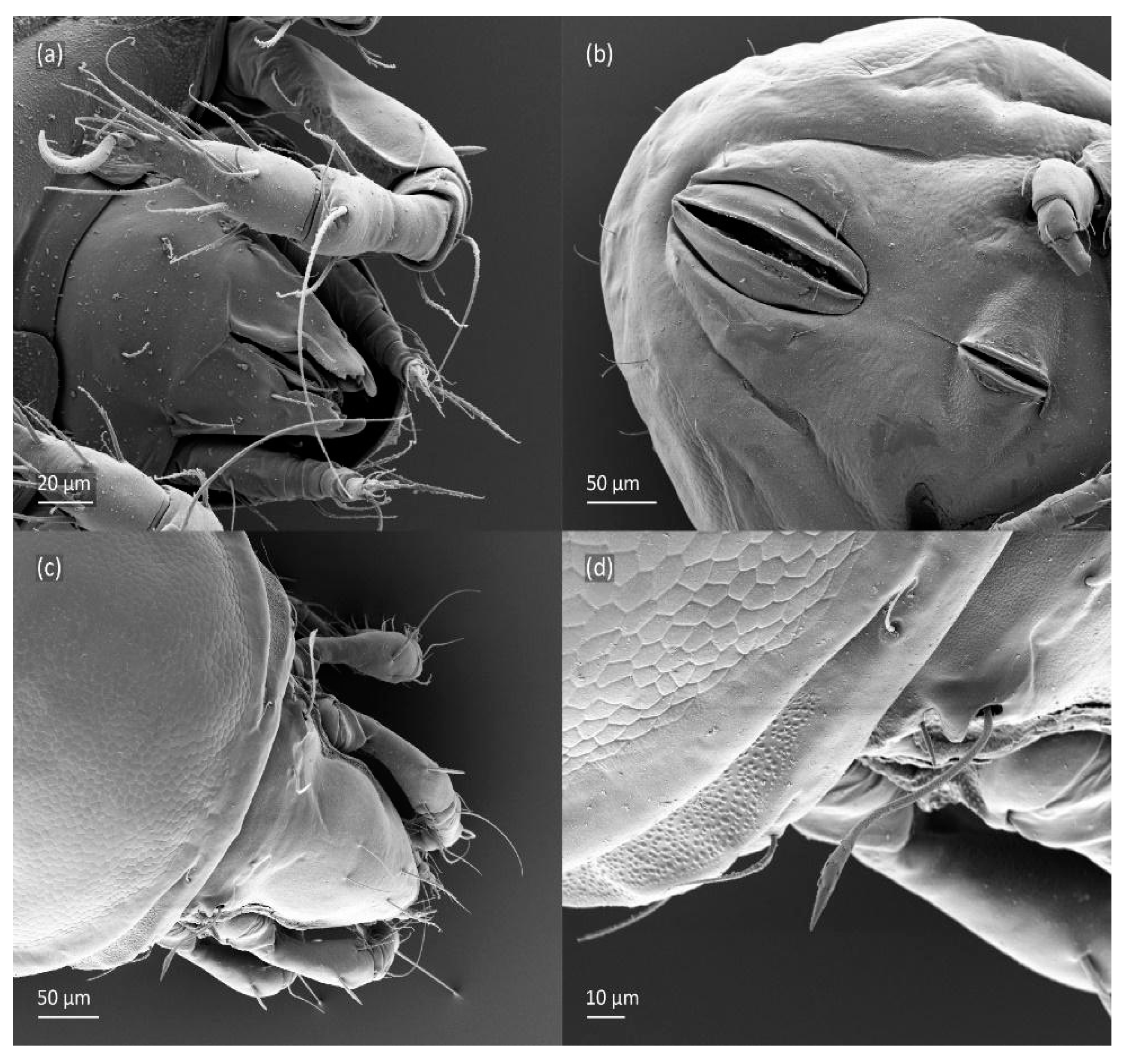
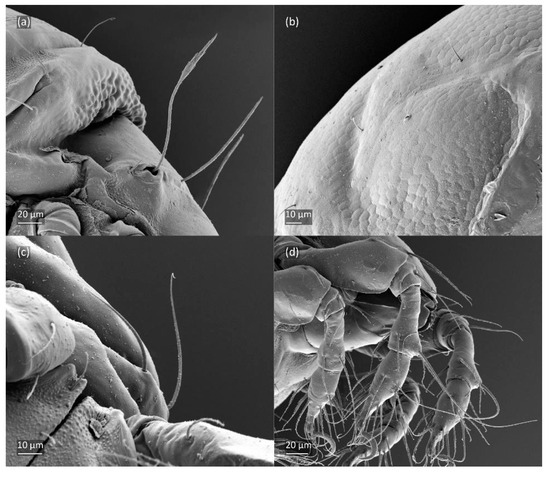
Figure 20.
SEM micrographs of Fuscozetes fuscipes tritonymph. Lateral view of (a) bothridial seta and (b) gla opening; (c) seta c2 and c3, ventral view; and (d) legs I and II, lateral view.
Figure 20.
SEM micrographs of Fuscozetes fuscipes tritonymph. Lateral view of (a) bothridial seta and (b) gla opening; (c) seta c2 and c3, ventral view; and (d) legs I and II, lateral view.
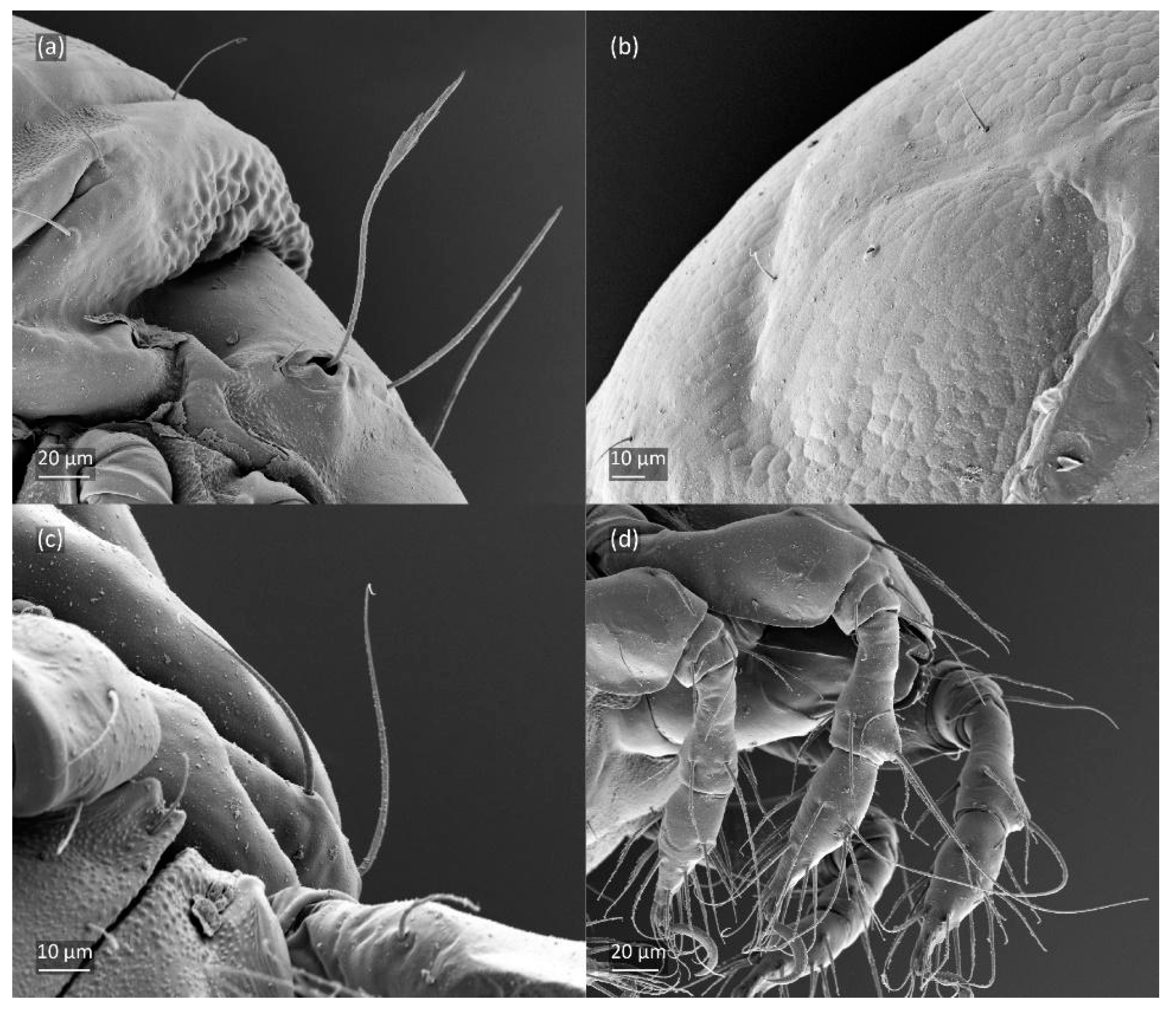
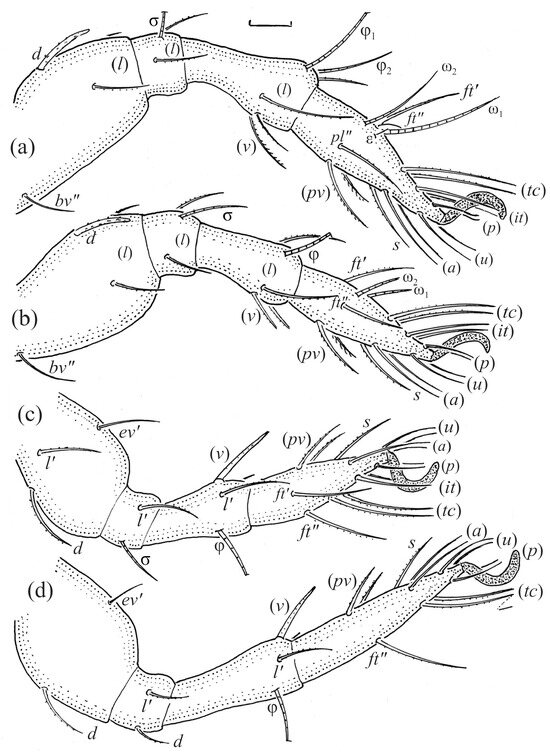
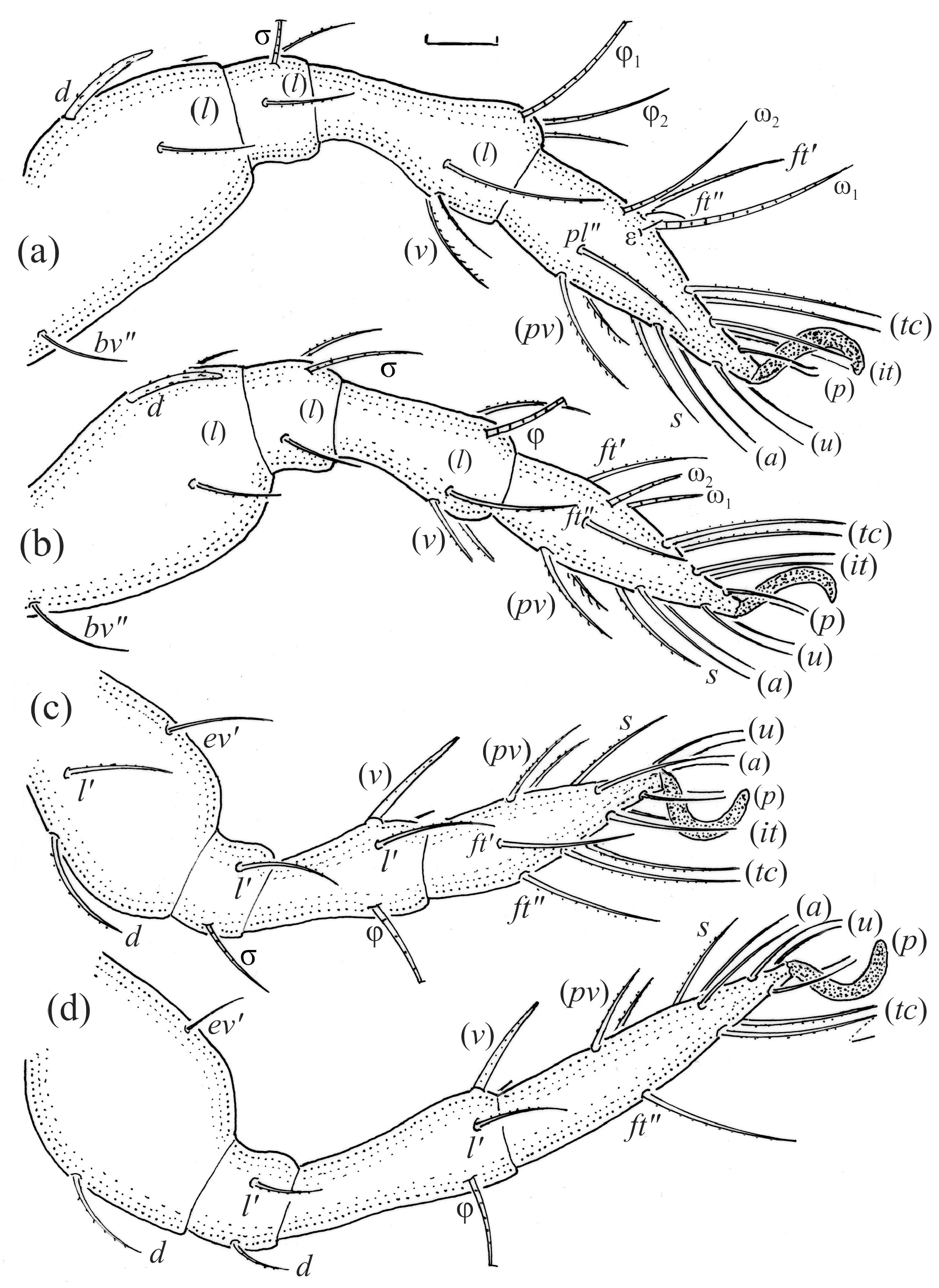
Figure 21.
Leg segments of Fuscozetes fuscipes tritonymph (femur to tarsus), right side; scale bars: 20 μm. (a) Leg I, tarsus (pl’ not illustrated); (b) leg II; (c) leg III; and (d) leg IV.
Figure 21.
Leg segments of Fuscozetes fuscipes tritonymph (femur to tarsus), right side; scale bars: 20 μm. (a) Leg I, tarsus (pl’ not illustrated); (b) leg II; (c) leg III; and (d) leg IV.
According to Shaldybina [8] and Weigmann [15], the porose area Aa was found to be larger than in the adults investigated herein and as reported by Bayartogtokh [47], which may reflect regional variation in this species. Shaldybina [8] drew dark areas around the notogastral setae, which are only observed in young, light-brown adults.
3.2.3. Redescription of Juveniles
The larva is egg-shaped in its dorsal and ventral view (Figure 9c, Figure 10a, Figure 11a and Figure 13b), and its body is light brown with a darker prodorsum, sclerites, epimeres, and legs. The prodorsum is subtriangular, the rostrum is rounded, setae ro and in are of a medium size and finely barbed, and the other setae are short and smooth (Figure 9c,d, Figure 10a, Figure 12a, Figure 13 and Table 2). The mutual distance between setal pair le is almost two times longer than that between setae ro, and the mutual distance between pair in is about four times longer than that between pair ro. Setal pair le is placed approximately midway between the setal pairs in and ro. The opening of the bothridium is rounded and the bothridial seta is fusiform, with a barbed head. A ridge is present between the opening of the bothridium and the insertion of seta in. The prodorsum is finely porose.
The gastronotum of the larva has 12 pairs of setae, including h3 inserted laterally to the anterior part of the anal valves (Figure 9d, Figure 11a, Figure 12a and Figure 13b). The gastronotal shield is poorly developed and most of the gastronotal setae are short and smooth (Figure 9c,d, Figure 10a, Figure 11a, Figure 12a, Figure 13a–c and Figure 14a,c), except for a slightly longer h2. Most of the setae are located on basal microsclerites except for h3. The humeral organ is rounded and located anteriorly to seta c3. The humeral sclerite is oval and porose, three or four other macrosclerites are present laterally to setae da and dm, and many microsclerites are present in the central and posterior parts of the gastronotum. Three large macrosclerites are present on the lateral side of the gastronotum, including one around the gla opening, along with 3–4 small sclerites (Figure 10a, Figure 11a and Figure 12a). A large sclerite is present posteriorly to leg III. Cupule ia is located posteriorly to seta c3, cupule im is located posteriorly to seta lm, im is located between setae h1 and h2, and ih is lateral to the anterior end of the anal opening (Figure 10a, Figure 11a and Figure 12a). The gland opening gla is located laterally to seta lp. The paraproctal valves (segment PS) are glabrous. The chelicera is chelate–dentate (Figure 14b,d). All the femora are oval in cross-section, a large ventral carina is absent, and most leg setae are finely barbed (Figure 9c,d, Figure 13, Figure 14b–d and Figure 15).
The shape and color of the protonymph and other nymphs are the same as in the larva, but the head of the bothridial seta is slimmer, and the prodorsum and gastronotal shield have a reticulate cuticle (Figure 12b, Figure 17, Figure 18, Figure 19b–d and Figure 20a,b). The gastronotum has 15 pairs of setae because the setae of the p-series appear in the protonymph and are retained in subsequent nymphs; all of these setae are short and smooth except for the medium-sized c-series setae (Figure 11b, Figure 12b, Figure 16, Figure 17, Figure 18, Figure 19b–d, Figure 20a–c and Table 2). The humeral organ is located in the same location as in the larva, the humeral sclerite has seta c1, and the other setae of the c-series are located on the basal microsclerites. The gastronotal shield is well developed, with 10 pairs of setae (d-, l-, and h-series, and p1), and setae p2 and p3 are placed on a large posteroventral sclerite. Small sclerites are present laterally to the setae of the l-series, and a large macrosclerite is present posteriorly to leg IV (Figure 11b, Figure 12b and Figure 16). In the protonymph, one pair of genital setae is present on the genital valves, and two pairs are added in both the deutonymph and the tritonymph (Figure 11b, Figure 16, Figure 18d and Figure 19b). The genital valves are placed on a large macrosclerite. In the deutonymph, one pair of aggenital setae and three pairs of adanal setae appear, and they remain in the other nymphs; all are short and smooth. In the tritonymph, cupules ia and im are placed in the same manner as in the larva, cupule ip is between setae p1 and h2, cupule iad is lateral to the anterior part of the anal valves, and cupules ih and ips are pushed laterally to cupule iad (Figure 11b, Figure 12b and Figure 16). The gland opening gla is placed anterolaterally to seta h3. The chelicerae are chelate–dentate (Figure 18d and Figure 19a). The anal valves of the protonymph and deutonymph are glabrous, and those of the tritonymph have two pairs of short and smooth setae. All the femora are oval in cross-section, a large ventral carina is absent, and most of the leg setae are finely barbed (Figure 18, Figure 19, Figure 20d and Figure 21). In one deutonymph, two setae v’ were present on trochanter III.
3.2.4. Summary of Ontogenetic Transformations
In the larva, the prodorsal setae ro and in are of a medium size, and the setae le and ex are short, whereas in the nymphs and adult, seta in is clearly longer than ro, and seta le is of a medium size. In all the juveniles, the bothridium is rounded, whereas in the adult, it is larger and gains scales. In all the instars, the bothridial seta is fusiform with a barbed head, but in the nymphs and adult, the head is slimmer than in the larva. The larva has 12 pairs of gastronotal setae (h3 is present), while the nymphs have 15 pairs. The notogaster of the adult loses setae c1, c3, and those of the d-series, such that 10 pairs of setae remain on the notogaster. The formula of gastronotal setae in F. fuscipes is 12-15-15-15-10 (from larva to adult). The formulae of the epimeral setae are: 3-1-2 (larva), 3-1-2-1 (protonymph), 3-1-2-2 (deutonymph), and 3-1-3-3 (tritonymph and adult); the formula of the genital setae is 1-3-5-6 (protonymph to adult); the formula of the aggenital setae is 1-1-1 (deutonymph to adult); and the formula of segments PS–AN is 03333-0333-022. The ontogeny of the leg setae and solenidia of F. fuscipes is given in Table 3.

Table 3.
Ontogeny of leg setae (Roman letters) and solenidia (Greek letters) of Fuscozetes fuscipes.
3.3. Mitochondrial Genetic Variation
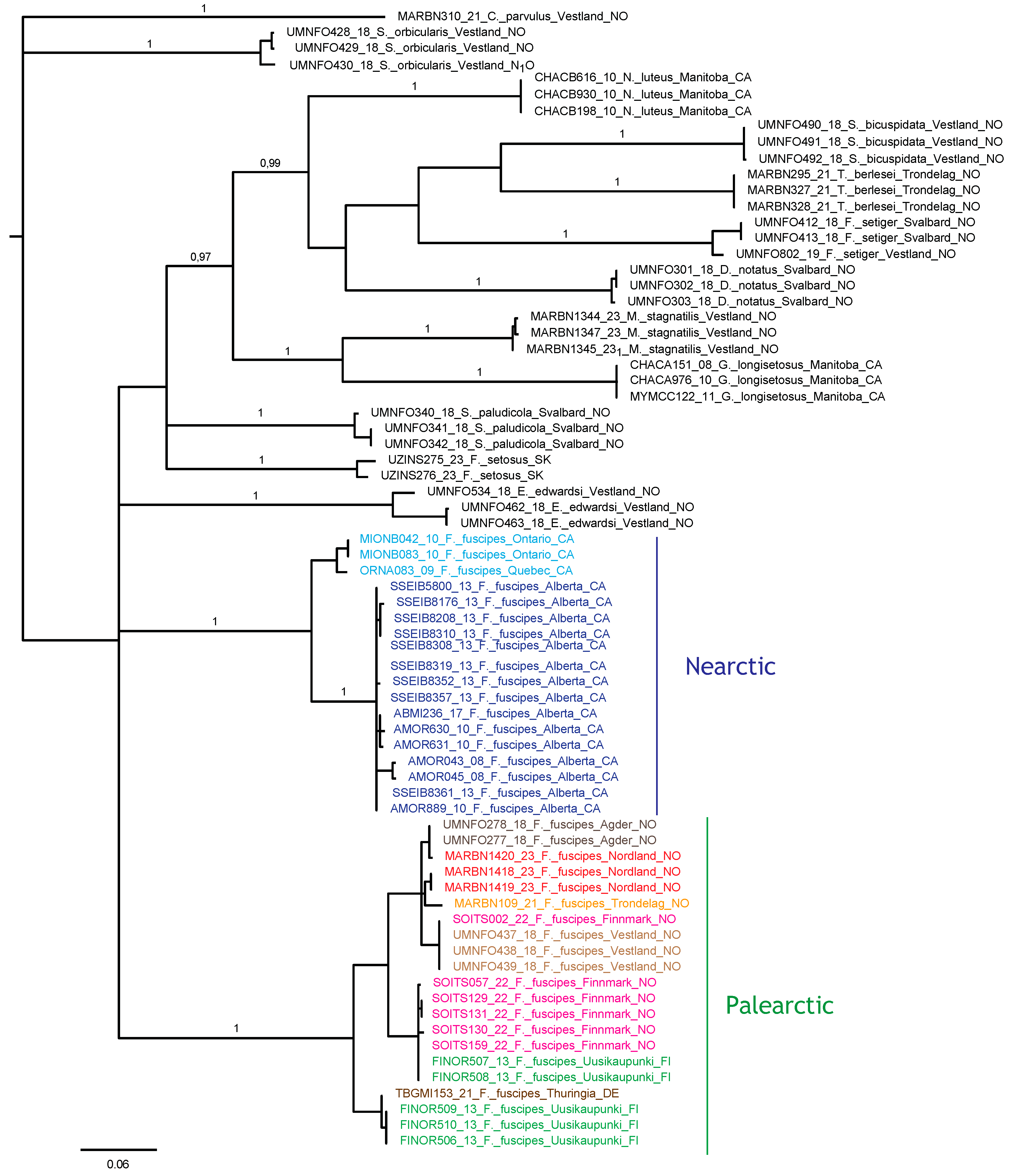
The Bayesian analysis of the COI sequences revealed two deeply diverged clades of F. fuscipes corresponding to exclusively Nearctic and Palearctic populations, respectively (Figure 22), and separated by a 15.5–18.4% uncorrected p-distance. Each regional clade contained 11 haplotypes, with a maximum of 7.2% divergence in the Nearctic clade and 5.8% in the Palearctic clade. Several Palearctic haplotypes were shared between distant locations, including Finnmark and Vestland in Norway, and one haplotype was shared between Finnmark and SW Finnland. Near-identical haplotypes were found in Agder and Nordland (Norway), and in SW Finland and Germany.
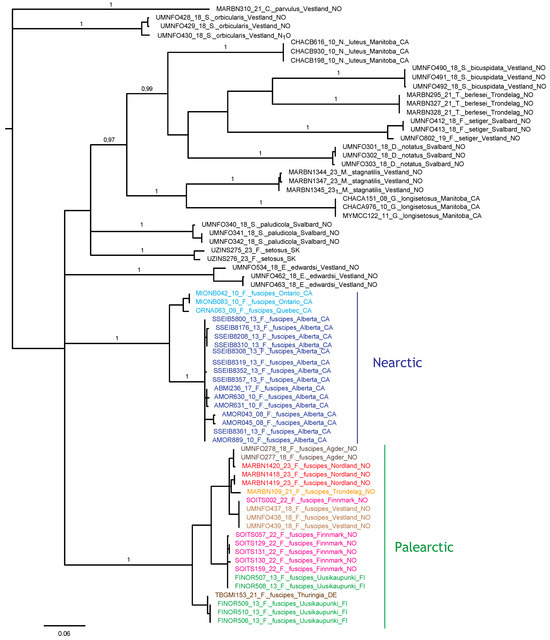
Figure 22.
Bayesian tree topology based on COI nucleotide sequences (658 bp). Specimen numbers correspond to those in the BOLD database (http://boldsystems.org/, accessed on 20 November 2023). Information about barcoded specimens is presented in Table 1.
3.4. Ecology and Biology
We collected F. fuscipes in Sphagnum mosses on the shore of lake Skomakerdiket (Bergen, Norway), where this species achieved a density of 102 individuals per 500 cm3. In this population, the juveniles made up 52% of all individuals, with the following stage structure: 8 larvae, 33 protonymphs, 4 deutonymphs, 7 tritonymphs, and 50 adults. The female-to-male sex ratio was 1:0.3, and 7% of the females were gravid and carried one or two large eggs (290 × 175), comprising 34% of the length of the females.
4. Discussion
4.1. Morphology and Development
The juvenile stages of F. fuscipes from Norway are generally similar to those from Russia [48] and Poland [17], except for the shape of some setae and sclerites and the number of microsclerites in the larvae. In the larvae from Norway, the prodorsal seta in is of a medium size and barbed, as in the larvae from Poland [17], whereas in the larvae from Russia [48], this seta is short and smooth. In all regions, the larvae of F. fuscipes lack a gastronotal shield, most of the gastronotal setae are located on the microsclerites and other sclerites, and microsclerites are also present on the gastronotum. However, the shape of the macrosclerites differs in these larvae, and the larvae from Norway and Russia have more microsclerites than those from Poland. In the tritonymphs from Norway and Poland, the gastronotal setae are slightly longer than in the specimens from Russia. All these differences probably illustrate regional variation in the species.
Seniczak et al. [14] compared a selection of morphological characters in several Fuscozetes species. In light of this comparison and this investigation, the largest is F. fuscipes, the smallest is F. setiger, and the body length of most species overlaps. These species also differ from one another in the shape of their bothridial seta, translamella, lamellar cusp, and porose area Aa, and in the number and shape of their notogastral setae. Most species have 10 pairs of notogastral setae (c2 is present); two species (F. novus Shaldybina, 1969, and F. tatricus) have 11 pairs (c2 and dp are present), and F. setosus has 10–13 pairs of setae (c2 and some or all the setae of the d-series are present).
Seniczak et al. [14] also compared 23 morphological characters of the larvae and tritonymphs of F. coulsoni, F. fuscipes, F. kamchatkicus, F. setiger, F. setosus, and F. tatricus. The juveniles of F. fuscipes are the most similar to those of F. setosus, differing from them in six morphological characters, and the most dissimilar from those of F. setiger, differing from them in 21 morphological characters. This is not too surprising, in view of the distant phylogenetic relationship to F. setiger indicated by our COI data analysis (Figure 22).
The morphological ontogeny of F. fuscipes is generally similar to that of F. coulsoni, F. kamchatkicus, F. pseudosetosus, F. setiger, F. setosus, and F. tatricus [8,12,13,14,17,49,50,51], except for the shape of the larval gastronotum. In F. fuscipes, F. pseudosetosus, and F. tatricus, the gastronotal shield is absent, but macrosclerites and microsclerites can be present, whereas in the other species, the gastronotal shield and a humeral macrosclerite are present, and sometimes other macrosclerites and microsclerites. Among these species, the ontogeny of the leg setae and solenidia were investigated in detail in F. coulsoni, F. kamchatkicus, and F. setiger [12,13,14]. The ontogeny of the leg setae and solenidia of F. fuscipes studied herein is most similar to that of F. setiger, differing from it in two morphological characters, and most dissimilar to that of F. kamchatkicus, differing from it in five morphological characters (Table 4). Some leg characters can be diagnostic.

Table 4.
Comparison of some leg characters in Fuscozetes fuscipes, F. coulsoni, F. kamchatkicus, and F. setiger.
The morphology of the adults and juveniles of Fuscozetes is generally similar to those of Melanozetes [9,10,11,52,53,54,55,56,57,58,59,60], except for the diagnostic characters for these genera. The adults of Fuscozetes have 10–13 pairs of notogastral setae, including c2 and some or all the setae of the d-series, whereas those of Melanozetes have 14 pairs of setae, including c2 and c3 [11,56,57,58,59].
The separation of the juveniles of Fuscozetes from those of Melanozetes is more difficult than the adults. The juveniles of Fuscozetes have a generally smaller area of sclerites on the gastronotum that those of Melanozetes, except for the larvae of some species. For example, the larva of Melanozetes avachai Seniczak et al. 2016 [58] has a weakly developed gastronotal shield, most of its gastronotal setae are located on microsclerites, femora I and II are oval in cross-section, and a large ventral carina is absent, as with that of F. fuscipes studied herein. The separation of the nymphs and adults of Fuscozetes from those of Melanozetes is easier than for the larva, mainly using the length and location of solenidion ω2 on tarsus I; in Fuscozetes, this solenidion is shorter than ω1 and is placed posterolaterally to ω1, whereas in Melanozetes, solenidion ω2 is as long as or longer than ω1 and is placed anteriorly to ω1.
The three-dimensional SEM figures of F. fuscipes correspond well with the line drawings of this species, which are, to some degree, subjective and depend on the technique of preparation and the author. For example, in the larva of F. fuscipes, some macrosclerites and microsclerites are observed in different aspects as depressions, which are rarely observed in SEM figures. In the tritonymph, the reticulation of the gastronotum and a humeral sclerite are well observed.
4.2. Ecology and Distribution
Fuscozetes fuscipes has a Holarctic distribution [1]. In Norway, it has been found in moist mosses in the north (Finnmark), west (Vestland), and south [60,61,62,63]. Fuscozetes fuscipes is a hygrophilous [64,65] or meso-hygrophilous species [66,67]. It inhabits wet tundra [10], Sphagnum mosses, and wet habitats [65,68,69,70] close to pools and lakes [71,72,73,74], as well as wet-to-humid forest soils and meadows [45]. In an oligotrophic bog in Norway, it was found only in the lower Sphagnum layer, 10–17 cm deep [63,75]. Solhøy Wunderle and Solhøy [76] consider F. fuscipes a true arctic and high-mountain species, and Schatz [77] and Murvanidze et al. [45] confirmed the dominance of this species in subalpine zones, while Solhøy found it in an alpine zone in Norway [75]. This species is sensitive to some pesticides [78], but it is cold-tolerant and able to survive winter temperatures of −28 °C [79].
Wallwork [80] investigated various biological aspects of F. fuscipes in the laboratory. Adults and nymphs were fed on the macerated leaf tissue of hemlock (Conium maculatum L.) and on the moist decaying leaves and petioles of yellow birch (Betula alleghaniensis Britt.), and the nymphs also fed on dead mites and springtails. Madge [65] observed no clear response by adults and juveniles to a higher air humidity in similar experiments, whereas in dry air, these wet-adapted mites quickly died. Nevertheless, this species survives much longer in a lower humidity than another hygrophilous species, H. rufulus C.L. Koch, 1835, probably due to its thicker waxy cuticle on the body [66,81].
Shaldybina [82] cultivated F. fuscipes under laboratory conditions at 18–20 °C; fed it on lichens, mosses, and raw potatoes; and estimated a development time of 86 days for this species. The mean time of the development of successive instars (+ immobile period between stages) was in days: egg, 12; larva, 11.5 + 4; protonymph, 12 + 4; deutonymph, 14.5 + 4; and tritonymph, 17.5 + 6.5. Among the 18 species cultivated by this author, the time of development varied between 40 and 180 days. Under natural conditions in cooler climates, the time of the development of F. fuscipes probably lasts longer than 86 days, which limits its population growth. In our investigation, only 7% of the females of this species were gravid, each carried one or two large eggs, and in June, the number of juveniles was approximately similar to that of the adults. In earlier studies carried out in a similar area at two lake shores, also in June, the juveniles were more abundant than the adults and made up 71% and 81% of each local population [83].
There are no specific data on the dispersal of F. fuscipes, but it can probably use many passive ways of spreading that are common in oribatid mites. Many oribatids can migrate over large distances with the wind (anemohydrochory) [84,85,86,87], via birds [88,89,90,91,92], by water currents or waves (together with the action of wind), or with objects drifting in water [84,86,93], including transport in seawater [94]. Even though Oribatida lack obvious morphological adaptations for active transport by phoresy, it has been demonstrated that they are carried on insects [95,96,97,98,99,100] and frogs [101].
The several shared or near-identical haplotypes between distant sites sampled in this study indicate high migration rates of this species in Europe, at least in modern times. A similar pattern of haplotype identity was found for Platynothrus peltifer (C.L. Koch, 1839) in Western Norway, Belgium, and Germany [102]; P. punctatus (L. Koch, 1879) in Svalbard, Western Norway, and Southern Spain [103]; and Nanhermannia coronata Berlese, 1913, in Northern, Central, and Southern Norway, Ireland, and Finland [104]. We may hypothesize that their migration in a latitudinal direction is largely influenced by migrating birds. In contrast, the longitudinal separation of subclades in Canada, and between Canada and Scandinavia, in two different clades of F. fuscipes indicates much less migration in this direction. The morphology of F. fuscipes from Canada has not been studied in detail, including the juvenile stages, so it is not possible to pinpoint any morphological differences between the Nearctic and Palearctic populations. To enable a firm test of species boundaries and taxon validity, more genetic data and morphological studies are needed, because a single and rapidly evolving mitochondrial marker is not sufficiently informative to conclude on such issues.
Furthermore, we note that other oribatid genera have mixed patterns of genetic differentiation across the Atlantic, e.g., Platynothrus troendelagicus Seniczak and Seniczak, 2022, which has identical COI haplotypes across Europe (Norway and Ireland) and Canada [105], whereas populations of P. peltifer have diverged significantly between Europe, USA, and Japan. It is, therefore, possible that more intensive sampling of F. fuscipes will support a similar cryptic-species scenario. In this context, it will be useful to study the ecological traits that may affect long-distance colonization in this species group.
5. Conclusions
- 1.
- Fuscozetes species are a well-formed morphological group of mites that differ clearly from closely related Melanozetes species, both as nymphs and adults. The adults of Fuscozetes have fewer notogastral setae (10–13 pairs, including c2 and some or all the setae of the d-series) than those of Melanozetes (14 pairs, including c2 and c3), and the adults and nymphs have a shorter solenidion ω2 on tarsus I than ω1, and it is placed posterolaterally to ω1. In Melanozetes, solenidion ω2 is as long as or longer than ω1 and is placed anteriorly to ω1.
- 2.
- Fuscozetes fuscipes is a hygrophilous species and prefers wet tundra, Sphagnum mosses, and wet habitats close to pools and lakes.
- 3.
- Mitochondrial genetic data revealed deeply diverged populations across the Holarctic, a high local and regional genetic diversity, and several examples of haplotypes shared between distant Scandinavian localities, indicating latitudinal long-distance migration.
Author Contributions
Conceptualization, S.S. and A.S.; methodology, S.S., B.H.J. and A.S.; line drawings, S.S.; DNA barcoding, B.H.J. and A.S.; SEM imaging, A.S.; writing—original draft, S.S. and A.S.; writing—review and editing, S.S., B.H.J. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by the Norwegian Taxonomy Initiative (grant No. 6-20, 70184243); some sequences were obtained with the grant to NIBIO from the Norwegian Taxonomy Initiative (grant No. 9-21, 70184244). The sequencing was financed by Norwegian Barcode of Life (NorBOL).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The COI sequences will be publicly available in GenBank and in BOLD.
Acknowledgments
We are grateful to all the reviewers for all their suggestions, which considerably increased the scientific value of this paper. We are also grateful to Cornelya F. C. Klütsch (Norwegian Institute of Bioeconomy Research, NIBIO, Svanhovd, Norway) for making sequences of Fusozetes fuscipes from Finnmark available; to Steffen Roth (University Museum of Bergen, University of Bergen, Bergen, Norway) for the collection of the Svalbard specimens used in this paper; and Irene Heggstad (Department of Earth Science, University of Bergen, Bergen, Norway) for her professional help with the scanning electron microscopy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Subías, L.S. Listado sistemático, sinonímico y biogeográfico de los Ácaros Oribátidos (Acariformes, Oribatida) del mundo (1758–2002). Graellsia 2004, 60, 3–305, updated 2023. [Google Scholar] [CrossRef]
- Shaldybina, E.S. Family Ceratozetidae Jacot, 1925. In Key to Soil-Inhabiting Mites–Sarcoptiformes; Ghilarov, M.S., Ed.; Nauka Publishing: Moscow, Russia, 1975; pp. 277–303. (In Russian) [Google Scholar]
- Sellnick, M. Formenkreis: Hornmilben, Oribatei. In Die Tierwelt Mitteleuropas; Brohmer, P., Ehrmann, P., Ulmer, G., Eds.; Series 3; Quelle und Meyer: Leipzig, Germany, 1928; Volume 9, pp. 1–42. [Google Scholar]
- Grandjean, F. Les segments post-larvaires de l’hystérosoma chez les Oribates (Acariens). Bull. Soc. Zool. 1939, 64, 273–284. [Google Scholar]
- Grandjean, F. Formules anales, gastronotiques, génitales et aggénitales du développement numériques des poils chez les Oribates. Bull. Soc. Zool. 1949, 74, 201–225. [Google Scholar]
- Shaldybina, E.S. Some morphological features of the ceratozetidoids (Oribatei). Notes Gorky Pedagog. Inst. Biol. Ser. 1972, 130, 35–66. (In Russian) [Google Scholar]
- Shaldybina, E.S. The postembryonic development of beetle mites of the superfamily Ceratozetoidea Balogh, 1961, and their systematics. 1. In Acarological Conference, Lecture Theses; Nauka: Moscow, Russia, 1966; pp. 225–226. (In Russian) [Google Scholar]
- Shaldybina, E.S. Moss Mites of the Superfamily Ceratozetoidea (Their Morphology, Biology, System and Role in the Anoplocephalid Epizooties). Ph.D. Thesis, Gorky State Pedagogical Institute, Moscow, Russia, 1969; p. 708. (In Russian). [Google Scholar]
- Behan-Pelletier, V.M. Ceratozetidae of the Western North American Arctic. Canad. Entomol. 1985, 117, 1287–1366. [Google Scholar] [CrossRef]
- Behan-Pelletier, V.M. Ceratozetidae (Acari: Oribatei) of the Western North American Subarctic. Canad. Entomol. 1986, 118, 991–1057. [Google Scholar] [CrossRef]
- Seniczak, S.; Behan-Pelletier, V.M.; Solhøy, T. Systematic value of some notogastral setae in adult Sphaerozetinae (Acari, Oribatida, Ceratozetoidea) in the light of ontogenetic studies. Acarologia 1990, 31, 385–400. [Google Scholar]
- Seniczak, S.; Kaczmarek, S.; Seniczak, A. Morphological ontogeny of Fuscozetes kamchatkicus sp. nov. (Acari: Oribatida: Ceratozetidae) from Kamchatka Peninsula (Russia), with comments on Fuscozetes. Syst. Appl. Acarol. 2016, 21, 1017–1030. [Google Scholar] [CrossRef]
- Seniczak, A.; Seniczak, S. Morphological ontogeny of Fuscozetes coulsoni sp. nov. (Acari: Oribatida: Ceratozetidae) from Svalbard, Norway. Syst. Appl. Acarol. 2020, 25, 680–696. [Google Scholar] [CrossRef]
- Seniczak, A.; Seniczak, S. Systematic position of Fuscozetes setiger (Acari, Oribatida, Ceratozetidae) in the light of ontogenetic studies. Syst. Appl. Acarol. 2022, 27, 1454–1474. [Google Scholar] [CrossRef]
- Weigmann, G. Hornmilben (Oribatida). In Die Tierwelt Deutschlands; Part 76; Dahl, F., Ed.; Goecke & Evers: Keltern, Germany, 2006; pp. 1–520. [Google Scholar]
- Norton, R.A.; Ermilov, S.G. Catalogue and historical overview of juvenile instars of oribatid mites (Acari: Oribatida). Zootaxa 2014, 3833, 1–132. [Google Scholar] [CrossRef] [PubMed]
- Seniczak, S. The morphology of juvenile stages of moss mites of the subfamily Sphaerozetinae (Acarida: Oribatida), II. Ann. Zool. 1989, 42, 237–248. [Google Scholar]
- Grandjean, F. Étude sur le développement des Oribates. Bull. Soc. Zool. 1933, 58, 30–61. [Google Scholar]
- Grandjean, F. Les poils des épimières chez les Oribates (Acariens). Bull. Mus. Nat. Hist. Nat. 1934, 6, 504–512. [Google Scholar]
- Grandjean, F. La notation des poils gastronotiques et des poils doreaux du propodosoma chez les Oribates (Acariens). Bull. Soc. Zool. 1934, 40, 12–44. [Google Scholar]
- Grandjean, F. Essai de classification des Oribates (Acariens). Bull. Soc. Zool. 1953, 78, 421–446. [Google Scholar]
- Norton, R.A.; Behan-Pelletier, V.M. Suborder Oribatida. Chapter 15. In A Manual of Acarology; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; Chapter 15; pp. 430–564. [Google Scholar]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; de Waard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Seniczak, A.; Seniczak, S.; Jordal, B.H. Integrated taxonomy approach: Molecular data and ontogeny studies clarify systematic status of Chamobates borealis (Acari, Oribatida). Syst. Appl. Acarol. 2019, 24, 2409–2426. [Google Scholar] [CrossRef]
- Young, M.R.; Proctor, H.C.; deWaard, J.R.; Hebert, P.D.N. DNA barcodes expose unexpected diversity in Canadian mites. Mol. Ecol. 2019, 28, 5347–5395. [Google Scholar] [CrossRef]
- Roslin, T.; Somervuo, P.; Pentinsaari, M.; Hebert, P.N.; Agda, J.; Ahlroth, P.; Anttonen, P.; Aspi, J.; Blagoev, G.; Blanco, S.; et al. A molecular-based identification resource for the arthropods of Finland. Mol. Ecol. Resour. 2022, 22, 803–882. [Google Scholar] [CrossRef] [PubMed]
- Young, M.R.; Behan-Pelletier, V.M.; Hebert, P.D.N. Revealing the Hyperdiverse Mite Fauna of Subarctic Canada through DNA Barcoding. PLoS ONE 2012, 7, e48755. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhn, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Michael, A.D. British Oribatidae; Ray Soc: London, UK, 1884; Volume I, pp. 1–336. [Google Scholar]
- Willmann, C. Moosmilben oder Oribatiden (Cryptostigmata). In Die Tierwelt Deutschlands; Dahl, F., Ed.; Gustav Fischer: Jena, Germany, 1931; Bd. 22; Volume 5, pp. 79–200. [Google Scholar]
- Mehl, R. Checklist of Norwegian ticks and mites (Acari). Fauna Norv. 1979, 26, 1–45. [Google Scholar]
- Karppinen, E.; Krivolutsky, D.A. List of oribatid mites (Acarina, Oribatei) of northern Palaearctic region. I. Europe. Acta Entomol. Fenn. 1982, 41, 1–18. [Google Scholar]
- Golosova, L.D.; Karppinen, E.; Krivolutsky, D.A. List of oribatid mites (Acarina, Oribatei) of northern Palaearctic region. II. Siberia and the Far East. Acta Entomol. Fenn. 1983, 43, 1–14. [Google Scholar]
- Karppinen, E.; Krivolutsky, D.A.; Poltavskaja, M.P. List of oribatid mites (Acarina, Oribatei) of northern Palaearctic region. III. Arid lands. Ann. Entomol. Fenn. 1986, 52, 81–94. [Google Scholar]
- Karppinen, E.; Krivolutsky, D.A.; Tarba, Z.M.; Shtanchaeva, U.Y.; Gordeeva, E.W. List of oribatid mites (Acarina, Oribatei) of northern Palaearctic region. IV. Caucasus and Crimea. Ann. Entomol. Fenn. 1987, 53, 119–137. [Google Scholar]
- Marshall, V.G.; Reeves, R.M.; Norton, R.A. Catalogue of the Oribatida (Acari) of Continental United States and Canada. Mem. Entomol. Soc. Canada 1987, 139, 1–418. [Google Scholar] [CrossRef]
- Olszanowski, Z.; Rajski, A.; Niedbała, W. Roztocze Acari–Mechowce Oribatida. In Katalog Fauny Polski–Catalogus Faunae Poloniae; Sorus: Poznań, Poland, 1996; Volume 34, pp. 1–243. [Google Scholar]
- Niemi, R.; Karppinen, E.; Uusitalo, M. Catalogue of the Oribatida (Acari) of Finland. Acta Zool. Fenn. 1997, 207, 1–39. [Google Scholar]
- Ryabinin, N.A.; Pankov, A.N. Catalogue of Oribatid Mites of the Far East of Russia. Part II. Continental Part of the Far East; DVO: Vladivostok, Russia; Khabarovsk, Russia, 2002; p. 92. (In Russian) [Google Scholar]
- Miko, L. Oribatid mites (Acarina, Oribatida) of the Czech Republic. Revised check-list with a proposal for Czech oribatid nomenclature. Klapalekiana 2016, 52, 1–302. [Google Scholar]
- Murvanidze, M.; Mumladze, L. Annotated checklist of Georgian oribatid mites. Zootaxa 2016, 4089, 1–81. [Google Scholar] [CrossRef]
- Schatz, H. Catalogue of oribatid mites (Acari: Oribatida) from Vorarlberg (Austria). Zootaxa 2020, 4783, 1–106. [Google Scholar] [CrossRef]
- Murvanidze, M.; Todria, N.; Maraun, M.; Mumladze, L. Annotated checklist of Georgian oribatid mites–II. Zootaxa 2023, 5227, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Bayartogtokh, B.; Weigmann, G. New and little known species of oribatid mites of the genera Arthrodamaeus and Fuscozetes (Arachnida: Acari: Oribatida) from Mongolia. Species Divers. 2005, 10, 75–84. [Google Scholar] [CrossRef][Green Version]
- Bayartogtokh, B. Oribatid mites of Mongolia (Acari: Oribatida). In Russian Academy of Sciences; KMK Scientific Press Ltd.: Moscow, Russia, 2010; 400p. [Google Scholar]
- Shaldybina, E.S. The postembryonal development of Fuscozetes fuscipes (C.L. Koch) (Oribatei, Ceratozetidae). In Fauna, Systematics, Biology and Ecology of Helminths and Their Intermediate Hosts; Gorky: Moskow, Russia, 1978; pp. 84–93. (In Russian) [Google Scholar]
- Shaldybina, E.S. A new species of the genus Fuscozetes (Oribatei, Ceratozetidae). Zool. J. 1977, 56, 709–713. (In Russian) [Google Scholar]
- Shaldybina, E.S. Juvenile instars of Ceratozetoidea (Oribatei). In Fauna, Classification, Biology and Ecology of Parasitic Worms and their Intermediate Hosts; Gorky State Pedagogy Institute: Moskow, Russia, 1977; pp. 76–89. [Google Scholar]
- Seniczak, S. Fuscozetes tatricus n. sp. a New Ceratozetoid Moss Mite (Acari, Oribatida, Ceratozetidae) from Poland. Zool. Anz. 1993, 230, 169–180. [Google Scholar]
- Shaldybina, E.S. The biology of Melanozetes mollicomus (Koch) (Oribatei, Ceratozetidae). Zool. J. 1967, 46, 1659–1667. (In Russian) [Google Scholar]
- Behan-Pelletier, V.M. Ceratozetidae (Acari: Oribatida) of arboreal habitats. Canad. Entomol. 2000, 132, 153–182. [Google Scholar] [CrossRef]
- Seniczak, S. The morphology of juvenile stages of moss mites of the subfamily Sphaerozetinae (Acarida: Oribatida), I. Ann. Zool. 1989, 42, 225–235. [Google Scholar]
- Seniczak, S. The morphology of juvenile stages of moss mites of the subfamily Sphaerozetinae (Acari, Oribatida). III. Zool. Anz. 1993, 231, 25–38. [Google Scholar]
- Seniczak, A.; Seniczak, S. Morphological ontogeny of Melanozetes stagnatilis (Acari, Oribatida, Ceratozetidae). Syst. Appl. Acarol. 2018, 23, 652–664. [Google Scholar] [CrossRef]
- Seniczak, S.; Seniczak, A.; Kaczmarek, S. Morphological ontogeny of Melanozetes azoricus with comments on Melanozetes (Acari: Oribatida: Ceratozetidae). Intern. J. Acarol. 2015, 41, 523–536. [Google Scholar] [CrossRef]
- Seniczak, S.; Kaczmarek, S.; Seniczak, A. Morphological ontogeny of Melanozetes avachai n. sp., a unique member of Melanozetes (Acari: Oribatida: Ceratozetidae). Acarologia 2016, 56, 463–484. [Google Scholar] [CrossRef]
- Seniczak, S.; Seniczak, A.; Kaczmarek, S.; Marquardt, T. Morphological ontogeny of Melanozetes interruptus (Acari, Oribatida, Ceratozetidae), and comments on Melanozetes Hull. Syst. Appl. Acarol. 2023, 28, 1897–1913. [Google Scholar] [CrossRef]
- Bayartogtokh, B.; Ermilov, S.G.; Shtanchaeva, U.Y.; Subías, L.S. Ontogenetic instars of Melanozetes paramollicomus sp. nov., with remarks on morphological ontogeny of Sphaerozetinae (Acari: Oribatida: Ceratozetidae). Zootaxa 2021, 5086, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Thor, S. Übersicht der norwegischen Cryptostigmata mit einzelnen Nebenbemerkungen. Nyt Mag. Nat. 1937, 77, 275–307. [Google Scholar]
- Willmann, C. Oribatiden von der Insel Herdla. Berg. Mus. Arbok Nat. Rekke 1929, 5, 1–16. [Google Scholar]
- Solhøy, T. Oribatids (Acari) from an oligotrophic bog in western Norway. Fauna Norv. Ser. B. 1979, 26, 91–94. [Google Scholar]
- Madge, D.S. The behaviour of free-living mites as affected by humidity (Acarina; Oribatoidea). Anim. Behav. 1961, 9, 108. [Google Scholar] [CrossRef]
- Madge, D.S. The humidity reactions of oribatid mites. Acarologia 1964, 6, 566–591. [Google Scholar]
- Madge, D.S. The longevity of fasting oribatid mites. Acarologia 1964, 6, 718–729. [Google Scholar]
- Ivan, O.; Calugar, A. The fauna of edaphic mites (Acari, Gamasida, Oribatida) in some peat bogs–Protected areas in North Moldavia (Romania). Ann. Compl. Muz. Bucov. 2003, 16–17, 127–150. [Google Scholar]
- Borcard, D. Les Oribates des tourbières du Jura Suisse (Acari, Oribatei). Faunistique VII. Oribatuloidea (Haplozetidae). Ceratozetoidea. Mitt. Schweiz. Entomol. Ges. 1995, 68, 363–372. [Google Scholar]
- Irmler, U. Long-term fluctuation of the soil fauna (Collembola and Oribatida) at groundwater-near sites in an alder wood. Pedobiologia 2004, 48, 349–363. [Google Scholar] [CrossRef]
- Weigmann, G. Recovery of the oribatid mite community in a floodplain after decline due to long time inundation. In Acarine Biodiversity in the Natural and Human Sphere; Weigmann, G., Alberti, G., Wohltmann, A., Ragusa, S., Eds.; Proceedings of the V Symposium of the European Association of Acarologists, Montpellier, Berlin, 21–25 July 2004. Phytophaga 2004, 14, 201–208. [Google Scholar]
- Seniczak, A.; Seniczak, S.; Maraun, M.; Graczyk, R.; Mistrzak, M. Oribatid mite species numbers increase, densities decline and parthenogens suffer during bog degradation. Exp. Appl. Acarol. 2016, 68, 409–428. [Google Scholar] [CrossRef] [PubMed]
- Seniczak, A.; Seniczak, S.; Iturrondobeitia, J.C.; Solhøy, T.; Flatberg, K.I. Diverse Sphagnum mosses support rich moss mite communities (Acari, Oribatida) in mires of western Norway. Wetlands 2020, 40, 1339–1351. [Google Scholar] [CrossRef]
- Seniczak, A.; Seniczak, S.; Graczyk, R.; Kaczmarek, S.; Jordal, B.H.; Kowalski, J.; Djursvoll, P.; Roth, S.; Bolger, T. A forest pool as a habitat island for mites in a limestone forest in southern Norway. Diversity 2021, 13, 578. [Google Scholar] [CrossRef]
- Seniczak, A.; Seniczak, S.; Iturrondobeitia, J.C.; Gwiazdowicz, D.J.; Waldon-Rudzionek, B.; Flatberg, K.I.; Bolger, T. Mites (Oribatida and Mesostigmata) and vegetation as complementary bioindicators in peatlands. Sci. Total Environ. 2022, 851, 158335. [Google Scholar] [CrossRef] [PubMed]
- Solhøy, T. Dynamics of oribatei populations on Hardangervidda. In Fennoscandian Tundra Ecosystems, Part 2 Animals and Systems Analysis; Wielgolaski, F.E., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1975; pp. 60–65. [Google Scholar]
- Solhøy Wunderle, I.; Solhøy, T. The fossil oribatid mite fauna (Acari, Oribatida) in late glacial and early holocene sediments in Krakenes Lake, Western Norway. J. Paleolim. 2000, 23, 35–47. [Google Scholar] [CrossRef]
- Schatz, H. Biogeography of oribatid mites (Acari, Oribatida) from the Cordillera de Talamanca, Costa Rica and Panama. In Acarology XI: Proceedings of the International Congress; Morales-Malacara, J.B., Behan-Pelletier, V., Ueckermann, E., Pérez, T.M., Estrada, E., Gispert, C., Badii, M., Eds.; Instituto de Biología UNAM., Facultad de Ciencias, UNAM., Sociedad Latinoamericana de Acarología: Mexico City, Mexico, 2007; pp. 151–167. [Google Scholar]
- Voronova, L.D. The effect of some pesticides on the soil invertebrate fauna in the South Taiga Zone in the Perm Region (USSR). Pedobiologia 1968, 8, 507–525. [Google Scholar] [CrossRef]
- Sömme, L. Overwintering ecology of alpine collembola and oribatid mites from the Austrian Alps. Ecol. Entomol. 1979, 4, 175–180. [Google Scholar] [CrossRef]
- Wallwork, J.A. Notes on the feeding-behaviour of some forest soil Acarina. Oikos 1958, 9, 260–271. [Google Scholar] [CrossRef]
- Madge, D.S. The effects of lethal temperatures on oribatid mites. Acarologia 1965, 7, 121–130. [Google Scholar]
- Shaldybina, E.S. Cultivation of some oribatid mite species in laboratory conditions with the purpose of studying their life cycles. In The First All-Union Conference on Zooculture Problems, Moscow (Extended Abstracts); Academy of Sciences of the USSR: Moscow, Russia, 1986; pp. 275–277. (In Russian) [Google Scholar]
- Seniczak, A.; Solhøy, T.; Seniczak, S.; Riva-Caballero, A. Species composition and abundance of the oribatid fauna (Acari, Oribatida) at two lakes in the Fløyen area, Bergen, Norway. Biological Lett. 2010, 47, 11–19. [Google Scholar] [CrossRef]
- Popp, E. Semiaquatile Lebensräume (Bülten) in Hoch- und Niedermooren. 2 Teil. Die Milbenfauna. Int. Rev. Ges. Hydrobiol. 1962, 47, 533–579. [Google Scholar] [CrossRef]
- Vanschoenwinkel, B.; Gielen, S.; Seaman, M.; Brendonck, L. Any way the wind blows–Frequent wind dispersal drives species sorting in ephemeral aquatic communities. Oikos 2008, 117, 125–134. [Google Scholar] [CrossRef]
- Vanschoenwinkel, B.; Gielen, S.; Vandewaerde, H.; Seaman, M.; Brendonck, L. Relative importance of different dispersal vectors for small aquatic invertebrates in a rock pool metacommunity. Ecography 2008, 31, 567–577. [Google Scholar] [CrossRef]
- Vanschoenwinkel, B.; Gielen, S.; Seaman, M.; Brendonck, L. Wind mediated dispersal of freshwater invertebrates in a rock pool metacommunity: Differences in dispersal capacities and modes. Hydrobiologia 2009, 635, 363–372. [Google Scholar] [CrossRef]
- Lebedeva, N.V.; Krivolutsky, D.A. Birds spread soil microarthropods to Arctic Islands. Dokl. Biol. Sci. 2003, 391, 329–332. [Google Scholar] [CrossRef]
- Lebedeva, N.V.; Lebedev, V.D. Transport of oribatid mites to the polar areas by birds. In Integrative Acarology; Bertrand, M., Kreiter, S., McCoy, K.D., Migeon, A., Navajas, M., Tixier, M.S., Vial, L., Eds.; European Association of Acarologists: Montpellier, France, 2008; pp. 359–367. [Google Scholar]
- Lebedeva, N.V.; Lebedev, V.D.; Melekhina, E.N. New data on the oribatid mite (Oribatei) fauna of Svalbard. Dokl. Biol. Sci. 2006, 407, 182–186. [Google Scholar] [CrossRef]
- Lebedeva, N.V.; Melekhina, E.N.; Gwiazdowicz, D.J. New data on soil mites in the nests of Larus hyperboreus in the Spitsbergen archipelago. Vestn. Juzn. Naucn. Cent. Ran 2012, 8, 70–75. [Google Scholar]
- Coulson, S.J. Association of the soil mite Diapterobates notatus (Thorell, 1871) (Acari, Oribatidae) with Cynomya mortuorum (Linnaeus, 1761) (Calliphoridae, Calliphorinae): Implications for the dispersal of oribatid mites. Int. J. Acarol. 2009, 35, 175–177. [Google Scholar] [CrossRef]
- Schuppenhauer, M.M.; Lehmitz, R. Floating Islands: A method to detect aquatic dispersal and colonisation potential of soil microarthropods. Soil Org. 2017, 89, 119–126. [Google Scholar]
- Coulson, S.J.; Hodkinson, I.D.; Block, W.; Webb, N.R.; Harrison, J.A. Survival of terrestrial soil-dwelling arthropods on and in seawater: Implications for trans-oceanic dispersal. Funct. Ecol. 2002, 16, 353–356. [Google Scholar] [CrossRef]
- Norton, R.A. Observations on phoresy by oribatid mites (Acari: Oribatei). Internat. J. Acarol. 1980, 6, 121–129. [Google Scholar] [CrossRef]
- Coulson, S.J.; Moe, B.; Monson, F.; Gabrielsen, G.W. The invertebrate fauna of High Arctic seabird nests: The microarthropod community inhabiting nests on Spitsbergen, Svalbard. Polar Biol. 2009, 32, 1041–1046. [Google Scholar] [CrossRef]
- Waleckx, E.; Montalvo-Balam, T.J.; Pinzón-Canul, A.; Arnal, A.; Gerardo, M.; Martínez, P.A. First report of phoresy by an oribatid mite (Acari: Oribatida) on a triatomine bug (Hemiptera: Reduviidae). Int. J. Acarol. 2018, 44, 210–211. [Google Scholar] [CrossRef]
- Ermilov, S.G. Oribatid mites (Acari: Oribatida) phoretic on passalid beetles (Coleoptera: Passalidae), with description of a new species from Indonesia. Ecol. Montenegrina 2019, 22, 90–96. [Google Scholar] [CrossRef]
- Ermilov, S.G.; Frolov, A.V. New and interesting oribatid mites (Acari, Oribatida) phoretic on Aceraius grandis (Coleoptera, Passalidae) from Vietnam. Syst. Appl. Acarol. 2019, 24, 945–961. [Google Scholar] [CrossRef]
- Ermilov, S.G.; Frolov, A.V. New data on oribatid mites (Acari, Oribatida) phoretic on passalid beetles (Coleoptera, Passalidae) from the Afrotropical and Oriental regions, with descriptions of three new species from Congo, Gabon and Ghana. Syst. Appl. Acarol. 2021, 26, 769–787. [Google Scholar] [CrossRef]
- Beaty, L.E.; Esser, H.J.; Miranda, R.; Norton, R.A. First report of phoresy by an oribatid mite (Trhypochthoniidae: Archegozetes magnus) on a frog (Leptodactylidae: Engystomops pustulosus). Int. J. Acarol. 2013, 39, 325–326. [Google Scholar] [CrossRef]
- Heethoff, M.; Domes, K.; Laumann, M.; Maraun, M.; Norton, R.A.; Scheu, S. High genetic divergences indicate ancient separation of parthenogenetic lineages of the oribatid mite Platynothrus peltifer (Acari, Oribatida). J. Evol. Biol. 2007, 20, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Seniczak, S.; Seniczak, A.; Kaczmarek, S.; Marquardt, T.; Ondoño, E.F.; Coulson, S.J. Morphological ontogeny and ecology of Platynothrus punctatus (Acari, Oribatida, Camisiidae), with comments on Platynothrus Berlese. Syst. Appl. Acarol. 2022, 27, 551–580. [Google Scholar] [CrossRef]
- Seniczak, S.; Seniczak, A. Morphological ontogeny and ecology of a common peatland mite, Nanhermannia coronata (Acari, Oribatida, Nanhermanniidae). Animals 2023, 13, 3590. [Google Scholar] [CrossRef] [PubMed]
- Seniczak, A.; Seniczak, S.; Hassel, K.; Flatberg, K.I. Morphological ontogeny of Platynothrus troendelagicus sp. nov. (Acari, Oribatida, Camisiidae) from Norway. Syst. Appl. Acarol. 2022, 27, 1702–1722. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).