Effects of Environmental Enrichments on Welfare and Hepatic Metabolic Regulation of Broiler Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animal Housing
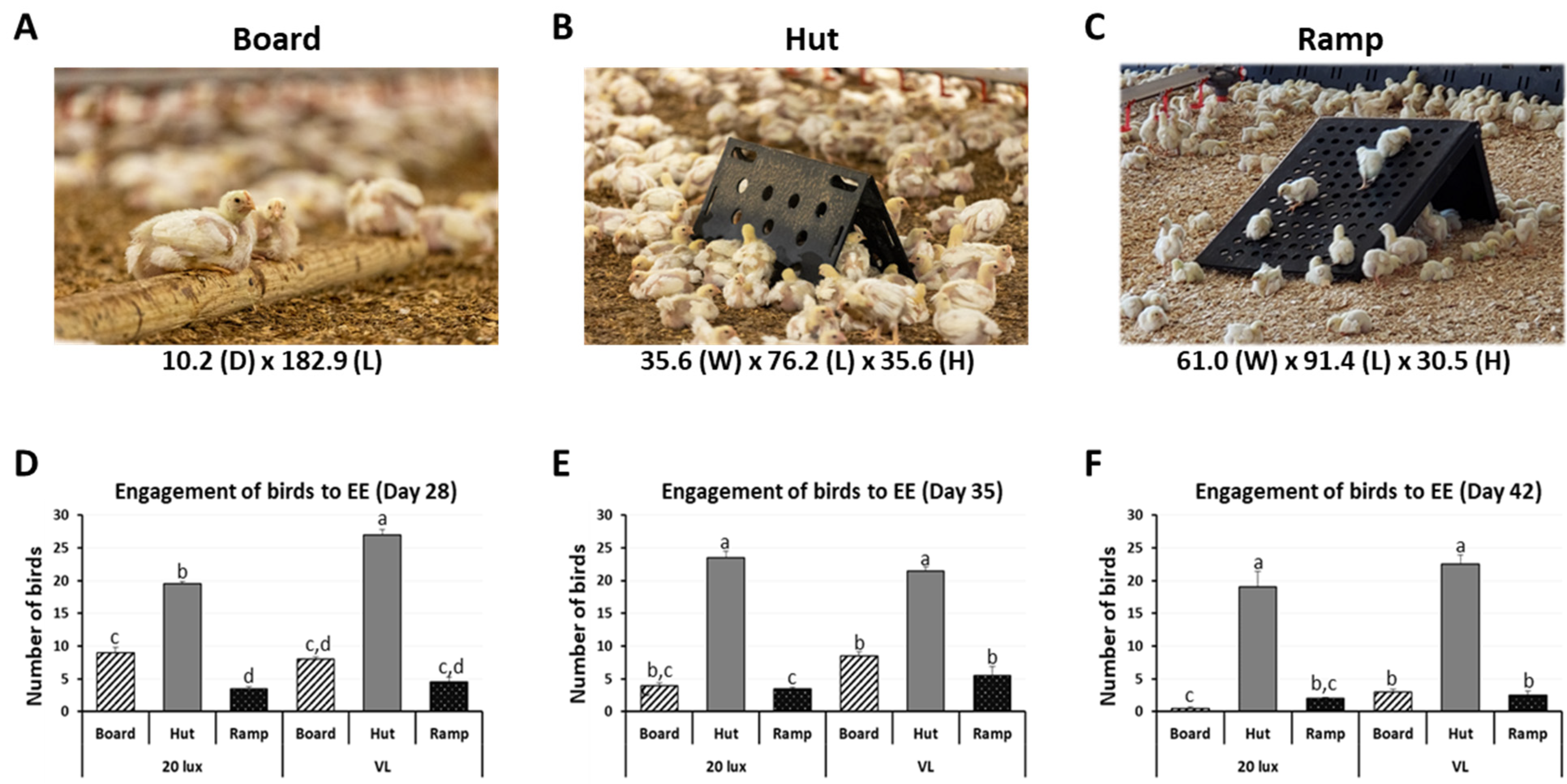
2.2. Behavioral Observations Affected by Enrichments
2.2.1. Number of Engaged Birds to Enrichments
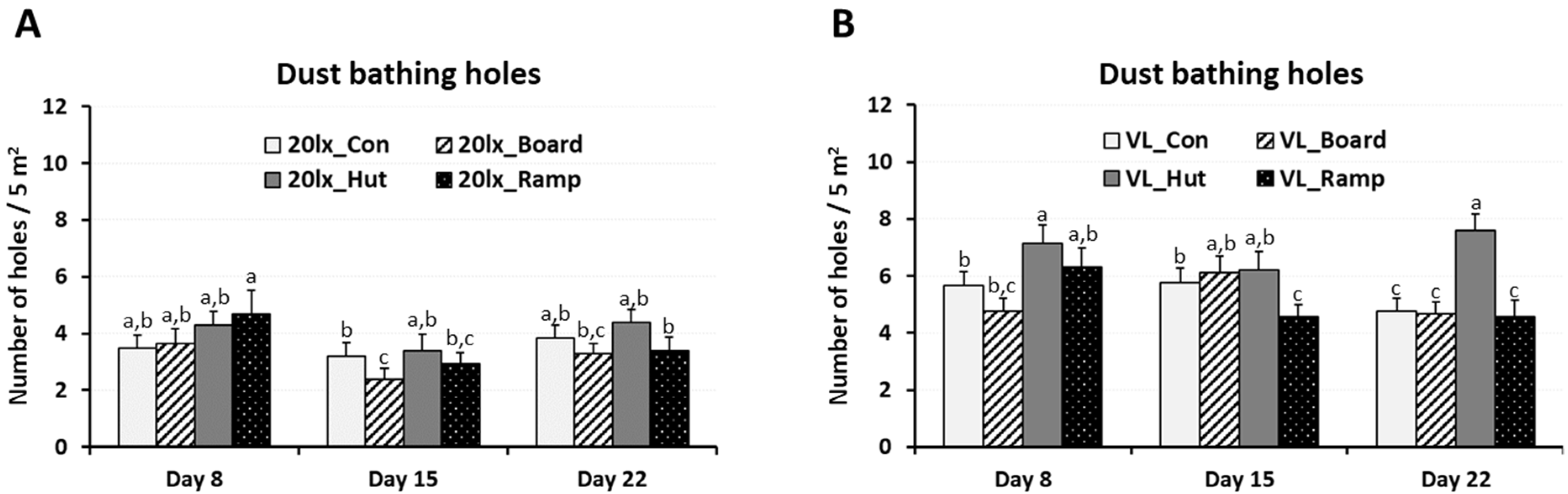
2.2.2. Dustbathing and Daily Physical Activity
2.3. Dissection of Ventral Tegmental Area (VTA) of Midbrain and Liver Sampling
2.4. RNA Isolation and Two-Step Real-Time Quantitative RT-PCR
2.5. Statistical Analyses
3. Results
3.1. Effects of Environmental Enrichments on Engagement, Dustbathing, and Daily Physical Activity of Broilers
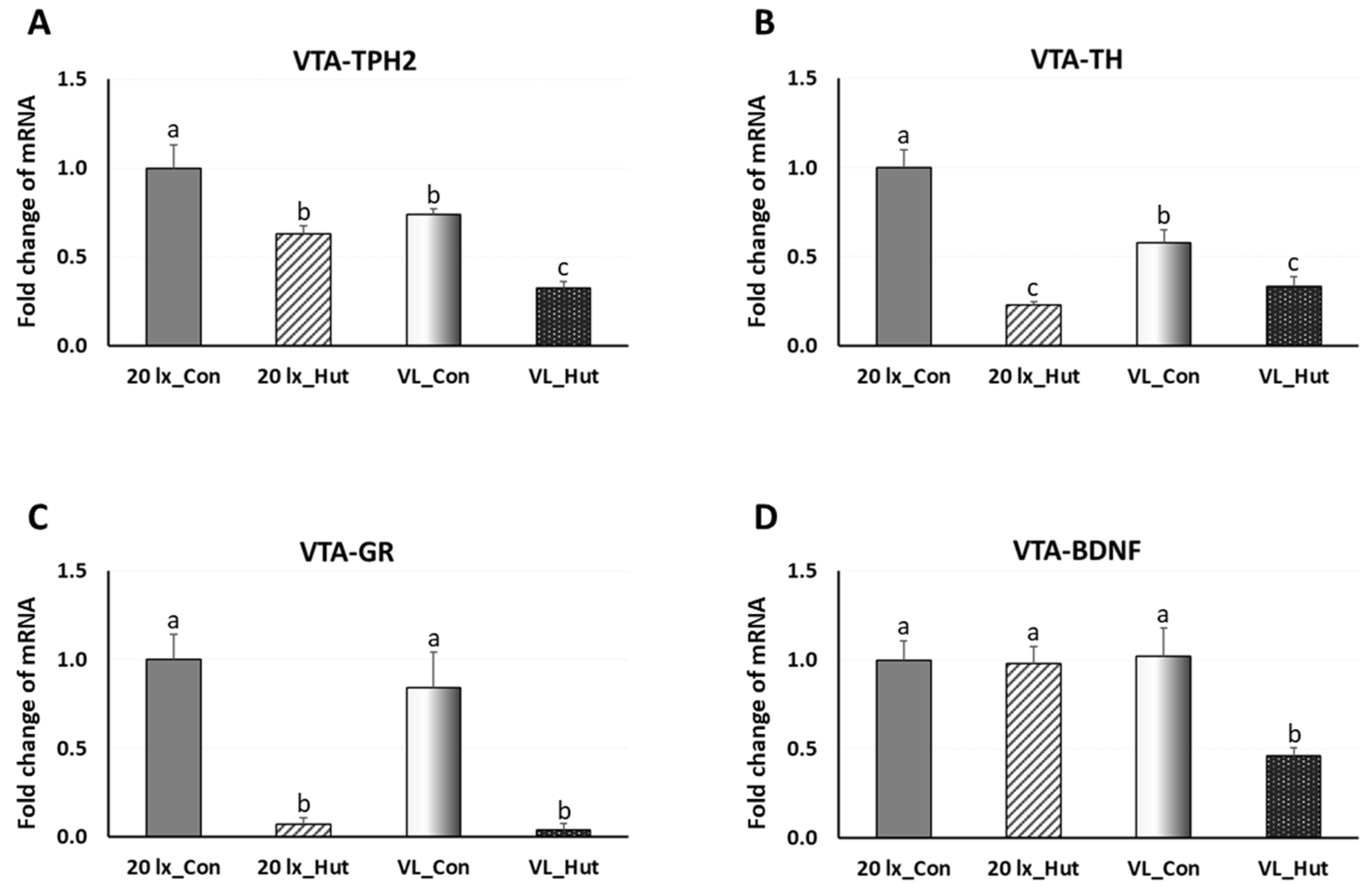
3.2. Effects of Light Programs and EHs on Regulation of Welfare Marker Genes in the Ventral Tegmental Area (VTA) in Commercial Broiler House
3.3. Combined Effects of Light Programs and EHs on Regulation of Hepatic Metabolic Pathway and Stress Response Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dal Bosco, A.; Mattioli, S.; Cartoni Mancinelli, A.; Cotozzolo, E.; Castellini, C. Extensive rearing systems in poultry production: The right chicken for the right farming system. A review of twenty years of scientific research in Perugia university, Italy. Animals 2021, 11, 1281. [Google Scholar] [CrossRef]
- Grzinic, G.; Piotrowicz-Cieslak, A.; Klimkowicz-Pawlas, A.; Gorny, R.L.; Lawniczek-Walczyk, A.; Piechowicz, L.; Olkowska, E.; Potrykus, M.; Tankiewicz, M.; Krupka, M.; et al. Intensive poultry farming: A review of the impact on the environment and human health. Sci. Total. Environ. 2023, 858, 160014. [Google Scholar] [CrossRef]
- Siviy, S.M.; Panksepp, J. In search of the neurobiological substrates for social playfulness in mammalian brains. Neurosci. Biobehav. Rev. 2011, 35, 1821–1830. [Google Scholar] [CrossRef]
- Jacobs, L.; Blatchford, R.A.; de Jong, I.C.; Erasmus, M.A.; Levengood, M.; Newberry, R.C.; Regmi, P.; Riber, A.B.; Weimer, S.L. Enhancing their quality of life: Environmental enrichment for poultry. Poult. Sci. 2023, 102, 102233. [Google Scholar] [CrossRef]
- Blatchford, R.A.; Archer, G.S.; Mench, J.A. Contrast in light intensity, rather than day length, influences the behavior and health of broiler chickens. Poult. Sci. 2012, 91, 1768–1774. [Google Scholar] [CrossRef]
- Olanrewaju, H.A.; Miller, W.W.; Maslin, W.R.; Collier, S.D.; Purswell, J.L.; Branton, S.L. Influence of light sources and photoperiod on growth performance, carcass characteristics, and health indices of broilers grown to heavy weights. Poult. Sci. 2018, 97, 1109–1116. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, J.; Quan, S.; Yang, Y. Light regimen on health and growth of broilers: An update review. Poult. Sci. 2022, 101, 101545. [Google Scholar] [CrossRef]
- Kang, S.W.; Christensen, K.D.; Aldridge, D.; Kuenzel, W.J. Effects of light intensity and dual light intensity choice on plasma corticosterone, central serotonergic and dopaminergic activities in birds, gallus gallus. Gen. Comp. Endocrinol. 2020, 285, 113289. [Google Scholar] [CrossRef] [PubMed]
- Raccoursier, M.; Thaxton, Y.V.; Christensen, K.; Aldridge, D.J.; Scanes, C.G. Light intensity preferences of broiler chickens: Implications for welfare. Animal 2019, 13, 2857–2863. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Christensen, K.D.; Kidd, M.T., Jr.; Orlowski, S.K.; Clark, J. Effects of a variable light intensity lighting program on the welfare and performance of commercial broiler chickens. Front. Physiol. 2023, 14, 1059055. [Google Scholar] [CrossRef] [PubMed]
- Riber, A.B.; van de Weerd, H.A.; de Jong, I.C.; Steenfeldt, S. Review of environmental enrichment for broiler chickens. Poult. Sci. 2018, 97, 378–396. [Google Scholar] [CrossRef]
- Coria-Avila, G.A.; Pfaus, J.G.; Orihuela, A.; Dominguez-Oliva, A.; Jose-Perez, N.; Hernandez, L.A.; Mota-Rojas, D. The neurobiology of behavior and its applicability for animal welfare: A review. Animals 2022, 12, 928. [Google Scholar] [CrossRef]
- Alcaro, A.; Panksepp, J. The seeking mind: Primal neuro-affective substrates for appetitive incentive states and their pathological dynamics in addictions and depression. Neurosci. Biobehav. Rev. 2011, 35, 1805–1820. [Google Scholar] [CrossRef]
- Derdeyn, P.; Hui, M.; Macchia, D.; Beier, K.T. Uncovering the connectivity logic of the ventral tegmental area. Front. Neural Circuits 2021, 15, 799688. [Google Scholar] [CrossRef]
- Geisler, C.E.; Hayes, M.R. Metabolic hormone action in the vta: Reward-directed behavior and mechanistic insights. Physiol. Behav. 2023, 268, 114236. [Google Scholar] [CrossRef]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- Rault, J.L.; Lawrence, A.J.; Ralph, C.R. Brain-derived neurotrophic factor in serum as an animal welfare indicator of environmental enrichment in pigs. Domest. Anim. Endocrinol. 2018, 65, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C. Brain-derived neurotrophic factor, depression, and physical activity: Making the neuroplastic connection. Neural Plast. 2017, 2017, 7260130. [Google Scholar] [CrossRef] [PubMed]
- Arosio, B.; Guerini, F.R.; Voshaar, R.C.O.; Aprahamian, I. Blood brain-derived neurotrophic factor (bdnf) and major depression: Do we have a translational perspective? Front. Behav. Neurosci. 2021, 15, 626906. [Google Scholar] [CrossRef] [PubMed]
- Hotting, K.; Roder, B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci. Biobehav. Rev. 2013, 37, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Ralph, C.R.; Tilbrook, A.J. Invited review: The usefulness of measuring glucocorticoids for assessing animal welfare. J. Anim. Sci. 2016, 94, 457–470. [Google Scholar] [CrossRef]
- Ferrario, C.R.; Labouebe, G.; Liu, S.; Nieh, E.H.; Routh, V.H.; Xu, S.; O’Connor, E.C. Homeostasis meets motivation in the battle to control food intake. J. Neurosci. 2016, 36, 11469–11481. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Banno, R.; Sun, R.; Yaginuma, H.; Taki, K.; Kobayashi, T.; Sugiyama, M.; Tsunekawa, T.; Onoue, T.; Takagi, H.; et al. Glucocorticoid receptor signaling in ventral tegmental area neurons increases the rewarding value of a high-fat diet in mice. Sci. Rep. 2021, 11, 12873. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W. Central nervous system associated with light perception and physiological responses of birds. Front. Physiol. 2021, 12, 723454. [Google Scholar] [CrossRef] [PubMed]
- Dumond Bourie, A.; Potier, J.B.; Pinget, M.; Bouzakri, K. Myokines: Crosstalk and consequences on liver physiopathology. Nutrients 2023, 15, 1729. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V. Aerobic exercise in the management of metabolic dysfunction associated fatty liver disease. Diabetes Metab. Syndr. Obes. 2021, 14, 3627–3645. [Google Scholar] [CrossRef] [PubMed]
- van der Windt, D.J.; Sud, V.; Zhang, H.; Tsung, A.; Huang, H. The effects of physical exercise on fatty liver disease. Gene Expr. 2018, 18, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Heald, P.J.; Badman, H.G. Lipid metabolism and the laying hen. I. Plasma-free fatty acids and the onset of laying in the domestic fowl. Biochim. Biophys. Acta 1963, 70, 381–388. [Google Scholar] [CrossRef]
- Leveille, G.A.; O’Hea, E.K.; Chakbabarty, K. In vivo lipogenesis in the domestic chicken. Proc. Soc. Exp. Biol. Med. 1968, 128, 398–401. [Google Scholar] [CrossRef]
- Hodson, L.; Gunn, P.J. The regulation of hepatic fatty acid synthesis and partitioning: The effect of nutritional state. Nat. Rev. Endocrinol. 2019, 15, 689–700. [Google Scholar] [CrossRef]
- Bechmann, L.P.; Hannivoort, R.A.; Gerken, G.; Hotamisligil, G.S.; Trauner, M.; Canbay, A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012, 56, 952–964. [Google Scholar] [CrossRef]
- Parhofer, K.G. Interaction between glucose and lipid metabolism: More than diabetic dyslipidemia. Diabetes Metab. J. 2015, 39, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Wondisford, F.E. Tracking the carbons supplying gluconeogenesis. J. Biol. Chem. 2020, 295, 14419–14429. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Tesseraud, S.; Simon, J. Insulin signaling in chicken liver and muscle. Gen. Comp. Endocrinol. 2009, 163, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Gorce, M.; Lebigot, E.; Arion, A.; Brassier, A.; Cano, A.; De Lonlay, P.; Feillet, F.; Gay, C.; Labarthe, F.; Nassogne, M.C.; et al. Fructose-1,6-bisphosphatase deficiency causes fatty liver disease and requires long-term hepatic follow-up. J. Inherit. Metab. Dis. 2022, 45, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Brust, K.B.; Corbell, K.A.; Al-Nakkash, L.; Babu, J.R.; Broderick, T.L. Expression of gluconeogenic enzymes and 11beta-hydroxysteroid dehydrogenase type 1 in liver of diabetic mice after acute exercise. Diabetes Metab. Syndr. Obes. 2014, 7, 495–504. [Google Scholar] [PubMed]
- Chapman, K.; Holmes, M.; Seckl, J. 11beta-hydroxysteroid dehydrogenases: Intracellular gate-keepers of tissue glucocorticoid action. Physiol. Rev. 2013, 93, 1139–1206. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Delgado, M.M.; Han, B.S.G.; Bain, M.J. Correction to: Domestic cats (Felis catus) prefer freely available food over food that requires effort. Anim. Cogn. 2022, 25, 493. [Google Scholar] [CrossRef] [PubMed]
- Kuenzel, W.J.; Masson, M. A Stereotaxic Atlas of the Brain of Chick (Gallus domesticus); Johns Hopkins University Press: Baltimore, MD, USA, 1988. [Google Scholar]
- Vestergaard, K.S.; Skadhauge, E.; Lawson, L.G. The stress of not being able to perform dustbathing in laying hens. Physiol. Behav. 1997, 62, 413–419. [Google Scholar] [CrossRef]
- Grandin, T. A practical approach to providing environmental enrichment to pigs and broiler chickens housed in intensive systems. Animals 2023, 13, 2372. [Google Scholar] [CrossRef]
- Alexander, R.; Aragon, O.R.; Bookwala, J.; Cherbuin, N.; Gatt, J.M.; Kahrilas, I.J.; Kastner, N.; Lawrence, A.; Lowe, L.; Morrison, R.G.; et al. The neuroscience of positive emotions and affect: Implications for cultivating happiness and wellbeing. Neurosci. Biobehav. Rev. 2021, 121, 220–249. [Google Scholar] [CrossRef] [PubMed]
- Marcet Rius, M.; Cozzi, A.; Bienboire-Frosini, C.; Teruel, E.; Chabaud, C.; Monneret, P.; Leclercq, J.; Lafont-Lecuelle, C.; Pageat, P. Providing straw to allow exploratory behaviour in a pig experimental system does not modify putative indicators of positive welfare: Peripheral oxytocin and serotonin. Animal 2018, 12, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Carkaci-Salli, N.; Salli, U.; Kuntz-Melcavage, K.L.; Pennock, M.M.; Ozgen, H.; Tekin, I.; Freeman, W.M.; Vrana, K.E. Tph2 in the ventral tegmental area of the male rat brain. Brain Res. Bull. 2011, 84, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, D.; Walsh, J.J.; Friedman, A.K.; Juarez, B.; Ku, S.M.; Koo, J.W.; Ferguson, D.; Tsai, H.C.; Pomeranz, L.; Christoffel, D.J.; et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 2013, 493, 532–536. [Google Scholar] [CrossRef]
- Bizeray, D.; Estevez, I.; Leterrier, C.; Faure, J.M. Influence of increased environmental complexity on leg condition, performance, and level of fearfulness in broilers. Poult. Sci. 2002, 81, 767–773. [Google Scholar] [CrossRef]
- Kristensen, H.H.; Aerts, J.M.; LeRoy, T.; Berckmans, D.; Wathes, C.M. Using light to control activity in broiler chickens. Br. Poult. Sci. 2004, 45, S30–S31. [Google Scholar] [CrossRef]
- Reiter, K.; Bessei, W. Effect of locomotor activity on leg disorder in fattening chicken. Berl. Munch. Tierarztl. Wochenschr. 2009, 122, 264–270. [Google Scholar]
- Berton, O.; McClung, C.A.; Dileone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rios, M.; et al. Essential role of bdnf in the mesolimbic dopamine pathway in social defeat stress. Science 2006, 311, 864–868. [Google Scholar] [CrossRef]
- Krishnan, V.; Han, M.H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; Laplant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef]
- Sikka, G.; Hussmann, G.P.; Pandey, D.; Cao, S.; Hori, D.; Park, J.T.; Steppan, J.; Kim, J.H.; Barodka, V.; Myers, A.C.; et al. Melanopsin mediates light-dependent relaxation in blood vessels. Proc. Natl. Acad. Sci. USA 2014, 111, 17977–17982. [Google Scholar] [CrossRef]
- Hermier, D.; Chapman, M.J.; Leclercq, B. Plasma lipoprotein profile in fasted and refed chickens of two strains selected for high or low adiposity. J. Nutr. 1984, 114, 1112–1121. [Google Scholar] [CrossRef]
- Leveille, G.A.; Romsos, D.R.; Yeh, Y.; O’Hea, E.K. Lipid biosynthesis in the chick. A consideration of site of synthesis, influence of diet and possible regulatory mechanisms. Poult. Sci. 1975, 54, 1075–1093. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.; Liu, R.; Zhao, G.; Zhang, Y.; Zheng, M.; Cui, H.; Li, P.; Cui, X.; Liu, J.; et al. Expression and methylation of microsomal triglyceride transfer protein and acetyl-coa carboxylase are associated with fatty liver syndrome in chicken. Poult. Sci. 2016, 95, 1387–1395. [Google Scholar] [CrossRef]
- Zhang, C.; Meng, S.; Li, C.; Yang, Z.; Wang, G.; Wang, X.; Ma, Y. Primary broiler hepatocytes for establishment of a steatosis model. Vet. Sci. 2022, 9, 316. [Google Scholar] [CrossRef]
- Meng, J.; Ma, N.; Liu, H.; Liu, J.; Liu, J.; Wang, J.; He, X.; Zhao, X. Untargeted and targeted metabolomics profiling reveals the underlying pathogenesis and abnormal arachidonic acid metabolism in laying hens with fatty liver hemorrhagic syndrome. Poult. Sci. 2021, 100, 101320. [Google Scholar] [CrossRef]
- Houten, S.M.; Violante, S.; Ventura, F.V.; Wanders, R.J. The biochemistry and physiology of mitochondrial fatty acid beta-oxidation and its genetic disorders. Annu. Rev. Physiol. 2016, 78, 23–44. [Google Scholar] [CrossRef]
- Schlaepfer, I.R.; Joshi, M. Cpt1a-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology 2020, 161, bqz046. [Google Scholar] [CrossRef]
- Liang, K. Mitochondrial cpt1a: Insights into structure, function, and basis for drug development. Front. Pharmacol. 2023, 14, 1160440. [Google Scholar] [CrossRef]
- Wang, M.D.; Wu, H.; Fu, G.B.; Zhang, H.L.; Zhou, X.; Tang, L.; Dong, L.W.; Qin, C.J.; Huang, S.; Zhao, L.H.; et al. Acetyl-coenzyme a carboxylase alpha promotion of glucose-mediated fatty acid synthesis enhances survival of hepatocellular carcinoma in mice and patients. Hepatology 2016, 63, 1272–1286. [Google Scholar] [CrossRef]
- Kalemba, K.M.; Wang, Y.; Xu, H.; Chiles, E.; McMillin, S.M.; Kwon, H.; Su, X.; Wondisford, F.E. Glycerol induces g6pc in primary mouse hepatocytes and is the preferred substrate for gluconeogenesis both in vitro and in vivo. J. Biol. Chem. 2019, 294, 18017–18028. [Google Scholar] [CrossRef]
- Whorwood, C.B.; Donovan, S.J.; Flanagan, D.; Phillips, D.I.; Byrne, C.D. Increased glucocorticoid receptor expression in human skeletal muscle cells may contribute to the pathogenesis of the metabolic syndrome. Diabetes 2002, 51, 1066–1075. [Google Scholar] [CrossRef]
- Kang, S.W.; Madkour, M.; Kuenzel, W.J. Tissue-specific expression of DNA methyltransferases involved in early-life nutritional stress of chicken, gallus gallus. Front. Genet. 2017, 8, 204. [Google Scholar] [CrossRef]
- Lattin, C.R.; Romero, L.M. Chronic stress alters concentrations of corticosterone receptors in a tissue-specific manner in wild house sparrows (Passer domesticus). J. Exp. Biol. 2014, 217, 2601–2608. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.A.; McCabe, E.L.; Gathercole, L.L.; Hassan-Smith, Z.K.; Larner, D.P.; Bujalska, I.J.; Stewart, P.M.; Tomlinson, J.W.; Lavery, G.G. 11beta-hsd1 is the major regulator of the tissue-specific effects of circulating glucocorticoid excess. Proc. Natl. Acad. Sci. USA 2014, 111, E2482–E2491. [Google Scholar] [CrossRef] [PubMed]
- Paterson, J.M.; Morton, N.M.; Fievet, C.; Kenyon, C.J.; Holmes, M.C.; Staels, B.; Seckl, J.R.; Mullins, J.J. Metabolic syndrome without obesity: Hepatic overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in transgenic mice. Proc. Natl. Acad. Sci. USA 2004, 101, 7088–7093. [Google Scholar] [CrossRef] [PubMed]



| Gene | GenBank # | Primer Sequences (5′-3′) | Size (bp) | Annealing Tm (°C) |
|---|---|---|---|---|
| TPH2 | NM_001001301.1 | F: AGGACCTCCGCAGTGATCTA R: CAGCATAAGCAGCTGACAACA | 111 | 58 |
| TH | NM_204805 | F: CTTTGATCCTGATGCTGCTG R: CCTCAGCTTGTTTTTGGCAT | 103 | 56 |
| GR | NM_001037826 | F: GCCATCGTGAAAAGAGAAGG R: TTTCAACCACATCGTGCAT | 95 | 54 |
| BDNF | NM_001031616 | F: GACATGGCAGCTTGGCTTAC R: GTTTTCCTCACTGGGCTGGA | 167 | 60 |
| CPT1A | NM_001012898.1 | F: GTGGCTGATGATGGTTACGGT R: CCCATGATGTCAACCAATGCT | 146 | 58 |
| ACCα | NM_205505.1 | F: GTTGCCATGGATTCGATCGTG R: GGAGTACAGGAAATCGATGCT | 128 | 58 |
| FBPase | AJ276212 | F: TTCCATTGGGACCATATTTGG R: ACCCGCTGCCACAAGATTAC | 100 | 58 |
| 11β-HSD1 | XM_417988 | F: CTGGGAACTGTCTGCACAAC R: GATTGCGAGGAACCATTTACAG | 96 | 56 |
| Opn4 | AY036061 | F: AAGGTTTCGCTGTCATCCAGC R: CTGCTGCTGTTCAAACCAAC | 128 | 58 |
| GAPDH | NM_204305 | F: CTTTGGCATTGTGGAGGGTC R: ACGCTGGGATGATGTTCTGG | 128 | 58–60 |
| β-actin | L08165 | F: CACAATGTACCCTGGCATTG R: ACATCTGCTGGAAGGTGGAC | 158 | 54–56 |
| 18S | AF173612 | F: TCCCCTCCCGTTACTTGGAT R: GCGCTCGTCGGCATGTA | 60 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.W.; Christensen, K.D.; Jr., M.T.K.; Orlowski, S.K. Effects of Environmental Enrichments on Welfare and Hepatic Metabolic Regulation of Broiler Chickens. Animals 2024, 14, 557. https://doi.org/10.3390/ani14040557
Kang SW, Christensen KD, Jr. MTK, Orlowski SK. Effects of Environmental Enrichments on Welfare and Hepatic Metabolic Regulation of Broiler Chickens. Animals. 2024; 14(4):557. https://doi.org/10.3390/ani14040557
Chicago/Turabian StyleKang, Seong W., Karen D. Christensen, Michael T. Kidd Jr., and Sara K. Orlowski. 2024. "Effects of Environmental Enrichments on Welfare and Hepatic Metabolic Regulation of Broiler Chickens" Animals 14, no. 4: 557. https://doi.org/10.3390/ani14040557
APA StyleKang, S. W., Christensen, K. D., Jr., M. T. K., & Orlowski, S. K. (2024). Effects of Environmental Enrichments on Welfare and Hepatic Metabolic Regulation of Broiler Chickens. Animals, 14(4), 557. https://doi.org/10.3390/ani14040557





