First Report of Caseous Lymphadenitis by Corynebacterium pseudotubercolosis and Pulmonary Verminosis in a Roe Deer (Capreolus capreolus Linnaeus, 1758) in Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Animal Identification
3.2. Gross Pathology
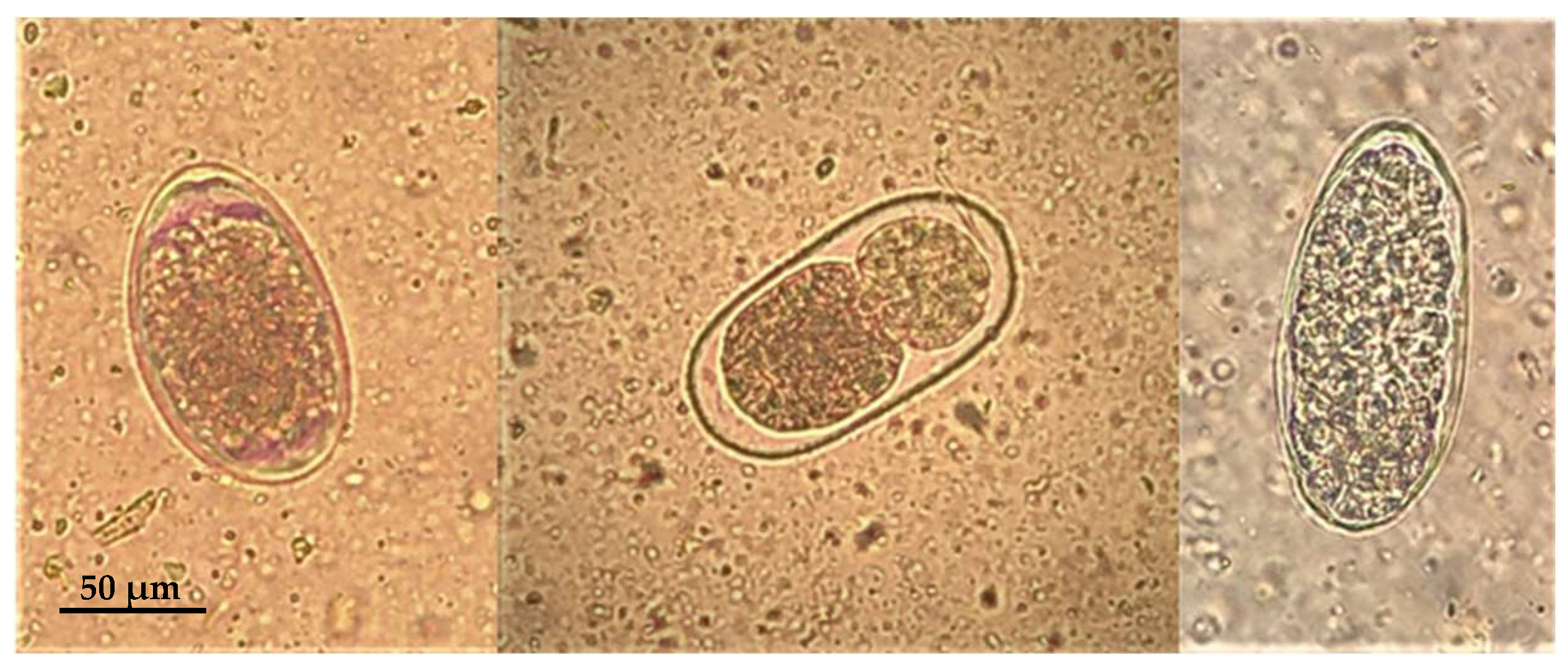
3.3. Microscopic Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pugh, D.G.; Baird, A.N.; Edmondson, M.A.; Passler, T. Diseases of the Integumentary System. In Sheep, Goat and Cervid Medicine, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 230–231. [Google Scholar]
- Gavier-Widén, D.; Duff, J.P.; Meredith, A. Other Bacterial Infections—Corynebacterium infections. In Infectious Diseases of Wild Mammals and Birds in Europe; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2012; pp. 438–439. [Google Scholar]
- Salam, N.; Jiao, J.Y.; Zhang, X.T.; Li, W.J. Update on the classification of higher ranks in the phylum Actinobacteria. Int. J. Syst. Evol. Microbiol. 2020, 70, 1331–1355. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.; Möller, J.; Burkovski, A.; Hotzel, H. Genome sequence of a pathogenic Corynebacterium ulcerans strain isolated from a wild boar with necrotizing lymphadenitis. BMC Res. Notes 2019, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.; Blazey, B.; Contzen, M.; Sting, R. Corynebacterium ulcerans infection in roe deer (Capreolus capreolus). Berl. Munch. Tierarztl. Wochenschr. 2012, 125, 159–162. [Google Scholar] [PubMed]
- Dorella, F.A.; Pacheco, L.G.C.; Oliveira, S.C.; Miyoshi, A.; Azevedo, V. Corynebacterium pseudotuberculosis: Microbiology, biochemical properties, pathogenesis and molecular studies of virulence. Vet. Res. 2006, 37, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Rzewuska, M.; Kwiecień, E.; Chrobak-Chmiel, D.; Kizerwetter-Świda, M.; Stefańska, I.; Gieryńska, M. Pathogenicity and Virulence of Trueperella pyogenes: A Review. Int. J. Mol. Sci. 2019, 20, 2737. [Google Scholar] [CrossRef]
- Baird, G.J.; Fontainey, M.C. Corynebacterium pseudotuberculosis and its Role in Ovine Caseous Lymphadenitis. J. Comp. Pathol. 2007, 137, 179–210. [Google Scholar] [CrossRef]
- De Pinho, R.B.; De Oliveira Silva, M.T.; Bezerra, F.S.B.; Borsuk, S. Vaccines for caseous lymphadenitis: Up-to-date and forward-looking strategies. Appl. Microbiol. Biotechnol. 2021, 105, 2287–2296. [Google Scholar] [CrossRef]
- Abebe, D.; Tessema, T.S. Determination of Corynebacterium pseudotuberculosis prevalence and antimicrobial susceptibility pattern of isolates from lymph nodes of sheep and goats at an organic export abattoir, Modjo, Ethiopia. Lett. Appl. Microbiol. 2015, 61, 469–476. [Google Scholar] [CrossRef]
- Join-Lambert, O.F.; Ouache, M.; Canioni, D.; Beretti, J.L.; Blanche, S.; Berche, P.; Kayal, S. Corynebacterium pseudotuberculosis necrotizing lymphadenitis in a twelve-year-old patient. Pediatr. Infect. Dis. J. 2006, 25, 848–851. [Google Scholar] [CrossRef]
- Boschert, V.; Berger, A.; Konrad, R.; Huber, I.; Hörmansdorfer, S.; Zöls, S.; Eddicks, M.; Ritzmann, M.; Sing, A. Corynebacterium species nasal carriage in pigs and their farmers in Bavaria, Germany: Implications for public health. Vet. Rec. 2014, 175, 148. [Google Scholar] [CrossRef]
- Heggelund, L.; Gaustad, P.; Håvelsrud, O.E.; Blom, J.; Borgen, L.; Sundset, A.; Sørum, H.; Frøland, S.S. Corynebacterium pseudotuberculosis Pneumonia in a Veterinary Student Infected During Laboratory Work. Open Forum. Infect. Dis. 2015, 15, ofv053. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.J.; Dorneles, E.M.S.; Spier, S.J.; Carroll, S.P.; Edman, J.; Azevedo, V.A.; Heinemann, M.B.; Lage, A.P. Molecular epidemiology of Corynebacterium pseudotuberculosis isolated from horses in California. Infect. Genet. Evol. 2016, 49, 1348–1567. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Vyas, S.; Kashnitah, J.; Sonawane, G.G.; Patil, N.V. Lymphadenitis Caused by Corynebacterium pseudotuberculosis in a Dromedary (Camelus dromedarius) Herd. J. Camel Pract. Res. 2018, 25, 45–48. [Google Scholar] [CrossRef]
- Terab, A.M.A.; Wahab, G.E.D.A.; Ishag, H.Z.A.; Khalil, N.A.H.; El Tigani-Asil, E.T.A.; Hashem, F.M.; Khalafalla, A.I.; Shah, A.A.M.; Al Muhairi, S.S.M. Pathology, bacteriology and molecular studies on caseous lymphadenitis in Camelus dromedarius in the Emirate of Abu Dhabi, UAE, 2015–2020. PLoS ONE 2021, 16, e0252893. [Google Scholar] [CrossRef] [PubMed]
- Shawaf, T.; Hussen, J. Cytological and microbiological evaluation of conjunctiva in camels with and without conjunctivitis. Vet. Ophthalmol. 2022, 26, 39–45. [Google Scholar] [CrossRef]
- Perveen, N.; Muzaffar, S.B.; Vijayan, R.; Al-Deeb, M.A. Assessing Temporal Changes in Microbial Communities in Hyalomma dromedarii Collected from Camels in the UAE Using High-Throughput Sequencing. Front. Vet. Sci. 2022, 9, e861233. [Google Scholar] [CrossRef]
- Oliveira, M.; Barroco, C.; Mottola, C.; Santos, R.; Lemsaddek, A.; Tavares, L.; Semedo-Lemsaddek, T. First report of Corynebacterium pseudotuberculosis from caseous lymphadenitis lesions in Black Alentejano pig (Sus scrofa domesticus). BMC Vet. Res. 2014, 10, 218. [Google Scholar] [CrossRef]
- Contzen, M.; Sting, R.; Blazey, B.; Rau, J. Corynebacterium ulcerans from Diseased Wild Boars. Zoonoses Public Health 2011, 58, 479–488. [Google Scholar] [CrossRef]
- Eisenberg, T.; Kutzer, P.; Peters, M.; Sing, A.; Contzen, M.; Rau, J. Nontoxigenic tox-bearing Corynebacterium ulcerans Infection among Game Animals, Germany. Emerg. Infect. Dis. 2014, 20, 448–452. [Google Scholar] [CrossRef]
- Dangel, A.; Berger, A.; Rau, J.; Eisenberg, T.; Kämpfer, P.; Margos, G.; Contzen, M.; Busse, H.-J.; Konrad, R.; Peters, M.; et al. Corynebacterium silvaticum sp. nov., a unique group of NTTB corynebacteria in wild boar and roe deer. Int. J. Syst. Evol. Microbiol. 2020, 70, 3614–3624. [Google Scholar] [CrossRef]
- Viana, M.; Profeta, R.; Da Silva, A.; Hurtado, R.; Cerqueira, J.; Ribeiro, B.; Almeida, M.; Morais-Rodrigues, F.; Soares, S.; Oliveira, M.; et al. Taxonomic classification of strain PO100/5 shows a broader geographic distribution and genetic markers of the recently described Corynebacterium silvaticum. PLoS ONE 2020, 15, e0244210. [Google Scholar] [CrossRef]
- Möller, J.; Musella, L.; Melnikov, V.; Rfer, W.G.Ö.; Burkovski, A.; Sangal, V. Phylogenomic characterisation of a novel corynebacterial species pathogenic to animals. Antonie Van Leeuwenhoek 2020, 113, 1225–1239. [Google Scholar] [CrossRef] [PubMed]
- Terio, K.A.; Mcaloose, D.; Leger, J.S. Pathology of Wildlife and Zoo Animals; Elsevier: Amsterdam, The Netherlands, 2018; 1067p. [Google Scholar]
- Varela-Castro, L.; Lara-Vergara, J.; Ortega, N.; Salinas, J.; Colom-Cadena, A.; Lavín, S.; Tizzani, P.; Velarde, R.; Serrano, E.; Mentaberre, G. Endemic caseous lymphadenitis in a wild Caprinae population. Vet. Rec. 2017, 180, 405. [Google Scholar] [CrossRef] [PubMed]
- Domenis, L.; Spedicato, R.; Orusa, R.; Goria, M.; Sant, S.; Guidetti, C.; Robetto, S. The diagnostic activity on wild animals through the description of a model case report (caseous lymphadenitis by Corynebacterium pseudotuberculosis associated with Pasteurella spp and parasites infection in an alpine ibex—Capra ibex). Open Vet. J. 2017, 7, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Domenis, L.; Spedicato, R.; Pepe, E.; Orusa, R.; Robetto, S. Caseous Lymphadenitis Caused by Corynebacterium pseudotuberculosis in Alpine Chamois (Rupicapra r. rupicapra): A Review of 98 Cases. J. Comp. Pathol. 2018, 161, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Stauber, E.; Armstrong, P.; Chamberlain, K. Caseous lymphadenitis in a white-tailed deer. J. Wildl. Dis. 1973, 9, 56–57. [Google Scholar] [CrossRef]
- Kelly, E.J.; Rood, K.A.; Skirpstunas, R. Abscesses in Captive Elk Associated with Corynebacterium pseudotuberculosis, Utah, USA. J. Wildl. Dis. 2012, 48, 803–805. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.C.; Dias, A.P.; Morais, M.; Figueira, L.; Martins, M.H.; Matos, M.; Pinto, M.L.; Coelho, A.C. Granuloma Coinfection with Mycobacterium bovis, Mycobacterium avium subsp. paratuberculosis, and Corynebacterium pseudotuberculosis in Five Hunted Red deer (Cervus elaphus) in Portugal. J. Wildl. Dis. 2015, 51, 793–794. [Google Scholar] [CrossRef]
- Morales, N.; Aldridge, D.; Bahamonde, A.; Cerda, J.; Araya, C.; Muñoz, R.; Saldías, M.; Lecocq, C.; Fresno, M.; Abalos, P.; et al. Corynebacterium pseudotuberculosis Infection in Patagonian Huemul (Hippocamelus bisulcus). J. Wildl. Dis. 2017, 53, 621–624. [Google Scholar] [CrossRef]
- Hussain, R.; Khaliq, S.A.; Siddique, A.B.; Khan, I.A.; Hassan, M.F.; Younus, M. Clinico-Pathological and Bacteriological Studies on Caseous Lymphadenitis in Small Ruminants. Pak. J. Agric. Sci. 2017, 54, 437–442. [Google Scholar]
- Pewsner, M.; Origgi, F.C.; Frey, J.; Ryser-Degiorgis, M.P. Assessing Fifty Years of General Health Surveillance of Roe Deer in Switzerland: A Retrospective Analysis of Necropsy Reports. PLoS ONE 2017, 12, e0170338. [Google Scholar] [CrossRef]
- Blackalu, P.; Angen, Ø.; Fegan, N.; Blackall, L.; Mutters, R.; Bisgaard, M. Characterisation of a novel Mannheimia sp from Australian feedlot cattle. Aust. Vet. J. 2001, 79, 634–639. [Google Scholar] [CrossRef]
- Bojesen, A.M.; Larsen, J.; Pedersen, A.G.; Mörner, T.; Mattson, R.; Bisgaard, M. Identification of a novel Mannheimia granulomatis lineage from lesions in roe deer (Capreolus capreolus). J. Wildl. Dis. 2007, 43, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Keel, M.K.; Keeler, S.; Brown, J.; Fenton, H.; Munk, B.; Gerhold, R.; Gottdenker, N.; Ruder, M.; Elsmo, E.; Nemeth, N. Granulomatous Inflammation of the Muzzle in White-Tailed Deer (Odocoileus virginianus) and Mule Deer (Odocoileus hemionus) Associated with Mannheimia granulomatis. Vet. Pathol. 2020, 57, 838–844. [Google Scholar] [CrossRef]
- Britton, A.P.; Redford, T.; Zabek, E.; Sojonky, K.R.; Scouras, A.P.; Lewis, D.; Joseph, T. Bronchopneumonia associated with Mannheimia granulomatis infection in a Belgian hare (Lepus europaeus). J. Vet. Diagn. Investig. 2017, 29, 566–569. [Google Scholar] [CrossRef]
- Carnevali, L.; Pedrotti, L.; Riga, F.; Toso, S. Banca Dati Ungulati: Status, distribuzione, consistenza, gestione e prelievo venatorio delle popolazioni di Ungulati in Italia. Biol. Cons. Fauna 2009, 117, 1–168. [Google Scholar]
- Burbaitè, L.; Csànyi, S. Roe deer population and harvest changes in Europe. Est. J. Ecol. 2009, 58, 169–180. [Google Scholar] [CrossRef]
- Ratcliffe, P.R.; Mayle, B. Roe Deer Biology and Management; Forestry Commission Bulletin: London, UK, 1992; p. 105. [Google Scholar]
- King, J.M.; Roth-Johnson, L.; Dodd, D.C.; Newsom, M.E. The Necropsy Book. A Guide for Veterinary Students, Residents, Clinicians, Pathologists and Biological Researchers; Mansbridge, B., Ed.; The Internet-First University Press: Ithaca, NY, USA, 2013. [Google Scholar]
- ISO 11133:2014; Microbiology of Food, Animal Feed and Water. Preparation, Production, Storage and Performance Testing of Culture Media. ISO: Geneva, Switzerland, 2014.
- CLSI Guideline M45: Methods for Antimicrobial Diluition and Disk Susceptibility Testing of Infrequently Isolated or Fastidius Bacteria, 3rd ed.; CLSI: Wayne, PA, USA, 2015.
- Müller, B.; De Klerk-Lorist, L.-M.; Henton, M.M.; Lane, E.; Parsons, S.; Van Pittius, N.C.G.; Kotze, A.; Van Helden, P.D.; Tanner, M. Mixed infections of Corynebacterium pseudotuberculosis and non-tuberculous mycobacteria in South African antelopes presenting with tuberculosis-like lesions. Vet. Microbiol. 2011, 147, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Mustoni, A.; Pedrotti, L.; Zanon, E.; Tosi, G. Ungulati delle Alpi. Biologia, Riconoscimento, Gestione; Nitida Immagine Editrice: Cles, Italy, 2003; p. 560. [Google Scholar]
- Panadero, R.; Carrillo, E.B.; López, C.; Díez-Baños, N.; Díez-Baños, P.; Morrondo, M.P. Bronchopulmonary helminths of roe deer (Capreolus capreolus) in the northwest of Spain. Vet. Parasitol. 2001, 99, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Bolukbas, C.S.; Gurler, A.T.; Beyhan, Y.E.; Acici, M.; Umur, S. Helminths of roe deer (Capreolus capreolus) in the Middle Black Sea Region of Turkey. Parasitol. Int. 2012, 61, 729–730. [Google Scholar] [CrossRef]
- Hora, F.S.; Genchi, C.; Ferrari, N.; Morariu, S.; Mederle, N.; Dărăbuș, G. Frequency of gastrointestinal and pulmonary helminth infections in wild deer from western Romania. Vet. Parasitol. Reg. Stud. Rep. 2017, 8, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Irvine, R.J.; Corbishley, H.; Pilkington, J.G.; Albon, S.D. Low-level parasitic worm burdens may reduce body condition in free-ranging red deer (Cervus elaphus). Parasitology 2006, 133, 465–475. [Google Scholar] [CrossRef] [PubMed]


| Study | Cervid Species | Animals Involved (N°) | Corynebacterium Species and Identification Method | Organs/Regions Affected |
|---|---|---|---|---|
| Staber 1973 [29] | White-tailed Deer (Odocoileus virginianus) | 1 | C. pseudotuberculosis Culture | Thoracic cavity |
| Kelly 2012 [30] | Rocky Mountain Elk (Cervus canadensis nelsoni) | 2 | C. pseudotuberculosis Culture | Head abscess |
| Matos 2014 [31] | Red Deer (Cervus elaphus) | 5 | C. pseudotuberculosis PCR | Mesenteric lymph nodes |
| Hussain 2017 [33] | Spotted Deer (Axis axis) | 3 | C. pseudotuberculosis PCR | Not defined |
| Morales 2017 [32] | Patagonian Huemul (Hippocamelus bisulcus) | 2 | C. pseudotuberculosis culture | Cutaneous abscess |
| Pewsner 2017 [34] | Roe deer | 1 | C. pseudotuberculosis culture | Systemic |
| Dangel 2020 [22] | Roe deer | 1 | C. sylvaticum | Cutaneous abscess |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Donato, A.; Gambi, L.; Ravaioli, V.; Perulli, S.; Cirasella, L.; Rossini, R.; Luppi, A.; Tosi, G.; Fiorentini, L. First Report of Caseous Lymphadenitis by Corynebacterium pseudotubercolosis and Pulmonary Verminosis in a Roe Deer (Capreolus capreolus Linnaeus, 1758) in Italy. Animals 2024, 14, 566. https://doi.org/10.3390/ani14040566
Di Donato A, Gambi L, Ravaioli V, Perulli S, Cirasella L, Rossini R, Luppi A, Tosi G, Fiorentini L. First Report of Caseous Lymphadenitis by Corynebacterium pseudotubercolosis and Pulmonary Verminosis in a Roe Deer (Capreolus capreolus Linnaeus, 1758) in Italy. Animals. 2024; 14(4):566. https://doi.org/10.3390/ani14040566
Chicago/Turabian StyleDi Donato, Alessandra, Lorenzo Gambi, Valentina Ravaioli, Simona Perulli, Letizia Cirasella, Rachele Rossini, Andrea Luppi, Giovanni Tosi, and Laura Fiorentini. 2024. "First Report of Caseous Lymphadenitis by Corynebacterium pseudotubercolosis and Pulmonary Verminosis in a Roe Deer (Capreolus capreolus Linnaeus, 1758) in Italy" Animals 14, no. 4: 566. https://doi.org/10.3390/ani14040566





